Abstract
The ability of the synthetic retinoid MDI-301, in which the carboxylic acid of 9-cis-retinoic acid (9-cis-RA) is replaced with an ester linkage, to induce epidermal and dermal thickening and skin irritation (erythema and flaking) in hairless (rhino) mice following its topical application was investigated in comparison with that of 14-all-trans-retinoic acid (14-all-trans-RA) and 9-cis-RA. MDI-301 induced epidermal proliferation leading to a thickened epidermis. Treated animals also demonstrated a prominent band of organized connective tissue immediately below the epidermis. In its ability to induce epidermal thickening, MDI-301 was quantitatively similar to 14-all-trans-RA and 9-cis-RA. However, unlike 14-all-trans-RA and 9-cis-RA, which produced skin irritation associated with a perivascular influx of mononuclear leukocytes into the dermis, there was no evidence of irritation with MDI-301 and little leukocyte infiltration. Intraperitoneal injection of either 14-all-trans-RA or MDI-301 also resulted in epidermal and dermal thickening. Irritation of skin was not observed in these animals but splenomegaly was prominent in animals treated with either agent.
Keywords: Retinoids, Epidermal hyperplasia, Dermal thickening, Irritation
Introduction
The naturally occurring vitamin A derivatives including 14-all-trans-retinoic acid (14-all-trans-RA) and 9-cis-retinoic acid (9-cis-RA) stimulate proliferation of epidermal keratinocytes when the cells are maintained in monolayer culture under conditions that promote quiescence (Varani et al. 1989). Growth-promoting activity can also be seen in retinoid-treated organ cultures of human skin (Varani et al. 1993; Varani et al. 2001) as well as in human skin (Weiss et al. 1988; Kligman et al. 1993; Kang et al. 1995) and rodent skin (Mezick et al. 1984; Ashton et al. 1984; Chen et al. 1995) following topical application. The same retinoids also stimulate proliferation of quiescent fibroblasts in monolayer culture (Varani et al. 1994). Production of type I procollagen is increased under the same conditions, while elaboration of collagen-degrading matrix metalloproteinases (MMP) is decreased. Fibroblast activation, leading to increased type I procollagen production and decreased MMP expression is also seen following topical retinoid treatment of human or rodent skin (Kligman et al. 1984; Griffiths et al. 1993; Fisher et al. 1996; Fisher et al. 1997).
While these potent vitamin A derivatives have stimulatory effects on the major cellular elements of the skin, the same agents also induce irritation (i.e., erythema and flaking) in the skin (Ashton et al. 1984; Chen et al. 1995). Mechanisms underlying irritation as well as the relationship between growth-promoting activity and induction of irritation are not fully understood. In the past, it has been thought that growth promotion and irritation are inextricably linked. According to this view, generation of excess keratinocytes is directly responsible for epidermal sloughing. At the same time, production of interleukin-1 (IL-1) as well as other cytokines in the growth-stimulated keratinocytes is responsible for proinflammatory effects on the vasculature (Wood et al. 1996). Studies with 14-all-trans-retinol (14-all-trans-ROL), the precursor of 14-all-trans-RA, suggest that growth-promoting activity and irritation may not be inseparable. While 14-all-trans-ROL is approximately fourfold less effective than RA (on a molar basis) in inducing proliferation in the epidermis and type I procollagen in the dermis following topical application, 14-all-trans-ROL tends to be less irritating than RA at efficacious doses (Kang et al. 1995).
In the present report, we describe studies with a synthetic retinoid in which the carboxylic acid of 9-cis-RA has been replaced with an ester linkage (Purcell 1998). The synthetic agent, referred to as MDI-301, stimulated human epidermal keratinocyte proliferation in monolayer culture as effectively as 9-cis-RA or 14-all-trans-RA. MDI-301 also induced epidermal and dermal thickening following systemic or topical application, but was non-irritating when applied topically to the skin of hairless (rhino) mice.
Materials and methods
MDI-301, 9-cis-RA and 14-all-trans-RA
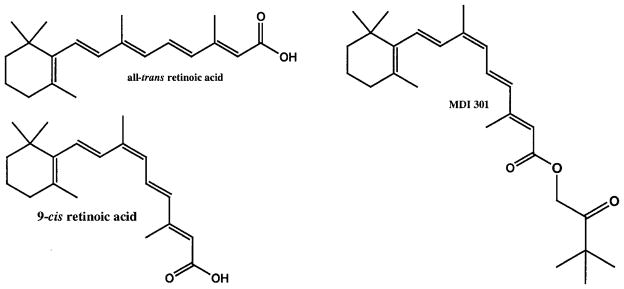
MDI-301 was synthesized from 9-cis-RA as described previously (Purcell 1998). Briefly, to a solution of 9-cis-RA (450 mg, 1.50 mM) in 17 ml dimethyl formamide were added at 0°C sequentially cesium carbonate (0.97 g, 3.0 mmol) and 1-chloropinacolone (0.25 ml, 1.9 mmol). The reaction mixture was stirred at room temperature for 2 h, and quenched with the addition of 20 ml water. The product was then extracted with methylene chloride (four times). The combined organic extract was dried over MgSO4 and concentrated under reduced pressure. Purification by silica gel column chromatography using 5:1 hexane/ethylacetate produced 480 mg of the final product, which contained a small amount of 1-chloropinacoline. Repeated recrystallization with ethanol at low temperature gave 320 mg of pure pinacolyl 9-cis-retinoate [(2E,4E,6Z,8E)-3,3-di-methyl-2-oxo-1-butyl 3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nonatetraenoate, MDI 301; mp 81°C]. The same chemical lot of 9-cis-RA that was used in the synthesis of MDI-301 for the present set of studies served as control. 14-All-trans-RA was purchased from Sigma Chemical Company (St. Louis, Mo.). Figure 1 shows the chemical structure of MDI-301 in relation to that of 9-cis-RA and 14-all-trans-RA.
