Abstract
A strain (S. fradiae ΔurdQ/R) with mutations in urdQ and urdR encoding a dTDP-hexose-3,4-dehydratase and a dTDP-hexose-4-ketoreductase, respectively, produces a new urdamycin analogue (urdamycin X) with changes in the polyketide structure. The structure of urdamycin X has been elucidated by NMR spectroscopy. Urdamycin X was not detectable, even in small amounts, in either S. fradiae ΔurdQ, in S. fradiae ΔurdR or in S. fradiae A0, a mutant lacking all glycosyltransferase genes. Complementation of S. fradiae ΔurdQ/R restored urdamycin A production indicating that the mutations did not cause any polar effect.
Keywords: Streptomyces fradiae, Secondary metabolism, Urdamycins, Deoxysugar biosynthetic enzymes
1. Introduction
Urdamycin A (Fig. 1), produced by Streptomyces fradiae Tü2717 (S. fradiae Tü2717), is an angucycline antibiotic with some anticancer activity (Drautz et al., 1986). It has been used as model substance for biosynthetic studies focusing especially on the biosynthesis of the sugar side chain (Hoffmeister et al., 2000; Trefzer et al., 2000). In addition, mutants of S. fradiae Tü2717 have been used as hosts for the production of hybrid natural products obtained by combinatorial biosynthesis (Trefzer et al., 2001; Luzhetskyy et al., 2005).
Fig. 1.
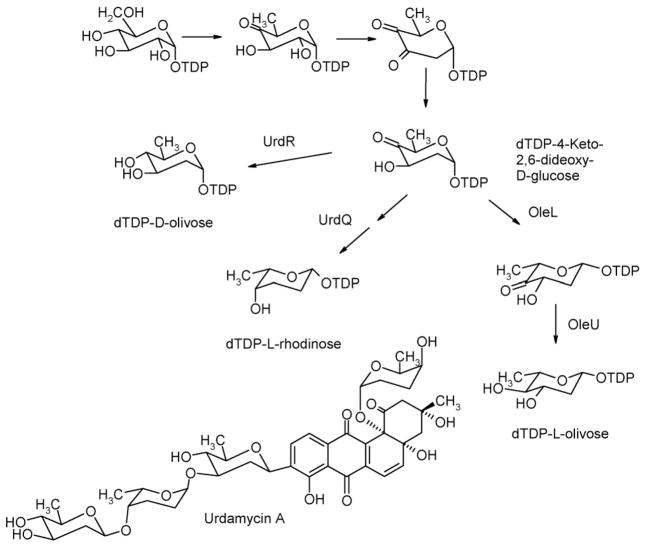
Biosynthetic pathway to dTDP-D-olivose, dTDP-L-rhodinose as sugar components of urdamycin A. A pathway to dTDP-L-olivose catalyzed by OleL and OleU is also shown.
Combinatorial biosynthesis has been described as an efficient implement for drug discovery and development, once the enzymology of the pathway being manipulated and the physiology of the organisms used for production is perfectly understood (Floss, 2006; Reeves, 2003). The enzymology for natural product biosynthesis ranges from single catalytic steps to multistep assemblies. Examples for single step reactions are glycosylation, hydroxylation and methylation, examples for multistep assemblies are polyketide synthases or non-ribosomal peptide syntethases (Reeves, 2003). Inactivation of a gene encoding a multifunctional protein very often leads to a non producing strain while inactivation of a gene responsible for a single step reaction very often leads to simple structural changes and these changes are often predictable. For example, the inactivation of a sugar biosynthetic gene generates a mutant producing a novel compound with changes in the glycan structure, and the inactivation of a gene involved in modifying the polyketide backbone results in a mutant producing a compound with changes in the aglycon structure (Reeves, 2003; Rix et al., 2002).
In this article, surprisingly and for the first time, we have observed that a mutant with modified sugar biosynthetic genes produces a new compound with changes in the polyketide structure.
