Abstract
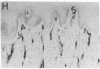
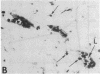
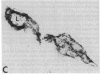
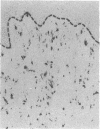
The ability of circulating white blood cells to enter inflamed tissues is mediated by specific cell adhesion molecules thought to be expressed in a programmed and sequential manner to form an "adhesion cascade." Because of the complexity of this process, it is becoming increasingly important to develop in vivo models. Two major problems have limited the utility of current animal models. The first is the inability of many of the antibodies developed against cell adhesion molecules in human cell culture models to cross-react in animals. The second is the uncertainty in extrapolating animal (particularly rodent) findings to humans. To circumvent these problems, full thickness human skin grafts were transplanted onto immunodeficient (severe combined immunodeficient) mice. After 4-6 wk, the transplanted skin grafts closely resembled normal skin histologically and maintained their human vasculature as determined by immunohistochemical staining with human-specific endothelial cell markers. Intradermal injection of tumor necrosis factor-alpha resulted in the reversible upregulation of the leukocyte-endothelial adhesion molecules E-selectin, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1, and in an active inflammatory reaction with migration of murine leukocytes into cytokine-injected areas. These results indicate that the severe combined immunodeficient mouse/human skin transplant model provides a useful in vivo system in which to study human endothelium during the process of inflammation.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Mette S. A., Elder D. E., Stewart R., Damjanovich L., Herlyn M., Buck C. A. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 1990 Oct 15;50(20):6757–6764. [PubMed] [Google Scholar]
- Albelda S. M., Muller W. A., Buck C. A., Newman P. J. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol. 1991 Sep;114(5):1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albelda S. M., Oliver P. D., Romer L. H., Buck C. A. EndoCAM: a novel endothelial cell-cell adhesion molecule. J Cell Biol. 1990 Apr;110(4):1227–1237. doi: 10.1083/jcb.110.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. N., Allen M. H., MacDonald D. M. The effect of in vivo interferon-gamma on the distribution of LFA-1 and ICAM-1 in normal human skin. J Invest Dermatol. 1989 Oct;93(4):439–442. doi: 10.1111/1523-1747.ep12284016. [DOI] [PubMed] [Google Scholar]
- Bogen S. A., Baldwin H. S., Watkins S. C., Albelda S. M., Abbas A. K. Association of murine CD31 with transmigrating lymphocytes following antigenic stimulation. Am J Pathol. 1992 Oct;141(4):843–854. [PMC free article] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Brigham K. L., Meyrick B. Endotoxin and lung injury. Am Rev Respir Dis. 1986 May;133(5):913–927. [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Harlan J. M. Membrane proteins involved in phagocyte adherence to endothelium. Immunol Rev. 1990 Apr;114:5–28. doi: 10.1111/j.1600-065x.1990.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Schwartz B. R., Kovach N. L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., Rosso M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990 Sep 1;76(5):965–970. [PubMed] [Google Scholar]
- Cotran R. S., Gimbrone M. A., Jr, Bevilacqua M. P., Mendrick D. L., Pober J. S. Induction and detection of a human endothelial activation antigen in vivo. J Exp Med. 1986 Aug 1;164(2):661–666. doi: 10.1084/jem.164.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custer R. P., Bosma G. C., Bosma M. J. Severe combined immunodeficiency (SCID) in the mouse. Pathology, reconstitution, neoplasms. Am J Pathol. 1985 Sep;120(3):464–477. [PMC free article] [PubMed] [Google Scholar]
- Damjanovich L., Albelda S. M., Mette S. A., Buck C. A. Distribution of integrin cell adhesion receptors in normal and malignant lung tissue. Am J Respir Cell Mol Biol. 1992 Feb;6(2):197–206. doi: 10.1165/ajrcmb/6.2.197. [DOI] [PubMed] [Google Scholar]