Fig. 1.
Structure of 14-all-trans-RA, 9-cis-RA and MDI-301
During development of MDI-301, the in vivo metabolic profile was assessed. By 2 h after the last of four daily injections, the vast majority of the recovered material (>95%) was still found in the plasma as the parent compound. Small amounts of 9-cis-RA and 4-oxo-9-cis-RA were also found. The same was true 24 h after the last injection, although the total amount of retinoid in the plasma had decreased by over 90% (Durand et al. 1996).
For the present studies, all three agents were prepared at 20 mg/ml in dimethyl sulfoxide and stored at −20°C protected from light. For in vitro studies and for intraperitoneal injection, the agents were diluted in phosphate-buffered saline. For topical treatment, the agents were diluted in either 70% ethanol/30% propylene glycol (v/v) containing 0.5 mg/ml butylated hydroxytoluene to inhibit oxidation or in 100% acetone.
Topical treatment studies
Rhino mice (Rhino-M, Taconic Farms, Germantown, N.Y.) were used to investigate skin irritation and epidermal and dermal thickening induced by MDI-301 in comparison with that induced by the other retinoids. For these studies, mice (five per group) were treated daily for 3 weeks with the test reagents. Treatment was accomplished by applying 100 μl of the retinoid solutions topically to the right side of the back. Animals were examined grossly for irritation (erythema and scaling) twice weekly throughout the treatment period. Animals were killed 1 day after the final treatment. After visual examination of the skin, samples of skin were fixed in 10% buffered formalin. Histological changes in the epidermis (epidermal thickness) and dermis (thickness of the organized collagen band in the subepidermal zone) were quantified. Histological sections were also evaluated for the presence of perivascular leukocytes.
Intraperitoneal treatment studies
Hairless, athymic (nu/nu) mice (Charles River, Wilmington, De.) were used for systemic treatment studies. Mice (seven per group) were treated daily for 4 weeks with the test reagents by the intraperitoneal route. Animals were killed 1 day after the final treatment. Tissue from liver, spleen and skin was removed, fixed in 10% buffered formalin and examined histologically after staining with hematoxylin and eosin. Histological changes in the epidermis (epidermal thickness) and dermis (thickness of the organized collagen band in the subepidermal zone) were quantified. All procedures involving animals were approved by the University of Michigan Committee on Use and Care of Animals.
In vitro studies
The effects of MDI-301, 9-cis-RA and 14-all-trans-RA on human epidermal keratinocyte and human dermal fibroblast proliferation were examined in monolayer culture. Fibroblasts were isolated from neonatal foreskin tissue obtained at circumcision as described previously (Varani et al. 1994). The cells were grown in Dulbecco’s Minimal Essential Medium supplemented with non-essential amino acids and 10% fetal bovine serum (DMEM-FBS) (MA Bioproducts, Walkersville, Md.). Cells were grown at 37°C in an atmosphere of 5% CO2 and 95% air, and subcultured by exposure to trypsin and EDTA as required. For experiments, cells at passage two to four were plated in wells of a 24-well dish at 5×104 cells per well. DMEM-FBS was used as plating medium. Once cells had attached and spread, the wells were washed two times in Keratinocyte Basal Medium (KBM) (MA Bioproducts) and incubated in this medium. KBM is a low-Ca2+ (0.15 mM) variant of MCDB-154 medium. Retinoids were added to the culture medium at the start of the incubation period, and the cells were then incubated for 2 days. At the end of the incubation period, the cells were harvested and counted with the aid of an electronic particle counter.
Epidermal keratinocytes were isolated from neonatal foreskin tissue obtained at circumcision as described previously (Varani et al. 1994). KGM (MA Bioproducts) was used as culture medium. KGM contains the same basal medium as KBM but is supplemented with a number of growth-promoting agents including epidermal growth factor, insulin and pituitary extract. Keratinocytes were grown at 37°C in an atmosphere of 5% CO2 and 95% air, and subcultured by exposure to trypsin and EDTA as required. Proliferation studies with these cells were conducted exactly as described for fibroblasts except that cells were initially plated at 4×104 cells per well and harvested and counted after 3 days of incubation.
The HaCaT line of immortalized human epidermal keratinocytes (Boukamp et al. 1988) was also used to compare the effects of the three retinoids on keratinocyte proliferation. HaCaT cells were handled exactly as described for epidermal keratinocytes.