2. Materials and methods
2.1. Baterial strains, plasmids and culture conditions
S. fradiae Tü2717 (Drautz et al., 1986), S. fradiae ΔurdR (Hoffmeister et al., 2000) and all mutants were grown on 1% malt extract, 0.4% yeast extract, 0.4% glucose and 1mM CaCl2, pH adjusted to 7.2 (HA medium) at 28 °C. DNA manipulation was carried out using Escherichia coli XL-1 Blue MRF′ (Strategene) as host strain. Plasmids pBluecript SK– was from Stratagene. Plasmid pKC1132 and pKC1218ermE were kind gifts from Eli Lilly and Company. For gene expression (complementation experiments) plasmid pUWL201 (Trefzer et al., 2000) and pIJ2925 (Kieser et al., 2000) were used. The construction of the gene inactivation plasmid pKurdQpm has been described (Hoffmeister et al., 2000). Plasmids pLR234Δ7 and pUC18U containing oleL and oleU (Aguirrezabalaga et al., 2000; Lombo et al., 2004; Salas and Mendez, 2005) were a kind gift of Prof. Dr. J.A. Salas, Oviedo, Spain. E. coli was grown on Luria-Bertani (LB) agar or liquid medium containing the appropriate antibiotic. For urdamycin production S. fradiae and all mutants were grown in NL111V medium as described (Trefzer et al., 2000).
2.2. General genetic manipulation
Standard molecular biology procedures were performed as described (Kieser et al., 2000). Isolation of E. coli DNA and DNA restriction, were performed by the protocols of the manufactures of kits, enzymes, and reagents (Amersham, Pharmacia, Boehringer, Mannheim, Promega, Stratagene).
2.3. Generation of S. fradiae ΔurdQ/R, a mutant lacking UrdQ and UrdR
Intergeneric conjugation between E. coli and S. fradiae ΔurdR was performed as described earlier (Luzhetskyy et al., 2006) using the plasmid pKurdQpm. After conjugation apramycin resistant colonies were obtained. Numerous colonies were grown on plates containing no apramycin to select for loss of resistance. Six apramycin-sensitive colonies were obtained suggesting that they were the consequence of a double crossover. One mutant named S. fradiae ΔurdQ/R was further examined. PCR fragments obtained from S. fradiae ΔurdQ/R using primers Q1: 5′-GGAACCACCGAATTCTGGCCGT-CC-3′ and Q2: 5′-CTAGCCACAAGCTTCGACGAACTCCTT-GAT-3′ were analyzed by restriction analysis. Fragments could not be restricted by NcoI which was the endonuclease employed for frame shifting. In contrast, PCR fragments amplified with the wt-DNA as template were digestible.
2.4. Construction of gene expression (complementation) plasmids
The construction of gene complementation plasmids containing either urdQ or urdR has been described (Hoffmeister et al., 2000). For the construction of pKColeUL oleL was amplified by PCR using pLR234Δ7 as template. SacI restriction sites were introduced 5′ to the ribosome binding site and 3′ to the termination codon using primers oleLF: 5′-TTGAATGGATCCGAGCTCCAG-3′ and oleLR: 5′-CGGCCGGAGCTCGGGTGCCGG-3′. The fragment containing oleL was cloned into the SacI site of pIJ2925 (Kieser et al., 2000) to yield plasmid pIJoleL. Then plasmid pUC18U was restricted by HindIII–XbaI. A 1.0 kb fragment containing oleU was ligated into pIJoleL previously restricted by the same enzymes to yield pIJoleUL. A 2,0 kb BglII fragment of pIJoleUL containing oleU and oleL was ligated behind the ermE promoter of pKC1218ermE to generate pKColeUL. For the construction of pKClanQR a 3.0 kb BamHI fragment from cosmid H2-26 (Westrich et al., 1999), containing lanQ and lanR, was ligated behind the ermE promoter into the BglII site of pKC1218ermE.
