Abstract
Purpose
The purpose of this study was to determine to what extent blood retina barrier (BRB) permeability occurred during experimental Bacillus cereus endophthalmitis and whether tight junction alterations were involved in permeability.
Methods
Mice were intravitreally injected with 100 CFU B. cereus and eyes were analyzed at specific times postinfection for permeability to fibrin and albumin, quantitation of intraocular plasma constituent leakage, production of inflammatory cytokines, and alterations in tight junction protein localization and expression at the level of the RPE.
Results
B. cereus-induced leakage of albumin and fibrin into the aqueous and vitreous humor by 8 h postinfection. BRB permeability occurred as early as 4 h and increased 13.30-fold compared to uninfected controls by 8 h. Production of proinflammatory cytokines IL-6, MIP-1α, IL-1β, and KC increased over the course of infection. In the retina, ZO-1 disruption begins by 4 h, followed by decreasing occludin and ZO-1 expression at 4 and 8 h, respectively. Tubulin condensation and RPE65 degradation occurred by 12 h. A quorum sensing mutant B. cereus strain caused BRB permeability comparable to that of wild-type B. cereus. Both wild-type and mutant B. cereus sterile supernatants induced blood ocular barrier permeability similarly to that of wild-type infection.
Conclusions
These results indicate that BRB permeability occurs during the early stages of experimental B. cereus endophthalmitis, beginning as early as 4 h postinfection. Disruption of tight junctions at the level of the RPE may contribute to barrier breakdown. Quorum-sensing dependent factors may not significantly contribute to BRB permeability.
Keywords: Bacillus cereus, toxins, tight junctions, blood retinal barrier, blood aqueous barrier, retinal pigment epithelium
INTRODUCTION
The introduction of bacteria into the posterior chamber of the eye can provoke rapid inflammation known as endophthalmitis. Pathogens usually enter the vitreous via a penetrating eye injury (post-traumatic), contamination during ocular surgery (post-surgical), or by spread from an extraocular infection into the eye (endogenous). The severity and outcome of endophthalmitis is dependent on the nature of the offending organism.1–4 Mild infection with a fairly avirulent organism may be cleared and visual function can be retained with prompt treatment. However, infection with a virulent organism, such as Bacillus cereus, can often result in loss of vision or of the eye itself in less than 48 h, regardless of antibiotic treatment or surgical intervention.5–12
B. cereus virulence has been generally attributed to toxin production.2 The majority of extracellular B. cereus toxins are produced under the control of a global regulator, plcR. However, non-plcR-regulated toxins have been found to be important in inducing RPE toxicity and blood retina barrier permeability in vitro.13 B. cereus and its toxins induce an explosive infection that poses a significant threat to vision, causing loss of retinal function and complete destruction of retinal architecture in a murine model by 12 h postinfection.14 In this mouse model, significant inflammation occurs, with polymorphonuclear leukocytes (PMN) infiltrating into the vitreous as early as 4 h postinfection.14 The presence of these inflammatory cells in the posterior chamber is atypical and may interfere with preserving clarity of the visual axis, which is necessary for proper visual function.
The eye is protected from inflammatory cells and blood constituents by blood ocular barriers (BOB), which include the blood aqueous barrier (BAB) and blood retina barrier (BRB). The BAB is comprised of iridial vessel endothelium, iridial epithelium, and ciliary epithelium. The inner and outer BRBs are comprised of retinal endothelium and retinal epithelial cells (RPE), respectively. These barriers manage blood supply to the retina and are responsible for the homeostasis of the neural retina. Intercellular tight junctions contribute to a tightly regulated diffusion barrier, resulting in highly selective permeability. Ocular inflammation can damage this delicate system, resulting in barrier breakdown, which may contribute to vision loss during endophthalmitis.
