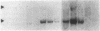Abstract
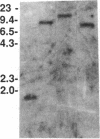
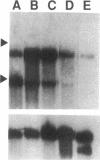
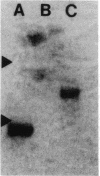
Gap junctions allow direct intercellular coupling between many cells including those in the blood vessel wall. They are formed by a group of related proteins called connexins, containing conserved transmembrane and extracellular domains, but unique cytoplasmic regions that may confer connexin-specific physiological properties. We used polymerase chain reaction amplification and cDNA library screening to clone DNA encoding a human gap junction protein, connexin37 (Cx37). The derived human Cx37 polypeptide contains 333 amino acids, with a predicted molecular mass of 37,238 D. RNA blots demonstrate that Cx37 is expressed in multiple organs and tissues (including heart, uterus, ovary, and blood vessel endothelium) and in primary cultures of vascular endothelial cells. Cx37 mRNA is coexpressed with connexin43 at similar levels in some endothelial cells, but at much lower levels in others. To demonstrate that Cx37 could form functional channels, we stably transfected communication-deficient Neuro2A cells with the Cx37 cDNA. The induced intercellular channels were studied by the double whole cell patch clamp technique. These channels were reversibly inhibited by the uncoupling agent, heptanol (2 mM). The expressed Cx37 channels exhibited multiple conductance levels and showed a pronounced voltage dependence. These electrophysiological characteristics are similar to, but distinct from, those of previously characterized connexins.
Full text
PDF


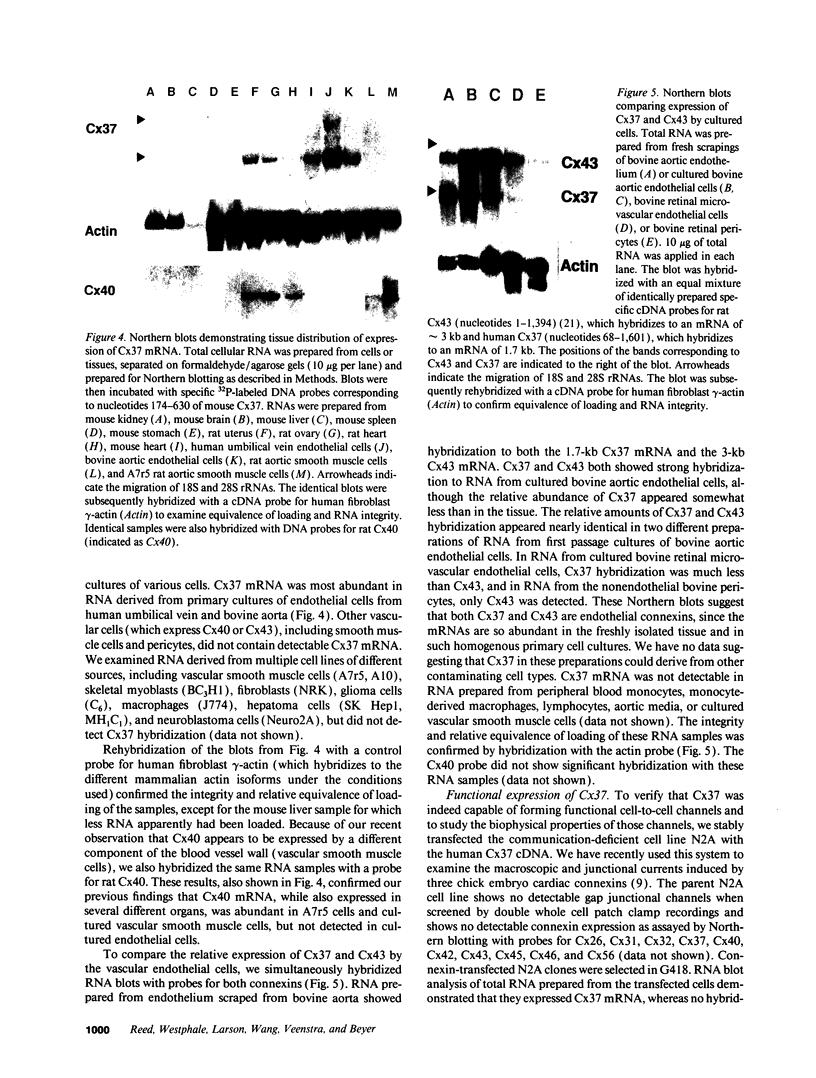
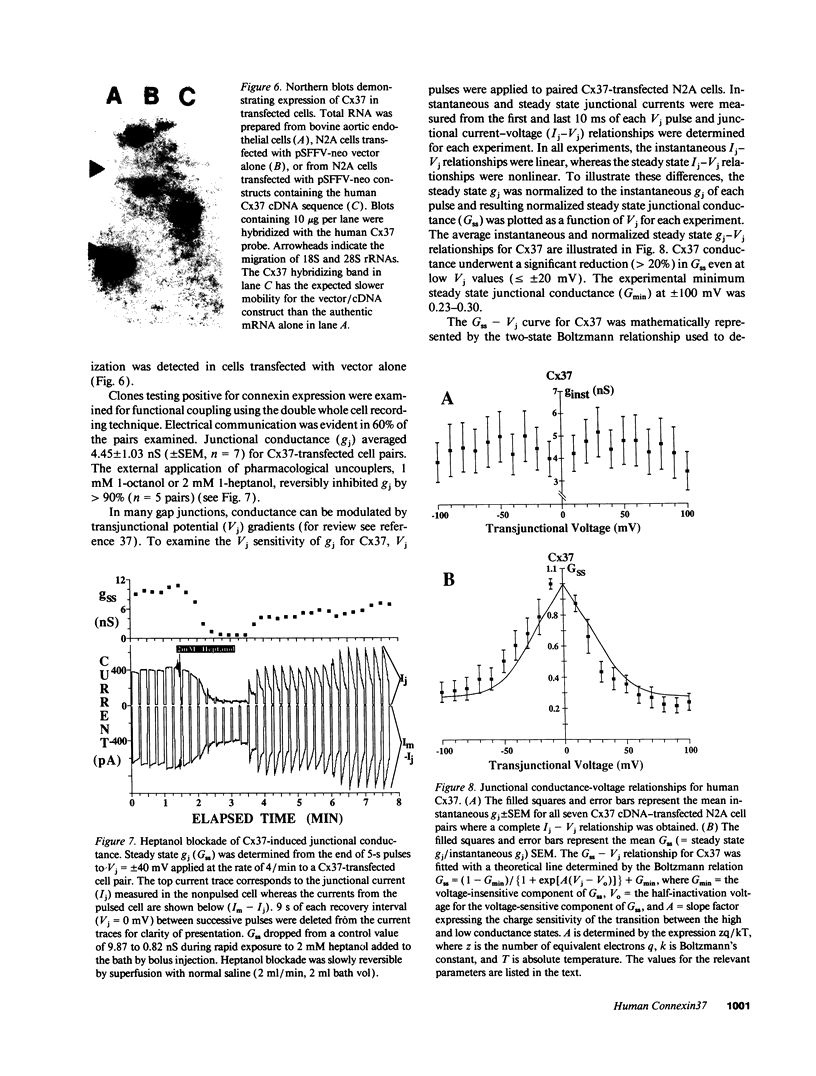
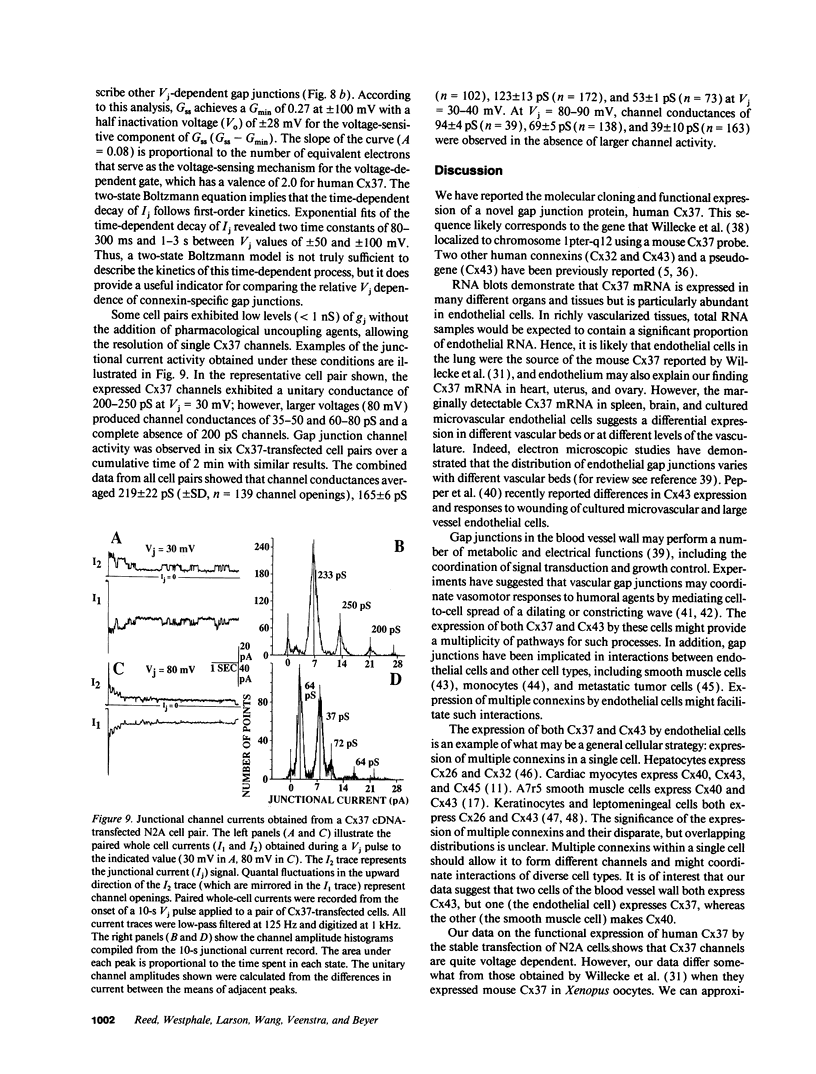


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrio L. C., Suchyna T., Bargiello T., Xu L. X., Roginski R. S., Bennett M. V., Nicholson B. J. Gap junctions formed by connexins 26 and 32 alone and in combination are differently affected by applied voltage. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8410–8414. doi: 10.1073/pnas.88.19.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V., Barrio L. C., Bargiello T. A., Spray D. C., Hertzberg E., Sáez J. C. Gap junctions: new tools, new answers, new questions. Neuron. 