2.5. Analysis of secondary metabolites produced by S. fradiae Tü2717 and by the mutants
Strains were cultured in production medium for 4 days at 28 °C in a rotary shaker (180 rpm) as described (Drautz et al., 1986). Samples (0.9 ml) were extracted with an equal volume of ethyl acetate. The solvent of the organic phase was removed and the residue was dissolved in 30 μl methanol. This solution was used for HPLC–UV–MS analyses. HPLC–UV–MS analysis was performed on an Agilent 1100 series LC/MS liquid chromatograph equipped with a photodiode array detector. For mass detection electrospray ionization (ESI) with detection in the positive and negative modes were used. The LC-system was equipped with a Hewlett Packard ZORBAX SB C-18 column (5 mm particle size, 4.6 mm × 150 mm), maintained at 35 °C. The gradient profile was: Solvent A: 0.5% acetic acid in H2O, Solvent B: 0.5% acetic acid in CH3CN. An initial hold for 3 min with 30% B was followed by a step gradient from 30 up to 60% B within 16 min and from 60 up to 95% within 3 min and that relationship was held for further 2 min. The solvent flow rate was 0.7 mL min−1.
2.6. Isolation of urdamycin X
Cultures of S. fradiae ΔurdQ/R were extracted with an equal volume of ethyl acetate. After evaporation of the solvent the dried extract was redissolved in methanol.
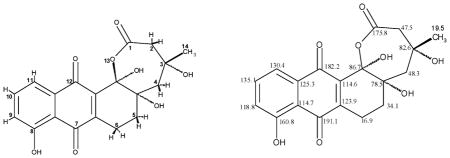
Urdamycin X was isolated from the extract using several chromatographic steps: (i) RP18 silica gel column (15 cm × 3 cm), eluted with water and acetonitrile (67:33), (ii) HPLC with gradient mixture of water (with 0.1% formic acid) and acetonitrile (standard gradient). A total of 23.8 mg of urdamycin X has been purified, and characterized by mass, 1H NMR, 13C NMR including 2D NMR (Table 1).
Table 1.
NMR spetral data; NMR analysis of urdamycin X
 | |||||
|---|---|---|---|---|---|
| Position | 1H NMR (multiplicity) | 13C NMR | HMBC | COSY | NOESY |
| 1 | – | 175.8 | – | – | |
| 2ax | 2.30 (d, 13.5) | 47.5 | 1, 3, 4 | 2eq, 4ax, 4eq 2ax, 4ax, 4eq | |
| 2eq | 1.95 (dd, 13.5, 3.6) | ||||
| 3 | – | 82.6 | – | – | |
| 3-OH | 4.05 (br s) | – | – | – | 4a-OH, 12b-OH |
| 4ax | 2.22 (d, 10.2) | 48.3 | 2, 3, 4a, 12b, 14 | 2eq, 4ax, 2eq 2ax, 2ax, 4eq | |
| 4eq | 1.99 (dd, 10.2, 3.6) | ||||
| 4a | – | 78.5 | – | – | |
| 4a-OH | 4.76 (br s) | – | – | – | |
| 5ax | 1.75 (ddd, 10.5, 10.2, 6.3) | 34.1 | 4, 4a, 6, 12b | 5eq, 6ax, 6eq | |
| 5eq | 2.16 (ddd, 10.5, 10.5, 4.8) | 5ax, 6ax, 6eq | |||
| 6ax | 2.84 (ddd, 10.2, 9.9, 4.8) | 16.9 | 4a, 5, 6a, 7 | 5eq, 5ax, 6eq | |
| 6eq | 2.64 (ddd, 10.5, 9.9, 6.3) | 5ax, 6ax, 5eq | |||
| 6a | – | 123.9 | – | – | |
| 7 | – | 191.1 | – | – | |
| 7a | – | 114.7 | – | – | |
| 8 | – | 160.8 | – | – | |
| 8-OH | 12.52 (br s) | – | – | – | |
| 9 | 7.57 (dd, 7.8, 1.2) | 125.3 | 7, 7a, 9,10, 11a | 10, 11 | |
| 10 | 7.63 (t, 7.8) | 135.1 | 9, 11, 11a | 9, 11 | |
| 11 | 7.26, (dd, 7.8, 1.2) | 118.8 | 9, 10, 11a, 12 | 9, 10 | |
| 11a | – | 130.4 | – | – | |
| 12 | – | 182.2 | – | – | |
| 12a | – | 114.6 | – | – | |
| 12b | – | 86.7 | – | – | |
| 12b-OH | 9.62 (br s) | – | – | – | |
| 14 | 1.48, (s) | 19.5 | 2, 3, 4 | – | 5ax, 4ax, 2ax, 6ax |
2.7. Structure elucidation of urdamycin X: high resolution NMR and ESI-MS measurement
All NMR assignments have been confirmed through combining 1H NMR, 13C NMR with 2D NMR spectra, including H, H-COSY, HSQC and HMBC. The stereochemistry at the chiral centers was determined through a NOESY spectrum, where a strong coupling has been found between 3-OH, 4a-OH, 12b-OH. The 3-CH3 group (C-14) showed coupling with the axial protons attached to C-2, C-4, C-5 and C-6.