Inflammation is linked to BRB permeability in many ocular diseases such as uveitis15–17 and glaucoma18. Barrier changes can be a result of dysfunction of tight junction proteins such as ZO-1 and occludin in mouse and rabbit models of experimental autoimmune uveoretinitis (EAU).19 Tight junctions of the BRB normally maintain a barrier between the blood and retinal tissues. Dysfunction or degradation of these proteins could cause unregulated permeability and contribute to the observed disease pathology. Endophthalmitis is an inflammatory disease, yet to our knowledge the integrity of the BOB and alterations in tight junction structure during active infection have not been analyzed. Barrier changes during endophthalmitis could contribute to loss of retinal structure and function. Outer BRB permeability has been demonstrated in an in vitro BRB system comprised of polarized RPE monolayers. B. cereus infection resulted in permeability of RPE monolayers and disruption and degradation of ZO-1 and occludin.13,20 Rapid BRB permeability occurred in vitro. However barrier integrity has not been analyzed during active infection.
We hypothesized that tight junction disruption and BOB permeability occur during experimental B. cereus endophthalmitis. Analyzing the changes in barrier function during B. cereus infection can lead to a greater understanding of mechanisms of inflammation and vision loss during B. cereus endophthalmitis. This may lead to the development of novel therapeutics aimed toward preventing barrier dysfunction, limiting inflammation, and preserving vision.
METHODS
Experimental Bacillus cereus Endophthalmitis
Male C57BL/6J mice (4–5 weeks of age; Jackson Laboratories, Bar Harbor, ME) were maintained according to institutional guidelines and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. All mice were anesthetized with a mixture of ketamine (85 mg/kg body weight, KetaVed [Phoenix Scientific, Inc. St. Joseph, MO]) and xylazine (14 mg/kg of body weight, Rompun [Bayer Corp., Shawnee Mission, KS]). Ophthalmic anesthetic (0.5% Proparacaine HCl [Ophthetic Allergan, Hormigueros, Puerto Rico]) was administered topically prior to intravitreal injections. Intravitreal injections were performed, as previously described.14 Briefly, 0.5 μl containing PBS (surgical control), brain heart infusion medium alone (BHI control), approximately 100 CFU of B. cereus, or sterile cell free B. cereus supernatants were injected into the mid-vitreous. The contralateral eye was not injected (absolute control).
Bacterial Inoculum and Sterile Supernatant Preparation
Wild-type B. cereus strain 14579 (American Type Culture Collection [ATCC], Manassas, VA) or a plcR-deficient quorum sensing isognenic mutant B. cereus (BCplcR::kanR)21 were used to induce experimental endophthalmitis. Wild-type or plcR-deficient B. cereus were grown to early stationary phase in BHI for 18 h and subcultured to 100 CFU/0.5 μl for injection into the mid-vitreous.
Sterile cell-free supernatants were prepared from 18 h bacterial cultures described above. Wild-type or plcR-deficient B. cereus bacterial cultures were centrifuged at 3716 × g for 15 min to pellet bacteria. Supernatants were filtered through Millex-GV 0.22 μm filters (Millipore, Billerica, MA). 0.5 μl supernatants were intravitreally injected into the mid-vitreous.
Histology
Eye globes were harvested at 0, 4, 8, and 12 h postinfection and were incubated in Perfix for 24 h at room temperature. Globes were then transferred to 70% ethanol and embedded in paraffin, sectioned, and analyzed by trichrome staining or immunohistochemistry. Trichrome staining was used to identify infiltrating fibrin in the vitreous. Sections were deparaffinized and treated with Bouin’s fixative solution overnight at room temperature. Sections were then stained with Weigert’s iron hematoxylin solution for 10 min followed by Biebrich scarlet-acid fucshin solution for 15 min and phosphomolybdic-phosphotungstic acid solution for 15 min. Sections were then counterstained with aniline blue for 10 min, differentiated in 1% acetic water for 5 min, dehydrated in 95% ethyl alcohol, absolute ethyl alcohol, and xylene (2 changes, 2 min each) and mounted. Images are representative of N≥3 eyes at each time point.