1991 Mar;6(3):305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- Beyer E. C. Molecular cloning and developmental expression of two chick embryo gap junction proteins. J Biol Chem. 1990 Aug 25;265(24):14439–14443. [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin family of gap junction proteins. J Membr Biol. 1990 Jul;116(3):187–194. doi: 10.1007/BF01868459. [DOI] [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol. 1987 Dec;105(6 Pt 1):2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. C., Reed K. E., Westphale E. M., Kanter H. L., Larson D. M. Molecular cloning and expression of rat connexin40, a gap junction protein expressed in vascular smooth muscle. J Membr Biol. 1992 Apr;127(1):69–76. doi: 10.1007/BF00232759. [DOI] [PubMed] [Google Scholar]
- Beyer E. C., Steinberg T. H. Evidence that the gap junction protein connexin-43 is the ATP-induced pore of mouse macrophages. J Biol Chem. 1991 May 5;266(13):7971–7974. [PubMed] [Google Scholar]
- Brissette J. L., Kumar N. M., Gilula N. B., Dotto G. P. The tumor promoter 12-O-tetradecanoylphorbol-13-acetate and the ras oncogene modulate expression and phosphorylation of gap junction proteins. Mol Cell Biol. 1991 Oct;11(10):5364–5371. doi: 10.1128/mcb.11.10.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. H., DeHaan R. L. Multiple-channel conductance states and voltage regulation of embryonic chick cardiac gap junctions. J Membr Biol. 1992 Apr;127(2):95–111. doi: 10.1007/BF00233282. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davies P. F., Truskey G. A., Warren H. B., O'Connor S. E., Eisenhaure B. H. Metabolic cooperation between vascular endothelial cells and smooth muscle cells in co-culture: changes in low density lipoprotein metabolism. J Cell Biol. 1985 Sep;101(3):871–879. doi: 10.1083/jcb.101.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R., Traub O., Hwang T. K., Beyer E., Bennett M. V., Spray D. C., Willecke K. Differential expression of three gap junction proteins in developing and mature brain tissues. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10148–10152. doi: 10.1073/pnas.86.24.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L., Beyer E. C., Swenson K. I., Paul D. L., Goodenough D. A. Cloning and expression of a Xenopus embryonic gap junction protein. Science. 1989 Mar 3;243(4895):1194–1195. doi: 10.1126/science.2466337. [DOI] [PubMed] [Google Scholar]