High resolution ESI-MS was measured using a Micromass QTOF2 mass spectrometer.
3. Results
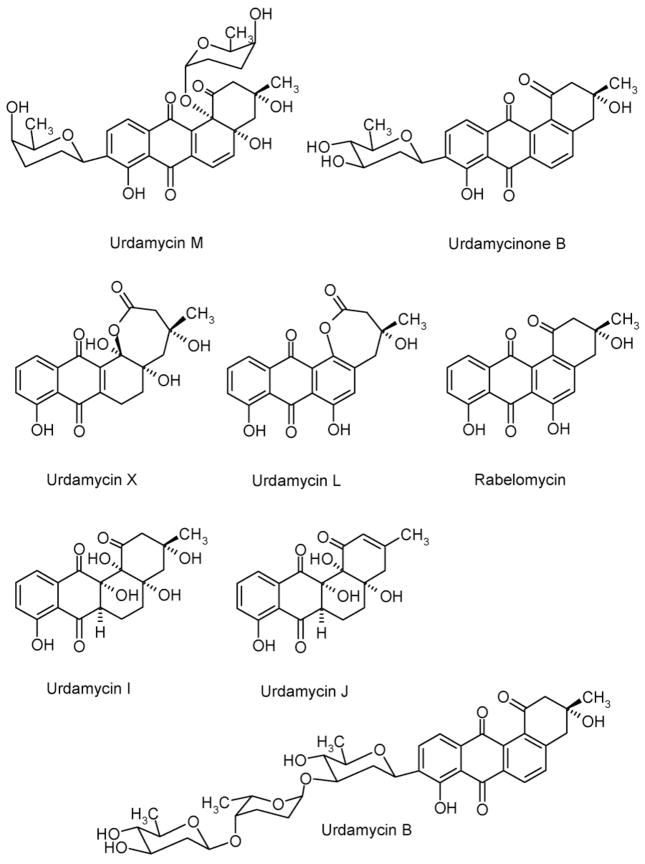
Urdamycin A (Fig. 1) served as a model compound for the investigation of the formation of a saccharide side chain during the past 10 years (Hoffmeister et al., 2000; Trefzer et al., 2000; Rix et al., 2003; Luzhetskyy et al., 2005). Functions of all glycosyltransferases as well as most enzymes involved in deoxy-sugars formation have been elucidated (Fig. 1) (Trefzer et al., 2000; Hoffmeister et al., 2000). The biosynthesis of D-olivose and L-rhodinose starts from dTDP-D-glucose. dTDP-4-keto-2,6-dideoxy-D-glucose was shown to be the central intermediate in the biosynthesis of dTDP-D-olivose and dTDP-L-rhodinose during urdamycin A biosynthesis. Inactivation of urdR involved in D-olivose biosynthesis led to the formation of urdamycin M (Fig. 2, Table 2), which carries a C-glycosidically attached D-rhodinose at the 9-position and inactivation of urdQ prevented the mutant strain from producing L-rhodinose resulting in the accumulation of urdamycinone B (Fig. 2, Table 2).
Fig. 2.