Results
Effects of MDI-301,14-all-trans-RA and 9-cis-RA on the skin of rhino mice following topical application
In a first series of experiments, mice were evaluated for epidermal and dermal thickening and for irritation after topical treatment with MDI-301, 9-cis-RA or 14-all-trans-RA in the ethanol/propylene glycol vehicle. Consistent with previous findings (Ashton et al. 1984; Chen et al. 1995), 14-all-trans-RA proved to be highly irritating. After 3 weeks of treatment, the skin of RA-treated animals was red and dry to the point where small cracks in the skin could be observed. Flaking of excess epidermis was readily apparent in these mice. Mice treated with 9-cis-RA demonstrated similar features of skin irritation. In contrast, there was no evidence of skin irritation in mice treated with MDI-301. As expected, none of the features of irritation were noted in untreated animals or those treated with vehicle alone. These observations are summarized in Table 1. Figure 2 demonstrates the appearance of skin from a mouse treated with MDI-301 (Fig. 2A) and from a mouse treated with 14-all-trans-RA (Fig. 2B). The differences in irritation were even more dramatic when the retinoids were prepared in acetone. The use of acetone allows much greater skin penetration of many reagents, including retinoids (Ashton et al. 1984). Consequently, biological responses tend to be enhanced. Mice treated topically with 14-all-trans-RA in this vehicle developed intense irritation, which was severe by the end of the first week of treatment. By day 10, treatment had to be suspended, and over the remaining days of the experiment, the intense irritation slowly subsided. In contrast, even when applied in acetone, MDI-301 was non-irritating (data not shown).
Table 1.
Irritation in rhino mouse skin following topical application of MDI-301, 14-all-trans-RA or 9-cis-RA. Erythema and skin flaking were assessed visually in each animal (n=3 for vehicle and 14-all-trans-RA and n=5 for other groups) and scored on a scale of 0–4+, with 4+ being maximal redness (flaking) and 0 being indistinguishable from the no-treatment group. Perivascular inflammation was assessed histologically, with 4+ indicating widespread foci of perivascular mononuclear cells (along with some polymorphonuclear leukocytes) throughout the dermis and 0 indicating no detectable foci of inflammatory cells. Values are means±SD (where no standard deviation is indicated, all animals received the same score)
| Treatment | Erythema | Flaking | Perivascular inflammation |
|---|---|---|---|
| None | 0 | 0 | 0 |
| Vehicle | 0 | 0 | 0 |
| 14-all-trans-RA (0.1%) | 3.7±0.5 | 4.0 | 3.8±0.3 |
| 9-cis-RA (0.03%) | 2.2±0.3 | 0 | 0.3±0.1 |
| 9-cis-RA (0.1%) | 3.4±0.3 | 0 | 4.0 |
| 9-cis-RA (0.3%) | 4.0 | 3.6±0.2 | 4.0 |
| MDI-301 (0.03%) | 0 | 0 | 0 |
| MDI-301 (0.1%) | 0.2±0.5 | 0 | 0.3±0.6 |
| MDI-301 (0.3%) | 0 | 0 | 0.2±03 |
Fig. 2.
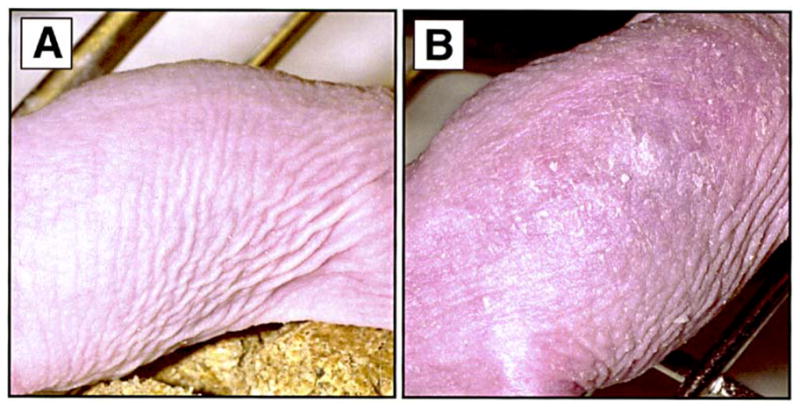
A, B Appearance of rhino mouse skin (A) after treatment for 3 weeks with 0.3% MDI-301 and (B) after treatment for 3 weeks with 0.1% 14-all-trans-RA. Both agents were prepared in the ethanol/propylene glycol vehicle
A perivascular influx of inflammatory cells was observed in animals treated with 14-all-trans-RA or 9-cis-RA (observed microscopically) (Table 1). Inflammatory cell infiltration was much reduced in animals treated with MDI-301. Figure 3 demonstrates the histological appearance of the typical perivascular inflammatory influx in an 14-all-trans-RA-treated animal and the lack of inflammatory cells in an animal treated with MDI-301. Most of the leukocytic infiltrate in the retinoid-treated animals consisted of mononuclear cells, although at the highest concentrations of 14-all-trans-RA and 9-cis-RA, dermal microabscesses with polymorphonuclear leukocytes were occasionally observed (not shown). Comparing erythema and flaking with inflammatory influx, it was apparent that the degree of inflammatory influx correlated closely with the level of erythema observed grossly. On the other hand, significant flaking was only observed at the highest concentration of both 9-cis-RA and 14-all-trans-RA (concentrations higher than needed to produce a perivascular leukocyte influx or erythema).