- Demarchez M., Hartmann D. J., Prunieras M. An immunohistological study of the revascularization process in human skin transplanted onto the nude mouse. Transplantation. 1987 Jun;43(6):896–903. [PubMed] [Google Scholar]
- Diamond M. S., Staunton D. E., Marlin S. D., Springer T. A. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 1991 Jun 14;65(6):961–971. doi: 10.1016/0092-8674(91)90548-d. [DOI] [PubMed] [Google Scholar]
- Dorshkind K., Pollack S. B., Bosma M. J., Phillips R. A. Natural killer (NK) cells are present in mice with severe combined immunodeficiency (scid). J Immunol. 1985 Jun;134(6):3798–3801. [PubMed] [Google Scholar]
- Griffiths C. E., Barker J. N., Kunkel S., Nickoloff B. J. Modulation of leucocyte adhesion molecules, a T-cell chemotaxin (IL-8) and a regulatory cytokine (TNF-alpha) in allergic contact dermatitis (rhus dermatitis). Br J Dermatol. 1991 Jun;124(6):519–526. doi: 10.1111/j.1365-2133.1991.tb04943.x. [DOI] [PubMed] [Google Scholar]
- Groves R. W., Allen M. H., Barker J. N., Haskard D. O., MacDonald D. M. Endothelial leucocyte adhesion molecule-1 (ELAM-1) expression in cutaneous inflammation. Br J Dermatol. 1991 Feb;124(2):117–123. doi: 10.1111/j.1365-2133.1991.tb00419.x. [DOI] [PubMed] [Google Scholar]
- Johnston S. C., Dustin M. L., Hibbs M. L., Springer T. A. On the species specificity of the interaction of LFA-1 with intercellular adhesion molecules. J Immunol. 1990 Aug 15;145(4):1181–1187. [PubMed] [Google Scholar]
- Kawamura T., Niguma T., Fechner J. H., Jr, Wolber R., Beeskau M. A., Hullett D. A., Sollinger H. W., Burlingham W. J. Chronic human skin graft rejection in severe combined immunodeficient mice engrafted with human PBL from an HLA-presensitized donor. Transplantation. 1992 Mar;53(3):659–665. doi: 10.1097/00007890-199203000-00032. [DOI] [PubMed] [Google Scholar]
- Kim Y. H., Woodley D. T., Wynn K. C., Giomi W., Bauer E. A. Recessive dystrophic epidermolysis bullosa phenotype is preserved in xenografts using SCID mice: development of an experimental in vivo model. J Invest Dermatol. 1992 Feb;98(2):191–197. doi: 10.1111/1523-1747.ep12555849. [DOI] [PubMed] [Google Scholar]
- Krueger G. G., Shelby J. Biology of human skin transplanted to the nude mouse: I. Response to agents which modify epidermal proliferation. J Invest Dermatol. 1981 Jun;76(6):506–510. doi: 10.1111/1523-1747.ep12521231. [DOI] [PubMed] [Google Scholar]
- Kyan-Aung U., Haskard D. O., Poston R. N., Thornhill M. H., Lee T. H. Endothelial leukocyte adhesion molecule-1 and intercellular adhesion molecule-1 mediate the adhesion of eosinophils to endothelial cells in vitro and are expressed by endothelium in allergic cutaneous inflammation in vivo. J Immunol. 1991 Jan 15;146(2):521–528. [PubMed] [Google Scholar]
- Lane A. T., Scott G. A., Day K. H. Development of human fetal skin transplanted to the nude mouse. J Invest Dermatol. 1989 Dec;93(6):787–791. doi: 10.1111/1523-1747.ep12284423. [DOI] [PubMed] [Google Scholar]
- Leung D. Y., Pober J. S., Cotran R. S. Expression of endothelial-leukocyte adhesion molecule-1 in elicited late phase allergic reactions. J Clin Invest. 1991 May;87(5):1805–1809. doi: 10.1172/JCI115201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. E., Buchsbaum M., Whitaker D., Murphy G. F. Intercellular adhesion molecule expression in the evolving human cutaneous delayed hypersensitivity reaction. J Invest Dermatol. 1989 Nov;93(5):672–677. doi: 10.1111/1523-1747.ep12319838. [DOI] [PubMed] [Google Scholar]
- Messadi D. V., Pober J. S., Fiers W., Gimbrone M. A., Jr, Murphy G. F. Induction of an activation antigen on postcapillary venular endothelium in human skin organ culture. J Immunol. 1987 Sep 1;139(5):1557–1562. [PubMed] [Google Scholar]
- Muller W. A., Ratti C. M., McDonnell S. L., Cohn Z. A. A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J Exp Med. 1989 Aug 1;170(2):399–414. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro J. M., Pober J. S., Cotran R. S. Recruitment of neutrophils in the local endotoxin response: association with de novo endothelial expression of endothelial leukocyte adhesion molecule-1. Lab Invest. 1991 Feb;64(2):295–299. [PubMed] [Google Scholar]