Structures of urdamycin M, urdamycinone B, urdamycin X, urdamycin L, rabelomycin, urdamycin I, urdamycin J and urdamycin B.
Table 2.
Mutated genes, gene constructs and resulting metabolites
| Mutated gene | Expressed gene | Main product produced by the recombinant strain |
|---|---|---|
| urdR | – | Urdamycin M |
| urdQ | – | Urdamycinone B |
| urdR and urdQ | – | Urdamycin X |
| urdR and urdQ | urdQ | Urdamycin M |
| urdR and urdQ | urdR | Urdamycinone B |
| urdR and urdQ | lanQ and lanR | Urdamycin A and urdamycin B |
| urdR and urdQ | oleL and oleU | Urdamycin I and urdamycin J |
| urdGT1a, urdGT1b, urdGT1c and urdGT2 | – | Urdamycin I and urdamycin J |
| urdM | – | Rabelomycin and urdamycin L |
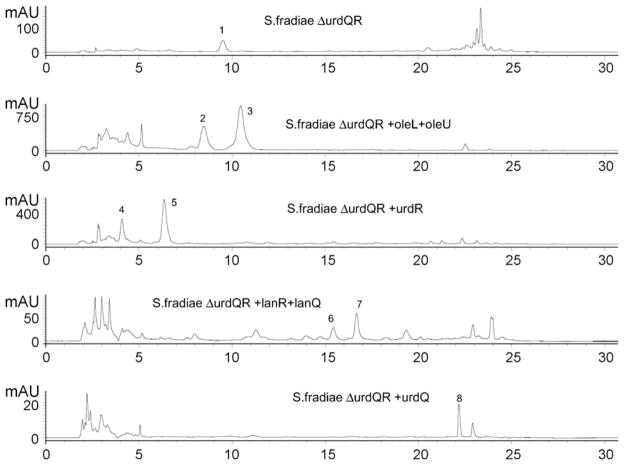
In this study, a strain (S. fradiae ΔurdQ/R) with mutations in urdQ and urdR encoding a dTDP-hexose-3,4-dehydratase and a dTDP-hexose-4-ketoreductase, respectively (Fig. 1) was generated. Analysis of the culture supernatant of S. fradiae ΔurdQ/R showed that one major compound was produced by the strain (Fig. 3) and its structure was elucidated using NMR spectroscopy (Table 1) and mass spectrometry.
Fig. 3.
HPLC chromatograms of crude extract from S. fradiae ΔurdQ/R and from S. fradiae ΔurdQ/R expressing different genes. First lane: detection of urdamycin X (1) in S. fradiae ΔurdQ/R; second lane: detection of urdamycin I (2) and urdamycin J (3) in S. fradiae ΔurdQ/R expressing oleL and oleU; third lane: detection of aquayamycin (4) and urdamycinone B (5) in S. fradiae ΔurdQ/R expressing urdR; fourth lane: detection of urdamycin A (6) and urdamycin B (7) in S. fradiae ΔurdQ/R expressing lanR and lanQ; fifth lane: detection of urdamycin M (8) in S. fradiae ΔurdQ/R expressing urdQ. Compounds were identified by UV- and MS-spectroscopy (structures of compounds are shown in Figs. 1 and 2).
The unusual hemiketal-lactone ring A followed from the 13C NMR data (δ 86.7 for C-12b, δ 175.8 for C-1) and indicative HMBC-couplings, e.g. 3JC–H between 5-Ha and C-12b, and between 4-Ha and C-12b), along with the HR mass of 374.1002 confirming the molecular formula of C19H18O8. The stereochemistry of the three centres at C-3, C-4a, and C-12b followed from observed NOESY couplings between the three OH groups located at these positions along with the given stereochemistry at C-3 from the biosynthetic process, while the 3-methyl group protons couple with the axial protons at 2-, 4-, 5- and 6-position. In addition to this new compound, named urdamycin X (Fig. 2), the mutant strain was also producing small amounts of urdamycinone B (Fig. 2, Table 2), detected by mass spectroscopy.