Fig. 3.
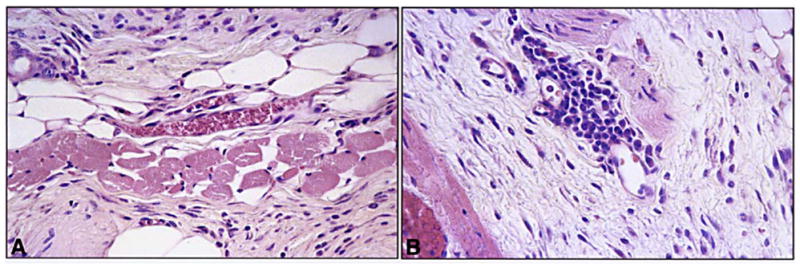
A, B Perivascular inflammation in retinoid-treated rhino mice. A Appearance of a dermal capillary in a rhino mouse after topical treatment for 3 weeks with 0.3% MDI-301. B Appearance of a dermal capillary in a rhino mouse after topical treatment for 3 weeks with 0.1% 14-all-trans-RA. There is no leukocytic infiltrate around the vessel in the MDI-301-treated animal, but an influx of inflammatory cells is evident in the vessel of the 14-all-trans-RA-treated mouse
Figure 4 demonstrates histological features of epidermis and dermis from control and retinoid-treated mice and Table 2 presents quantitative data on epidermal thickness and thickness of the organized band of collagen immediately below the epidermis from the same animals. In untreated and vehicle-treated animals, the epidermis was thin (one or two layers of viable keratinocytes) and the dermis consisted of a thin, disorganized “wispy” collagenous matrix. In contrast, mice treated with any of the three retinoids in either vehicle had a much thicker epidermis and a thick, organized layer of dermal collagen immediately below the epidermis. Utriculus formation, extensive in untreated and vehicle-treated animals, was much reduced in the retinoid-treated rhino mice. The responses to high concentrations of all three retinoids were similar (Table 2). In addition, it can be seen from Table 2 that MDI-301 and 9-cis-RA were dose-responsive over the same range of concentrations. However, with neither retinoid did we reach a concentration at which no response was observed.
Fig. 4.
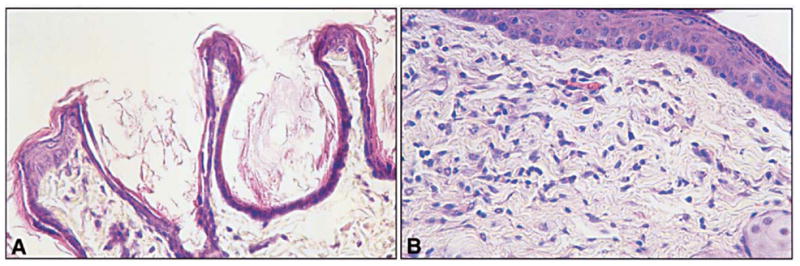
A, B Histological appearance of skin from a control rhino mouse (A), and a rhino mouse after topical treatment for 3 weeks with 0.1% MDI-301 (B). A The skin of the control animal is thin. The epidermis consists of one or two layers of viable cells and the dermis has no organized subepidermal band of connective tissue. Utriculus formation is prominent. B Skin from the retinoid-treated animal demonstrates a thickened epidermis with additional layers of keratinocytes. A prominent band of connective tissue is apparent beneath the epidermis. There is a significant reduction in utriculus formation. The histological appearance of the skin samples shown here are representative of their respective groups
Table 2.
Thickening of the skin of rhino mice following topical application of MDI-301, 14-all-trans-RA or 9-cis-RA. Epidermal thickness was taken as the distance (in microns) from the base of the epidermis to the upper layer of viable cells, and dermal thickness as the distance (in microns) from the base of the epidermis to the lower edge of the dense, organized connective tissue band immediately below the epidermis. Values are means±SD from five (epidermis) or two (dermis) random measurements per tissue section in three animals from each group. Statistical significance was determined by ANOVA, followed by paired group comparisons. All of the retinoid values are significantly different from the no-treatment value at the P<0.01 level
| Treatment | Epidermis | Dermis |
|---|---|---|
| None | 9± 4 | 8± 4 |
| Vehicle | 8± 3 | 7+ 2 |
| 14-all-trans-RA (0.1%) | 46±14 | 75±12 |
| 9-cis-RA (0.03%) | 30±11 | 40±11 |
| 9-cis-RA (0.1%) | 38± 9 | 60±15 |
| 9-cis-RA (0.3%) | 46± 8 | 77±12 |
| MDI-301 (0.03%) | 35±13 | 55±12 |
| MDI-301 (0.1%) | 39±16 | 67±13 |
| MDI-301 (0.3%) | 47±31 | 67± 9 |
Effects of MDI-301 and 14-all-trans-RA on mouse skin following systemic treatment
Mice were treated for 4 weeks with either 14-all-trans-RA or MDI-301 given by the intraperitoneal route. This treatment led to histological changes in the skin that resembled those observed following topical application. In control mice, the epidermis was thin (two or three layers of viable keratinocytes) and there was no organized band of connective tissue below the epidermis in these animals. In contrast, retinoid-treated mice had a much thicker epidermis and a thick, organized layer of dermal collagen immediately below the epidermis. Histological features of skin from control and retinoid-treated mice are shown in Fig. 5 and the results of morphometric analysis are presented in Table 3. In spite of these profound changes in both the epidermis and dermis, there was no evidence of skin irritation (erythema and flaking) with either MDI-301 or 14-all-trans-RA. Careful histological examination revealed little influx of inflammatory cells into the skin.