Immunohisthochemistry
Fluorescence Detection
Sections were deparaffinized and incubated in a surfactant (PBS + 0.02% Tween 20) for 10 min. Samples were blocked in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) for 1 h at room temperature (Gibco, Grand Island, NY). Slides were treated with primary antibody diluted in 0.5% bovine serum albumin (BSA) in PBS with 0.02% NaN3 overnight at 4°C, followed by secondary antibody for 1.5 h at room temperature. Coverslips were mounted with fluorescent mounting medium (Dako, Carpinteria, CA) and slides were viewed by confocal microscopy (Olympus FV 500, San Jose, CA) or fluorescence microscopy (Nikon Eclipse E800, Tokyo, Japan). Images were analyzed by Olympus Fluoview (version 4.3) and are representative of N≥3 eyes at each time point. Secondary-alone controls were used to verify the absence of non-specific fluorescence. Primary antibodies included rabbit anti-rat albumin (1:700, Accurate Chemical & Scientific Corp, Westbury, NY), rabbit anti-ZO-1 (1:200, Zymed, San Francisco, CA), mouse anti-occludin (1:200, Zymed), rat anti-tubulin (1:200, Chemicon), or mouse anti-RPE65 (1:200, Chemicon). Secondary antibodies included goat anti-rabbit (Alexafluor 594) or goat anti-mouse (Alexafluor 488) prepared at 1:200 (Molecular Probes-Invitrogen, Carlsbad, CA). The mouse anti-ZO-1 antibody required epitope unmasking immediately following deparaffinization (1 mg/ml protease, 10 min at 25°C).
Polymer Detection
Sections were deparaffinized and incubated in a steam bath of 1mM EDTA (pH 8.0) for 75 m to retrieve antigens. This was followed by a 20 min cool down and wash in 0.3% potassium permanganate for 10 sec to remove pigment from the RPE. This was followed by a 5 sec 1% oxalic acid wash and a 10 min water wash. Samples were blocked with DMEM + 10% FBS. Detection methods were in accordance with the UltraVision LP Detection System (Thermo Fisher Scientific, Fremont, CA). Endogenous peroxidases were blocked by adding 3% H2O2. Primary antibody (mouse anti-ZO-1 [1:200, Zymed]) was added for 15 min at 25°C. The secondary antibody (goat anti-mouse [1:200, Alexafluor 488, Molecular Probes]), conjugated to horseradish peroxidase, was incubated with samples for 30 min at 25°C. The substrate, 3,3 diaminobenzidine (DAB), was added to the sample for 1 min. Enzyme cleavage of DAB produced an insoluble brown product.
Quantitation of Vascular Permeability
Albumin leakage from blood vessels into the retina was measured to quantify vascular permeability using a modified Evans blue protocol.22 The Evans blue dye (Sigma, St Louis, MO) was dissolved in normal saline (30 mg/ml), sonicated for 5 min, and filtered (0.45 μm, Millipore, Bedford, MA). After intravitreal injection of B. cereus, and 2 h prior to enucleation of the eye, mice were anesthetized and Evans blue dye (30 mg/kg) was injected into the tail vein. Mice were kept on a warm pad for 2 h to ensure complete distribution of the dye. Mice were perfused with 1% paraformaldehyde in citrate buffer (pH 4.2, 37°C, 120 mmHg). Eyes were harvested every 2 h postinfection up to 12 h. Retinas were dissected out and placed in 150 μl formamide to extract the dye, incubated at 70°C for 18 h, and then centrifuged for 20 min at 70,000 rpm. Optical densities of 100 μl supernatants were determined by spectrophotometery (OD620). The concentration of Evans blue was calculated from a standard curve and normalized to the total protein per sample (calculated by bicinchoninic acid [BCA] protein assay). Results were expressed in micrograms of Evans blue per milligrams of total protein content. Values represent mean ± standard error of the mean (SEM) for N=12 per time point.