Complementation of the double mutant with either urdQ or urdR resulted in a complete disappearing of urdamycin X; instead urdamycin M (after complementation with urdQ) and aquayamycin and urdamycinone B (after complementation with urdR) (Figs. 2 and 3, Table 2) were accumulated in both strains. Complementation with lanQ (encoding a dTDP-hexose-3,4-dehydratase) and lanR (encoding a dTDP-hexose-4-ketoreductase), two genes from the landomycin biosynthetic gene cluster from S. cyanogenus S136 resulted in urdamycin A and urdamycin B production (Figs. 1 and 3, Table 2).
Expression of oleL and oleU, genes encoding a dTDP-4-keto-6-deoxyglucose 3,5-epimerase and a dTDP-hexose-4-ketoreductase, respectively, in S. fradiae ΔurdQ/R again resulted in disappearing of urdamycin X. Now urdamycin I and urdamycin J (Figs. 2 and 3, Table 2) were accumulated in the strain.
4. Discussion
The modifications observed in the polyketide-derived urdamycin X, particularly the hemiketal-lactone and the saturated 5,6 bond, were very surprising. We can rule out any polar effects caused by the gene inactivation experiments as complementation of our mutants restored activity. Urdamycin X was not detectable, even in small amounts, in either S. fradiae ΔurdQ, in S. fradiae ΔurdR or in S. fradiae A0, a mutant lacking all glycosyltransferase genes. Major compounds produced by S. fradiae A0 were urdamycin I and J (Fig. 2).
An explaination for the unusual production of urdamycin X might be that the biosynthesis of urdamycin A takes place in a complex. If two enzymes of the complex are not active or cannot interact with other enzymes, biosynthetic intermediates are not transferred correctly along the biosynthetic machinery. In our case it might influence the activities of two oxygenases UrdE and/or UrdM involved in urdamycin A biosynthesis.
There are reports in the literature indicating that polyketide biosynthesis takes place in a complex. The recent structural study by Stroud, Khosla and coworkers, in which the authors were able to ‘catch a type II PKS in action’, was a breakthrough. They provided the direct proof for a KS heterodimer in showing that KS and CLF (or KSβ) have evolved highly complementary contacts. In addition, they deduced from the structure that a protein cleft keeps the nascent polyketide chain extended. In this fashion the reactive ketide groups are separated and probably contact the KS-CLF mainly as enols (Keatinge-Clay et al., 2004). This study well complements the previously available structures of type II PKS components, mainly an X-ray structure of a priming KS (KAS III homolog) from the R1128 pathway (Pan et al., 2002), and NMR solution structures of ACPs from the actinorhodin (Crump et al., 1997), oxytetracycline (Findlow et al., 2003), and frenolicin (Li et al., 2003) PKSs.
Interestingly a very similar compound to urdamycin X, urdamycin L (Fig. 2) has been detected in an urdM mutant of S. fradiae as a minor compound (Rix et al., 2003). Urdamycin X differs from urdamycin L in possessing an almost saturated ring B (additional OH groups at the angular 4a- and 12b-positions, and a saturated 5,6-bond), while ring B in urdamycin L is an phenolic arene ring with an OH group in 6-position. However, both compounds are related by having an unusual ε-lactone as ring A. It was discussed that urdamycin L accumulates in the cell as a consequence of a Baeyer-Villiger-oxygenation process in context with the 12b-oxygenation. Our finding now might indicate that the 12b-oxygenation catalyzed by UrdM is even more complex than suggested, and leads here to an over-oxidized 12-carbon.
When oleU and oleL, two genes involved in dTDP-L-oleandrose biosynthesis in S. antibioticus were expressed in S. fradiae ΔurdQ/R, urdamycin I and J were produced by the strain but not urdamycin X. OleL (dTDP-4-keto-2,6-dideoxy-D-glucose-3,5-epimerase) and OleU (dTDP-4-deoxy-4-keto-L-oliose-4-ketoreductase) are known to convert dTDP-4-keto-2,6-dideoxy-D-glucose to dTDP-L-olivose. dTDP-L-olivose is obviously not accepted as substrate by UrdGT2 but interestingly, OleL and OleU are influenzing product formation.