- Fishman G. I., Eddy R. L., Shows T. B., Rosenthal L., Leinwand L. A. The human connexin gene family of gap junction proteins: distinct chromosomal locations but similar structures. Genomics. 1991 May;10(1):250–256. doi: 10.1016/0888-7543(91)90507-b. [DOI] [PubMed] [Google Scholar]
- Fishman G. I., Spray D. C., Leinwand L. A. Molecular characterization and functional expression of the human cardiac gap junction channel. J Cell Biol. 1990 Aug;111(2):589–598. doi: 10.1083/jcb.111.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M., Shenk T. The sequence 5'-AAUAAA-3'forms parts of the recognition site for polyadenylation of late SV40 mRNAs. Cell. 1981 Apr;24(1):251–260. doi: 10.1016/0092-8674(81)90521-3. [DOI] [PubMed] [Google Scholar]
- Fuhlbrigge R. C., Fine S. M., Unanue E. R., Chaplin D. D. Expression of membrane interleukin 1 by fibroblasts transfected with murine pro-interleukin 1 alpha cDNA. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5649–5653. doi: 10.1073/pnas.85.15.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefliger J. A., Bruzzone R., Jenkins N. A., Gilbert D. J., Copeland N. G., Paul D. L. Four novel members of the connexin family of gap junction proteins. Molecular cloning, expression, and chromosome mapping. J Biol Chem. 1992 Jan 25;267(3):2057–2064. [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Kanter H. L., Saffitz J. E., Beyer E. C. Cardiac myocytes express multiple gap junction proteins. Circ Res. 1992 Feb;70(2):438–444. doi: 10.1161/01.res.70.2.438. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N. M., Gilula N. B. Cloning and characterization of human and rat liver cDNAs coding for a gap junction protein. J Cell Biol. 1986 Sep;103(3):767–776. doi: 10.1083/jcb.103.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang L. M., Beyer E. C., Schwartz A. L., Gitlin J. D. Molecular cloning of a rat uterine gap junction protein and analysis of gene expression during gestation. Am J Physiol. 1991 May;260(5 Pt 1):E787–E793. doi: 10.1152/ajpendo.1991.260.5.E787. [DOI] [PubMed] [Google Scholar]
- Larson D. M., Haudenschild C. C., Beyer E. C. Gap junction messenger RNA expression by vascular wall cells. Circ Res. 1990 Apr;66(4):1074–1080. doi: 10.1161/01.res.66.4.1074. [DOI] [PubMed] [Google Scholar]