Western Blot of Tight Junction Proteins
Eyes were harvested and dissected to remove the retina. For each sample, two retinas were pooled in 200 μl of 1× lysis buffer (Cell Signaling, Danvers, MA) supplemented with phenylmethylsulfonyl fluoride (PMSF, 1 mM final concentration) and sonciated. Lysates were centrifuged at 16,000 rpm for 5 min. Supernatant protein concentrations were determined by BCA. Lysate samples prepared at 50 μg were analyzed by gel electrophoresis. Proteins were transferred to nitrocellulose for immunoblottting. Membranes were blocked with 5% nonfat milk in 0.1% Tween-20 in Tris-buffered saline (TBST) for 1 h at 25°C. The same membrane was probed for both actin and occludin or ZO-1. Membranes were treated with primary antibody (rabbit anti-occludin [1:200] or rabbit anti-ZO-1 [1:100], Zymed, San Francisco, CA or mouse anti-actin, 1:500, Affinity Bioreagents, Golden, CO) overnight at 4°C and secondary antibody (anti-mouse or anti-rabbit immunoglobulin G-horseradish peroxidase, 1:2,500, Sigma, St. Louis, MO) for 1 h at 25°C. All antibodies were prepared in TBST with 0.02% NaN3. Membranes were developed with the ECL Plus Detection System (GE Healthcare, Pscataway, NJ) and imaged on a Storm 860 Imager (Amersham Biosciences, Pittsburg, PA) or ImageStation 2000R (Eastman Kodak, Rochester, NY). Densitometry was performed using Kodak IM software. Expression was calculated in arbitrary units (AU), normalized to actin, and presented as percent change compared to 0 h.
Quantitation of Cytokines and Chemokines
After intravitreal injection of B. cereus, eyes were analyzed for presence of proinflammatory cytokines shown to be involved in models of ocular inflammation.15–18 Globes were harvested and homogenized with glass beads in a protease inhibitor cocktail (Triton X-100, 0.5 M EDTA, 10 mM sodium orthovanadate [Sigma, St. Louis, MO] and Protease Inhibitor [Calbiochem, La Jolla, CA] in PBS, pH 7.4). Supernatants were then analyzed for the presence of interleukin-6 (IL-6), macrophage inflammatory protein 1 alpha (MIP-1α), interleukin-1 beta (IL-1β), or keratinocyte-derived chemokine (KC) using commercial ELISA kits (Quantikine, R&D Systems, Minneapolis, Minn) in accordance with the manufacturer’s instructions. The cytokine and chemokine concentrations were interpolated from standard curves. Values are expressed as mean ± SEM for n ≥ 6 analyses per time point.
Statistics
Results were the arithmetic means ± SEM of all of the samples in the same experimental group. A two-tailed Student t test was used to determine the statistical significance of the data. Statistical significance was determined at P ≤ 0.05.
RESULTS
Blood Ocular Barrier Permeability
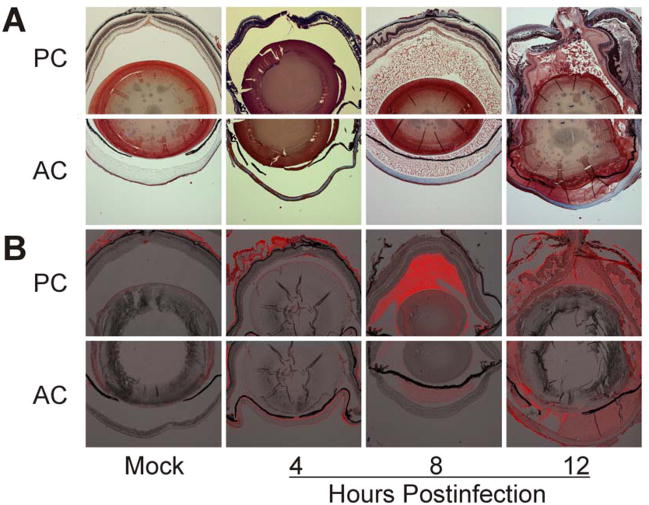
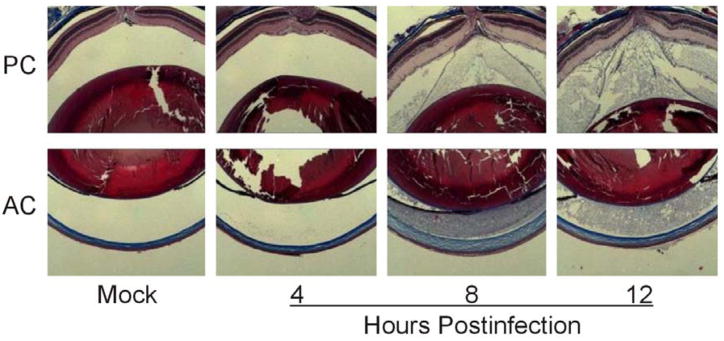
Our experimental B. cereus endophthalmitis infection course was consistent with that previously published.14 100 CFU B. cereus injected into the mid-vitreous resulted in similar intraocular bacterial growth, inflammation, and loss of globe architecture. Leakage of blood constituents within the eye during B. cereus endophthalmitis is depicted in Figure 1. Trichrome staining of infected eyes demonstrated that fibrin had seeped into and filled the anterior and posterior chamber by 8 h postinfection (Figure 1a). Fibrin was still visible in both the posterior and anterior chambers at 12 h as eyes began to lose structural integrity and severe retinal detachment was apparent. Mock-infected and uninjected eyes retained structural integrity and no fibrin was detected in the vitreous or aqueous humor of these control eyes.