S. fradiae ΔurdQ/R was also producing small amounts of urdamycinone B. We believe that an unspecific ketoreductase is converting dTDP-4-keto-2,6-dideoxyglucose to dTDP-D-olivose which is the substrate for glycosyltransferase UrdGT2 that form the C-glycosidic link. However, the major sugar component accumulating in the mutant strain is presumably dTDP-4-keto-2,6-dideoxy-D-glucose (4, Scheme 2), which appears to be unsuited as a substrate of UrdGT2. Therefore urdamycin X remains not glycosylated.
Acknowledgments
This work was supported by the Deutsche Forschungs-gemeinschaft (DFG) grant 6-1 to A.B. and by NIH grant CA 102102 to J.R. We thank Prof. Dr. J. Salas for providing plasmids pLR234Δ7 and pUC18U.
Abbreviations
- PCR
polymerase chain reaction
- dTDP
deoxythymidine phosphate
- NMR
nuclear magnetic resonance
- NAD(H)
nicotinamide adenine dinucleotide
- NADP(H)
nicotinamide adenine dinucleotide phosphate
- PMP
pyridoxamine 5′-phosphate
Contributor Information
J. Rohr, Email: jrohr2@email.uky.edu.
A. Bechthold, Email: andreas.bechthold@pharmazie.uni-freiburg.de.
References
- Aguirrezabalaga I, Olano C, Allende N, Rodriguez L, Brana AF, Mendez C, Salas JA. Identification and expression of genes involved in biosynthesis of L-oleandrose and its intermediate L-olivose in the oleandomycin producer Streptomyces antibioticus. Antimicrob Agents Chemother. 2000;44:1266–1275. doi: 10.1128/aac.44.5.1266-1275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump MP, Crosby J, Dempsey CE, Parkinson JA, Murray M, Hopwood DA, Simpson TJ. Solution structure of the actinorhodin polyketide synthase acyl carrier protein from Streptomyces coelicolor A3(2) Biochemistry. 1997;20:6000–6008. doi: 10.1021/bi970006+. [DOI] [PubMed] [Google Scholar]
- Drautz H, Zähner H, Rohr J, Zeeck A. Metabolic products of microorganisms. 234 Urdamyins, new angucycline antibiotics from Streptomyces fradiae 1: Isolation, characterization and biological properties. J Antibiot. 1986;39:1657–1669. doi: 10.7164/antibiotics.39.1657. [DOI] [PubMed] [Google Scholar]
- Findlow SC, Winsor C, Simpson TJ, Crosby J, Crump MP. Solution structure and dynamics of oxytetracycline polyketide synthase acyl carrier protein from Streptomyces rimosus. Biochemistry. 2003;42:8423–8433. doi: 10.1021/bi0342259. [DOI] [PubMed] [Google Scholar]
- Floss HG. Combinatorial biosynthesis—potential and problems. J Biotechnol. 2006;124:242–257. doi: 10.1016/j.jbiotec.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeister D, Ichinose K, Domann S, Faust B, Trefzer A, Dräger G, Kirschning A, Fischer C, Künzel E, Bearden W, Rohr J, Bechthold A. The NDP-sugar co-substrate concentration and the enzyme expression level influence the substrate specificity of glycosyltransferases: cloning and characterization of deoxysugar biosynthetic genes of the urdamycin biosynthetic gene cluster. ChemBiol. 2000;7:821–831. doi: 10.1016/s1074-5521(00)00029-6. [DOI] [PubMed] [Google Scholar]
- Keatinge-Clay AT, Maltby DA, Medzihradszky KF, Khosla C, Stroud RM. An antibiotic factory caught in action. Nat Struct Mol Biol. 2004;11:888–893. doi: 10.1038/nsmb808. [DOI] [PubMed] [Google Scholar]
- Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA. Practical Streptomyces Genetics. John Innes Foundation; Norwich: 2000. [Google Scholar]