Fig. 5.
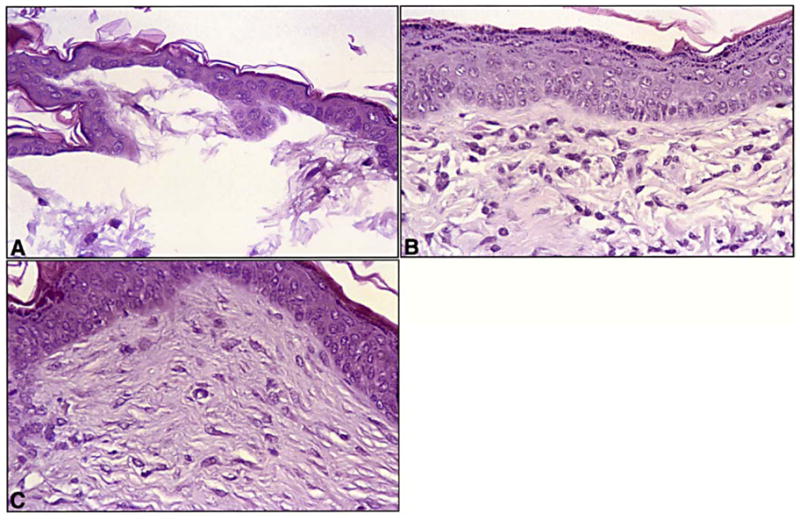
A–C Histological appearance of skin from a control mouse (A), and a mouse following treatment for 4 weeks with 14-all-trans-RA at 10 mg/kg (B) or with MDI-301 at 50 mg/kg (C). A Skin of the control animal is thin. The epidermis consists of one or two layers of viable cells and the dermis has no organized subepidermal band of connective tissue. B, C Skin from the retinoid-treated animals shows a thickened epidermis with additional layers of keratinocytes. A prominent band of connective tissue is apparent beneath the epidermis. The histological appearances of the skin samples shown here are representative of their respective groups
Table 3.
Thickening of the skin of hairless mice following in-traperitoneal injection of MDI-301 or 14-all trans RA. Epidermal thickness was taken as the distance (in microns) from the base of the epidermis to the upper layer of viable cells, and dermal thickness as the distance (in microns) from the base of the epidermis to the lower edge of the dense, organized connective tissue band immediately below the epidermis. Values are means±SD from ten (epidermis) or five (dermis) random measurements per tissue section in two animals from each group. Statistical significance was determined by ANOVA, followed by paired group comparisons. All of the retinoid values are significantly different from the no-treatment value at the P<0.01 level
| Treatment | Epidermis | Dermis |
|---|---|---|
| None | 10± 4 | 13±11 |
| 14-all trans RA (10 mg/kg) | 35±16 | 62±18 |
| MDI-301 (10 mg/kg) | 30±18 | 90±11 |
| MDI-301 (50 mg/kg) | 31±17 | 73±15 |
In addition to the skin, we also examined spleen and liver tissue from control and retinoid-treated mice. There was nothing remarkable detected in the liver tissue from retinoid-treated animals either grossly or at the light microscopic level, but gross examination revealed that the spleens from all of the treated mice were enlarged relative to spleens from control mice (approximately 140 mg in control mice versus 180–220 mg per spleen in mice treated with 14-all-trans-RA or MDI-301). Histological evaluation demonstrated enlargement of the periartereolar sheaths with an increased number of lymphoid cells in the retinoid-treated tissue. There were no apparent differences among the retinoid-treated animals (not shown). Splenomegaly is commonly observed in animals exposed to high doses of retinoids (Look et al. 1995; Standeven et al. 1996) and is reflective of a systemic inflammatory response.
Effects of MDI-301, 14-all-trans-RA and 9-cis-RA on human epidermal keratinocytes and dermal fibroblasts in vitro
Figure 6 demonstrates the effects of the three retinoids on human keratinocyte and fibroblast proliferation. Consistent with the findings of previous studies (Varani et al. 1994), 14-all-trans-RA induced proliferation of both cell types. Keratinocyte proliferation was maximal at 0.05 μg/ml (approximately 0.15 μM) and the net increase was 3.13-fold as compared to a net increase of 0.25-fold under control conditions. The other two agents produced a similar net increase (2.75-fold with 9-cis-RA and 3.15-fold with MDI-301) at the same concentration. The results described were obtained using the HaCaT keratinocyte cell line, but virtually identical results were obtained with primary cultures of neonatal foreskin keratinocytes (not shown).