- Munro J. M., Pober J. S., Cotran R. S. Tumor necrosis factor and interferon-gamma induce distinct patterns of endothelial activation and associated leukocyte accumulation in skin of Papio anubis. Am J Pathol. 1989 Jul;135(1):121–133. [PMC free article] [PubMed] [Google Scholar]
- Newman P. J., Berndt M. C., Gorski J., White G. C., 2nd, Lyman S., Paddock C., Muller W. A. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990 Mar 9;247(4947):1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- Norris P., Poston R. N., Thomas D. S., Thornhill M., Hawk J., Haskard D. O. The expression of endothelial leukocyte adhesion molecule-1 (ELAM-1), intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in experimental cutaneous inflammation: a comparison of ultraviolet B erythema and delayed hypersensitivity. J Invest Dermatol. 1991 May;96(5):763–770. doi: 10.1111/1523-1747.ep12471720. [DOI] [PubMed] [Google Scholar]
- Nortamo P., Li R., Renkonen R., Timonen T., Prieto J., Patarroyo M., Gahmberg C. G. The expression of human intercellular adhesion molecule-2 is refractory to inflammatory cytokines. Eur J Immunol. 1991 Oct;21(10):2629–2632. doi: 10.1002/eji.1830211049. [DOI] [PubMed] [Google Scholar]
- Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990 Jul 13;62(1):3–6. doi: 10.1016/0092-8674(90)90230-c. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. The role of endothelial cells in inflammation. Transplantation. 1990 Oct;50(4):537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Pober J., Cotran R. S. What can be learned from the expression of endothelial adhesion molecules in tissues? Lab Invest. 1991 Mar;64(3):301–305. [PubMed] [Google Scholar]
- Reddy S., Piccione D., Takita H., Bankert R. B. Human lung tumor growth established in the lung and subcutaneous tissue of mice with severe combined immunodeficiency. Cancer Res. 1987 May 1;47(9):2456–2460. [PubMed] [Google Scholar]
- Rice G. E., Munro J. M., Bevilacqua M. P. Inducible cell adhesion molecule 110 (INCAM-110) is an endothelial receptor for lymphocytes. A CD11/CD18-independent adhesion mechanism. J Exp Med. 1990 Apr 1;171(4):1369–1374. doi: 10.1084/jem.171.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlein R., Dustin M. L., Marlin S. D., Springer T. A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986 Aug 15;137(4):1270–1274. [PubMed] [Google Scholar]
- Rygaard J. Skin grafts in nude mice. 3. Fate of grafts from man and donors of other taxonomic classes. Acta Pathol Microbiol Scand A. 1974 Jan;82(1):105–112. [PubMed] [Google Scholar]
- Sanchez-Madrid F., Simon P., Thompson S., Springer T. A. Mapping of antigenic and functional epitopes on the alpha- and beta-subunits of two related mouse glycoproteins involved in cell interactions, LFA-1 and Mac-1. J Exp Med. 1983 Aug 1;158(2):586–602. doi: 10.1084/jem.158.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Tryka A. F., Witschi H., Gosslee D. G., McArthur A. H., Clapp N. K. Patterns of cell proliferation during recovery from oxygen injury. Species differences. Am Rev Respir Dis. 1986 Jun;133(6):1055–1059. doi: 10.1164/arrd.1986.133.6.1055. [DOI] [PubMed] [Google Scholar]
- Waldorf H. A., Walsh L. J., Schechter N. M., Murphy G. F. Early cellular events in evolving cutaneous delayed hypersensitivity in humans. Am J Pathol. 1991 Feb;138(2):477–486. [PMC free article] [PubMed] [Google Scholar]
- Wu N. W., Jalkanen S., Streeter P. R., Butcher E. C. Evolutionary conservation of tissue-specific lymphocyte-endothelial cell recognition mechanisms involved in lymphocyte homing. J Cell Biol. 1988 Nov;107(5):1845–1851. doi: 10.1083/jcb.107.5.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fougerolles A. R., Stacker S. A., Schwarting R., Springer T. A. Characterization of ICAM-2 and evidence for a third counter-receptor for LFA-1. J Exp Med. 1991 Jul 1;174(1):253–267. doi: 10.1084/jem.174.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]