- Li Q, Khosla C, Puglisi JD, Liu CW. Solution structure and backbone dynamics of the holo form of the frenolicin acyl carrier protein. Biochemistry. 2003;42:4648–4657. doi: 10.1021/bi0274120. [DOI] [PubMed] [Google Scholar]
- Lombo F, Gibson M, Greenwell L, Brana AF, Rohr J, Salas JA, Mendez C. Engineering biosynthetic pathways for deoxysugars: branched-chain sugar pathways and derivatives from the antitumor tetracenomycin. ChemBiol. 2004;11:1709–1718. doi: 10.1016/j.chembiol.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Luzhetskyy A, Fedoryshyn M, Gromyko O, Ostash B, Rebets Y, Bechthold A, Fedorenko V. IncP plasmids are most effective in mediating conjugation between Escherichia coli and streptomycetes. Genetika. 2006;42:595–601. [PubMed] [Google Scholar]
- Luzhetskyy A, Vente A, Bechthold A. Glycosyltransferases involved in the biosynthesis of biologically active natural products that contain oligosaccharides. Mol BioSyst. 2005;1:117–126. doi: 10.1039/b503215f. [DOI] [PubMed] [Google Scholar]
- Pan H, Tsai S, Meadows ES, Miercke LJ, Keatinge-Clay AT, O’Connell J, Khosla C, Stroud RM. Crystal structure of the priming beta-ketosynthase from the R1128 polyketide biosynthetic pathway. Structure. 2002;10:1559–1568. doi: 10.1016/s0969-2126(02)00889-4. [DOI] [PubMed] [Google Scholar]
- Reeves CD. The enzymology of combinatorial biosynthesis. Crit Rev Biotechnol. 2003;23:95–147. doi: 10.1080/713609311. [DOI] [PubMed] [Google Scholar]
- Rix U, Fischer C, Remsing LL, Rohr J. Modification of post-PKS tailoring steps through combinatorial biosynthesis. Nat Prod Rep. 2002;19:542–580. doi: 10.1039/b103920m. [DOI] [PubMed] [Google Scholar]
- Rix U, Remsing L, Hoffmeister D, Bechthold A, Rohr J. Urdamycin L: a novel metabolic shunt product that provides evidence for the role of the urdM gene in the urdamycin A biosynthetic pathway of Streptomyces fradiae TU 2717. Chembiochem. 2003;1:109–111. doi: 10.1002/cbic.200390002. [DOI] [PubMed] [Google Scholar]
- Salas JA, Mendez C. Biosynthesis pathways for deoxysugars in antibiotic-producing actinomycetes: isolation, characterization and generation of novel glycosylated derivatives. J Mol Microbiol Biotechnol. 2005;9:77–85. doi: 10.1159/000088838. [DOI] [PubMed] [Google Scholar]
- Trefzer A, Hoffmeister D, Westrich L, Weitnauer G, Stockert S, Künzel E, Rohr J, Fuchser J, Bindseil K, Bechthold A. Function of glycosyltransferase genes involved in the biosynthesis of urdamycin A. ChemBiol. 2000;7:133–142. doi: 10.1016/s1074-5521(00)00079-x. [DOI] [PubMed] [Google Scholar]
- Trefzer A, Fischer C, Stockert S, Westrich L, Künzel E, Girreser U, Rohr J, Bechthold A. Elucidation of the function of two glycosyltransferase genes (lanGT1 and lanGT4) involved in landomycin biosynthesis and generation of new oligosaccharide antibiotics. ChemBiol. 2001;8:1239–1252. doi: 10.1016/s1074-5521(01)00091-6. [DOI] [PubMed] [Google Scholar]
- Westrich L, Domann S, Faust B, Bedford D, Hopwood DA, Bechthold A. Cloning and characterization of the landomycin biosynthetic gene cluster of Streptomyces cyanogenus S136. FEMS Microbiol Lett. 1999;170:381–387. doi: 10.1111/j.1574-6968.1999.tb13398.x. [DOI] [PubMed] [Google Scholar]