Fig. 6.
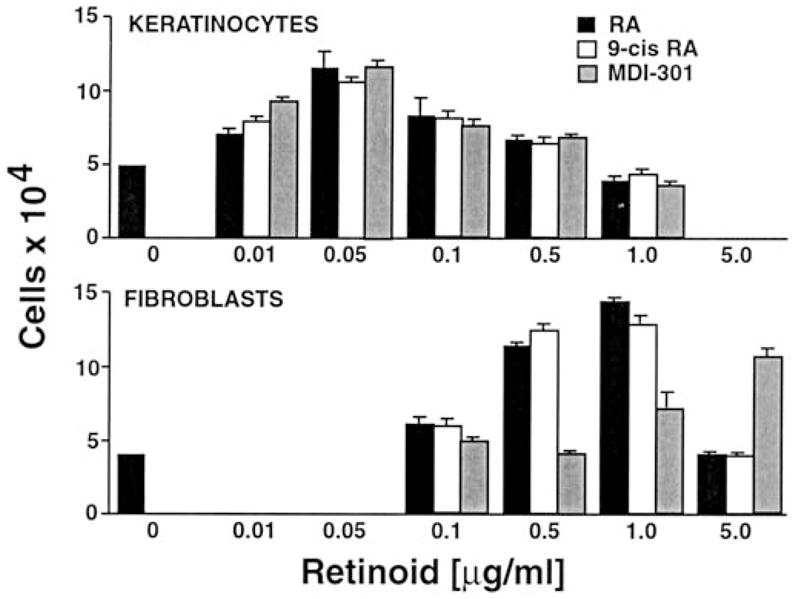
Effects of MDI-301, 14-all-trans-RA and 9-cis-RA on human epidermal keratinocytes and human dermal fibroblasts in monolayer culture. Cell numbers were determined as described in Materials and methods. Values shown are the average number of cells ±SD present on day 3 for keratinocytes and day 2 for fibroblasts. Statistical significance was determined by ANOVA, followed by paired group comparisons. Compared with cultures with no retinoid, keratinocyte numbers were significantly increased by all three retinoids at 0.01–0.5 μg/ml (P<0.05), and fibroblast numbers were significantly increased by 14-all-trans-RA and 9-cis-RA at 0.5 and 1.0 μg/ml (P<0.05) and by MDI-301 at 5 μg/ml (P<0.05)
Fibroblast proliferation was induced by 14-all-trans-RA and 9-cis-RA over a similar concentration range (1.75-fold and 1.63-fold increases with 14-all-trans-RA and 9-cis-RA, respectively, at 1.0 μg/ml as compared to a 0.5-fold decrease under control conditions). MDI-301 stimulated fibroblast growth by 1.40-fold, an increase similar to that seen with the other agents. However, as compared to the other retinoids, an approximately 5-fold higher concentration was required to achieve the same response (Fig. 6).
Discussion
Topical retinoid treatment of the skin stimulates events in both the epidermis and dermis that lead to the repair of skin damaged through the natural aging process (Kligman et al. 1993; Varani et al. 2000) or from chronic sun exposure (photodamage) (Weiss et al. 1988). The same retinoid treatment protocols that repair skin damage, however, also cause irritation, manifested primarily be erythema and scaling (Kang et al. 1995). The relationship between events that lead to skin repair and irritation is not fully understood. The results presented in this report help to address this issue.
In the past it was thought that the major manifestations of skin irritation – i.e., epidermal scaling and erythema – were the direct and indirect consequences of retinoid-induced epidermal hyperplasia. According to this understanding, epidermal flaking is simply the “shedding” of excess epidermis. Erythema, on the other hand, reflects the production of epidermal cytokines such as IL-1, either as a result of retinoid-stimulated keratinocyte proliferation directly or as a consequence of the disrupted epidermal barrier accompanying retinoid stimulation (Wood et al. 1996). IL-1 induces a number of events in vascular endothelial cells that result, ultimately, in increased vascular permeability and leukocyte influx (Kupper 1990; Luger and Schwartz 1990). If this view is correct, it should be possible to inhibit erythema and perivascular leukocyte infiltration into retinoid-treated skin by suppressing the epidermal retinoid response. Since recent studies have demonstrated that epidermal retinoid responses can be separated, at least in part, from dermal responses (Xiao et al. 1999; Varani et al. 2001), to the extent that irritation is a manifestation of excessive keratinocyte proliferation, it should be possible to prevent irritation without compromising events in the dermis (induction of procollagen production and inhibition of MMP function) that are necessary for skin repair.