- Lash J. A., Critser E. S., Pressler M. L. Cloning of a gap junctional protein from vascular smooth muscle and expression in two-cell mouse embryos. J Biol Chem. 1990 Aug 5;265(22):13113–13117. [PubMed] [Google Scholar]
- Miller T., Dahl G., Werner R. Structure of a gap junction gene: rat connexin-32. Biosci Rep. 1988 Oct;8(5):455–464. doi: 10.1007/BF01121644. [DOI] [PubMed] [Google Scholar]
- Moore L. K., Beyer E. C., Burt J. M. Characterization of gap junction channels in A7r5 vascular smooth muscle cells. Am J Physiol. 1991 May;260(5 Pt 1):C975–C981. doi: 10.1152/ajpcell.1991.260.5.C975. [DOI] [PubMed] [Google Scholar]
- Moreno A. P., Eghbali B., Spray D. C. Connexin32 gap junction channels in stably transfected cells: unitary conductance. Biophys J. 1991 Nov;60(5):1254–1266. doi: 10.1016/S0006-3495(91)82159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M., Liao F., Hough G. P., Ross L. A., Van Lenten B. J., Rajavashisth T. B., Lusis A. J., Laks H., Drinkwater D. C., Fogelman A. M. Interaction of monocytes with cocultures of human aortic wall cells involves interleukins 1 and 6 with marked increases in connexin43 message. J Clin Invest. 1991 May;87(5):1763–1772. doi: 10.1172/JCI115195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M. S., Montesano R., el Aoumari A., Gros D., Orci L., Meda P. Coupling and connexin 43 expression in microvascular and large vessel endothelial cells. Am J Physiol. 1992 May;262(5 Pt 1):C1246–C1257. doi: 10.1152/ajpcell.1992.262.5.C1246. [DOI] [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler J. E., Shelton-Inloes B. B., Sorace J. M., Harlan J. M., Titani K., Davie E. W. Cloning and characterization of two cDNAs coding for human von Willebrand factor. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6394–6398. doi: 10.1073/pnas.82.19.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Segal S. S., Damon D. N., Duling B. R. Propagation of vasomotor responses coordinates arteriolar resistances. Am J Physiol. 1989 Mar;256(3 Pt 2):H832–H837. doi: 10.1152/ajpheart.1989.256.3.H832. [DOI] [PubMed] [Google Scholar]
- Segal S. S., Duling B. R. Flow control among microvessels coordinated by intercellular conduction. Science. 1986 Nov 14;234(4778):868–870. doi: 10.1126/science.3775368. [DOI] [PubMed] [Google Scholar]
- Swenson K. I., Jordan J. R., Beyer E. C., Paul D. L. Formation of gap junctions by expression of connexins in Xenopus oocyte pairs. Cell. 1989 Apr 7;57(1):145–155. doi: 10.1016/0092-8674(89)90180-3. [DOI] [PubMed] [Google Scholar]