The present studies present new insight into retinoid irritancy. These studies suggest that epidermal hyperplasia itself can be separated from events that underlie retinoid irritation. Induction of hyperplasia in the skin of hairless mice by MDI-301 was not associated with either scaling or erythema. In parallel, topical treatment of mice with this agent resulted in almost no inflammatory infiltrate in the dermis. In contrast, both 14-all-trans-RA and 9-cis-RA elicited erythema and flaking of mouse skin and this was associated with perivascular infiltration of mononuclear cells into the treated skin. While a relationship between leukocyte infiltration into the skin and erythema is not difficult to understand (both depend on vascular changes), the relationship between inflammatory influx and epidermal scaling is less intuitive. It may be that inflammatory cell products promote in some way the separation of large, visible pieces of the keratinized epidermal layer from the underlying viable cells. Epidermal hyperplasia may be necessary but not sufficient for excessive flaking. The fact that epidermal scaling was significant only at the highest retinoid concentrations while both hyperplasia and erythema were seen at lower doses suggests that epidermal flaking is a complex response and, perhaps, depends on additional factors.
An alternative hypothesis is that retinoid-induced skin irritation reflects a direct response of cells in the dermis to topical treatment. If this were the case, then one might envision MDI-301 (with its reduced activity for fibroblasts in vitro relative to 14-all-trans RA and 9-cis-RA) being inherently less irritating than the naturally occurring analogues. However, this is not likely to be the entire explanation since there was at least a tenfold difference between MDI-301 and the other retinoids in regard to irritation, but a fivefold difference in stimulation of fibroblast growth.
What accounts for the differential sensitivity of dermal fibroblasts to MDI-301 on the one hand, and to 14-all-trans-RA and 9-cis-RA on the other, and the lack of a differential keratinocyte response to the same agents? The results of past in vitro studies along with the data in Fig. 6 provide insight. Keratinocyte proliferation was maximal with all three agents at a concentration of 0.05 μg/ml (approximately 150 nM). At this concentration, retinoid receptors are activated (Duell et al. 1992), but biophysical effects on the membrane are insignificant. At 1–5 μg/ml (micromolar range), retinoids have a mild, detergent-like effect on cellular membranes (Meeks et al. 1981). Past studies have demonstrated that mild detergents can stimulate fibroblast proliferation at concentrations below levels at which they are toxic (Varani et al. 1991). It may be that the keratinocyte response reflects receptor occupancy only, while the fibroblast response involves both receptor activation and membrane-active effects.
In summary, it has in the past been thought that retinoid efficacy and retinoid-induced skin irritation are inextricably linked. The present studies do not support this view. It is clear from our data that the propensity to induce skin irritation (a major undesired consequence of topical retinoid use) can be distinguished from induction of epidermal/dermal thickening. A drug such as MDI-301, with capacity to induce skin thickening but little capacity for irritation, should find wide acceptance in the dermatological marketplace, where irritation is the major cause of non-compliance among users.
Acknowledgments
This study was supported in part by grants AR 49621 and DK 59169 from the USPHS. The authors would like to thank Molecular Design International, Inc. (MDI) of Memphis, TN, for supplying MDI-301.
References
- Ashton RE, Connor MJ, Lowe NJ. Histological changes in the skin of the rhino mouse (hrrhhrrh) induced by retinoids. J Invest Dermatol. 1984;82:632–635. doi: 10.1111/1523-1747.ep12261472. [DOI] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Ostowski J, Whiting G, Hammer L, Currier SJ, Reczek PR. Retinoic acid receptor gamma mediates topical retinoid efficacy and irritation in animal models. J Invest Dermatol. 1995;104:779–783. doi: 10.1111/1523-1747.ep12606988. [DOI] [PubMed] [Google Scholar]
- Duell EA, Astrom A, Griffiths EC, Chambon P, Voorhees JJ. Human skin levels of retinoic acid and cytochrome P-450-derived 4-hydroxyretinoic acid after topical application or retinoic acid in vivo as compared to concentrations required to stimulate retinoic acid-mediated transcription in vitro. J Clin Invest. 1992;90:1269–1274. doi: 10.1172/JCI115990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand A, Bun H, Marchetti MN. Preliminary development of a bioanalytical method for measuring MDI-301 and potential metabolites and its application to rat plasma samples (Technical Report) Molecular Design International; Memphis, TN: 1996. [Google Scholar]