- Traub O., Look J., Dermietzel R., Brümmer F., Hülser D., Willecke K. Comparative characterization of the 21-kD and 26-kD gap junction proteins in murine liver and cultured hepatocytes. J Cell Biol. 1989 Mar;108(3):1039–1051. doi: 10.1083/jcb.108.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra R. D., DeHaan R. L. Measurement of single channel currents from cardiac gap junctions. Science. 1986 Aug 29;233(4767):972–974. doi: 10.1126/science.2426781. [DOI] [PubMed] [Google Scholar]
- Veenstra R. D. Developmental changes in regulation of embryonic chick heart gap junctions. J Membr Biol. 1991 Feb;119(3):253–265. doi: 10.1007/BF01868730. [DOI] [PubMed] [Google Scholar]
- Veenstra R. D. Voltage-dependent gating of gap junction channels in embryonic chick ventricular cell pairs. Am J Physiol. 1990 Apr;258(4 Pt 1):C662–C672. doi: 10.1152/ajpcell.1990.258.4.C662. [DOI] [PubMed] [Google Scholar]
- Veenstra R. D., Wang H. Z., Westphale E. M., Beyer E. C. Multiple connexins confer distinct regulatory and conductance properties of gap junctions in developing heart. Circ Res. 1992 Nov;71(5):1277–1283. doi: 10.1161/01.res.71.5.1277. [DOI] [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner R., Levine E., Rabadan-Diehl C., Dahl G. Formation of hybrid cell-cell channels. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5380–5384. doi: 10.1073/pnas.86.14.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willecke K., Heynkes R., Dahl E., Stutenkemper R., Hennemann H., Jungbluth S., Suchyna T., Nicholson B. J. Mouse connexin37: cloning and functional expression of a gap junction gene highly expressed in lung. J Cell Biol. 1991 Sep;114(5):1049–1057. doi: 10.1083/jcb.114.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willecke K., Jungbluth S., Dahl E., Hennemann H., Heynkes R., Grzeschik K. H. Six genes of the human connexin gene family coding for gap junctional proteins are assigned to four different human chromosomes. Eur J Cell Biol. 1990 Dec;53(2):275–280. [PubMed] [Google Scholar]
- el-Sabban M. E., Pauli B. U. Cytoplasmic dye transfer between metastatic tumor cells and vascular endothelium. J Cell Biol. 1991 Dec;115(5):1375–1382. doi: 10.1083/jcb.115.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]