- Fisher GJ, Datta SC, Talwar HS, Wang Z-Q, Varani J, Kang S, Voorhees JJ. The molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–338. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Wang Z-Q, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. New Engl J Med. 1997;337:1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- Griffiths CEM, Russman G, Majmudar G, Singer RS, Hamilton TA, Voorhees JJ. Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid) New Engl J Med. 1993;329:530–534. doi: 10.1056/NEJM199308193290803. [DOI] [PubMed] [Google Scholar]
- Kang S, Duell EA, Fisher GJ, Datta SC, Wang Z-Q, Reddy AP, Tavakkol A, Yi Y, Griffiths CEM, Elder JT, Voorhees JJ. Application of retinol to human skin in vivo induces epidermal hyperplasia and cellular retinoid binding proteins characteristic of retinoic acid but without measurable retinoic acid levels or irritation. J Invest Dermatol. 1995;105:549–556. doi: 10.1111/1523-1747.ep12323445. [DOI] [PubMed] [Google Scholar]
- Kligman AM, Dogadkina D, Lavker RM. Effects of topical tretinoin on non-sun-exposed skin of the elderly. J Am Acad Dermatol. 1993;29:25–33. doi: 10.1016/0190-9622(93)70147-l. [DOI] [PubMed] [Google Scholar]
- Kligman LH, Duo CH, Kligman AM. Topical retinoic acid enhances the repair of ultraviolet damaged dermal connective tissue. Connect Tissue Res. 1984;12:139–150. doi: 10.3109/03008208408992779. [DOI] [PubMed] [Google Scholar]
- Kupper TS. Immune and inflammatory processes in cutaneous tissues. Mechanisms and speculation. J Clin Invest. 1990;86:1783–1789. doi: 10.1172/JCI114907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Look J, Landwehr J, Bauer F, Hoffmann AS, Bluethmann H, Lemotte P. Marked resistance of RARγ-deficient mice to the toxic effects of retinoic acid. Am J Physiol. 1995;269:E91–E98. doi: 10.1152/ajpendo.1995.269.1.E91. [DOI] [PubMed] [Google Scholar]
- Luger TA, Schwartz T. Evidence for an epidermal cytokine network. J Invest Dermatol. 1990;935:100s–104s. doi: 10.1111/1523-1747.ep12874944. [DOI] [PubMed] [Google Scholar]
- Meeks RG, Zaharevitz D, Chen RF. Membrane effects of retinoids: possible correlation with toxicity. Arch Biochem Biophys. 1981;207:141–147. doi: 10.1016/0003-9861(81)90019-9. [DOI] [PubMed] [Google Scholar]
- Mezick JA, Bhatia M, Capetola RJ. Topical and systemic effects of retinoids on horn-filled utriculus size in the rhino mouse. A model to quantify “anti-keratinizing” effects of retinoids. J Invest Dermatol. 1984;83:110–113. doi: 10.1111/1523-1747.ep12263280. [DOI] [PubMed] [Google Scholar]
- Purcell WP. 5,837,728. US Patent. 1998 November 17;
- Standeven AM, Johnson AT, Escobar M, Chandraratna RAS. Specific antagonist of retinoid toxicity in mice. Toxicol Applied Pharmacol. 1996;138:169–175. doi: 10.1006/taap.1996.0110. [DOI] [PubMed] [Google Scholar]
- Varani J, Nickoloff BJ, Dixit VM, Mitra RS, Voorhees JJ. All-trans retinoic acid stimulates growth of adult human keratinocytes cultured in growth factor-deficient medium, inhibits the production of thrombospondin and fibronectin and reduces adhesion. J Invest Dermatol. 1989;93:449–454. doi: 10.1111/1523-1747.ep12284020. [DOI] [PubMed] [Google Scholar]
- Varani J, Astrom A, Griffiths CEM, Voorhees JJ. Induction of proliferation of growth inhibited keratinocytes and fibroblasts in monolayer culture by sodium lauryl sulfate; comparison with all-trans retinoic acid. J Invest Dermatol. 1991;97:917–921. doi: 10.1111/1523-1747.ep12491682. [DOI] [PubMed] [Google Scholar]
- Varani J, Fligiel SEG, Schuger L, Perone P, Inman D, Griffiths CEM, Voorhees JJ. Effects of all-trans retinoic acid and Ca++ on human skin in organ culture. Am J Pathol. 1993;142:189–198. [PMC free article] [PubMed] [Google Scholar]
- Varani J, Perone P, Griffiths CEM, Inman DR, Fligiel SEG, Voorhees JJ. All-trans retinoic acid (RA) stimulates events in organ-cultured human skin that underlie repair. J Clin Invest. 1994;94:1747–1753. doi: 10.1172/JCI117522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J, Warner RL, Mehrnaz G-K, Phan SH, Kang S, Chung JH, Wang ZQ, Datta SC, Fisher GJ, Voorhees JJ. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000;114:480–486. doi: 10.1046/j.1523-1747.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- Varani J, Zeigler M, Dame MK, Kang S, Fisher GJ, Voorhees JJ. Heparin-binding epidermal growth factor-like growth factor activation of keratinocyte ErbB receptors mediates epidermal hyperplasia, a prominent side-effect of retinoid therapy. J Invest Dermatol. 2001;117:1335–1341. doi: 10.1046/j.0022-202x.2001.01564.x. [DOI] [PubMed] [Google Scholar]
- Weiss JS, Ellis CN, Headington JT, Tincoff T, Hamilton TA, Voorhees JJ. Topical tretinoin improves photoaged skin: a double-blind, vehicle controlled study. JAMA. 1988;259:527–532. [PubMed] [Google Scholar]
- Wood LC, Elias PM, Calhoun C, Tsai JC, Grunfeld C, Feingold KR. Barrier disruption stimulates interleukin-1α expression and release from a pre-formed pool in murine epidermis. J Invest Dermatol. 1996;106:397–403. doi: 10.1111/1523-1747.ep12343392. [DOI] [PubMed] [Google Scholar]
- Xiao JH, Feng XW, Peng ZH, Li LA, Chambon P, Voorhees JJ. Identification of heparin-binding EGF-like growth factor as a target in intercellular regulation of epidermal basal cell growth by suprabasal retinoic acid receptors. EMBO J. 1999;18:1539–1548. doi: 10.1093/emboj/18.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]