Figure 1. Fibrin and albumin infiltrate the posterior and anterior chamber during experimental B. cereus endophthalmitis.
C57BL/6J mouse eyes were intravitreally injected with 100 CFU of B. cereus, injected with BHI (mock), or left untreated. Fibrin (A) and albumin (B) leakage into the anterior (AC) and posterior (PC) chambers was analyzed by trichrome staining and immunohistochemistry. Immunofluorescent images were overlaid onto brightfield images to enhance localization of albumin in the eye. Images represent three or more individual experiments. Images were acquired at 4× magnification.
Leakage of blood albumin into the eye was analyzed by immunohistochemistry (Figure 1b). Leakage was not detected at 4 h postinfection. At 8 h postinfection, albumin had accumulated in the posterior and anterior chambers. Intensity of the albumin signal in the posterior chamber suggested a greater concentration of albumin in the vitreous than the aqueous humor. These data suggested that the BRB may have become leaky prior to the BAB. By 12 h postinfection, albumin had diffused through the vitreous and aqueous humor, and appeared to be equally distributed.
Blood Retina Barrier Disruption
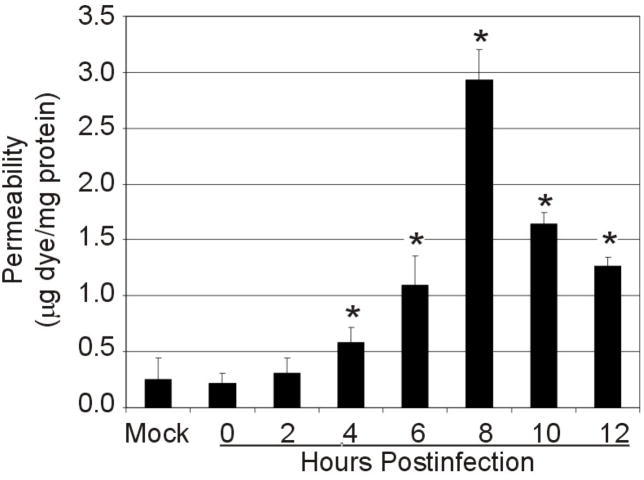
Due to the parallel of inflammatory eye disease and outer BRB breakdown,19, 23–24 as well as the early leakage of albumin into the vitreous, we analyzed and quantified BRB leakage (Figure 2). There was minimal leakage of dye in retinas after BHI mock injection when compared to uninjected controls (P=0.9). Leakage of albumin into the retina increased above mock and uninjected controls as early as 4 h postinfection (P=0.04). These results correlated with PMN infiltration into the eye at 4 h postinfection.14 By 8 h postinfection, BRB permeability had increased 13.30-fold over controls (P=0.003) (Figure 2). Decreasing retinal albumin after 8 h was attributed to the loss of structural integrity of eyes and consequential leakage of ocular fluids in highly inflamed eyes.
Figure 2. BRB permeability to albumin during experimental B. cereus endophthalmitis.
C57BL/6J mouse eyes were intravitreally injected with 100 CFU of B. cereus, injected with BHI (mock) or were not injected. Mice were injected into the tail vein with Evans blue dye 2 h prior to each time point. Albumin leakage into the retina was measured by dye quantification against a standard curve and normalized to total protein in each retina (data represents mean ± standard error of the mean of 8 or more eyes per group). Permeability increased significantly over mock-injected controls as early as 4 h postinfection (P=0.04).
Tight Junction Alterations in the Outer BRB
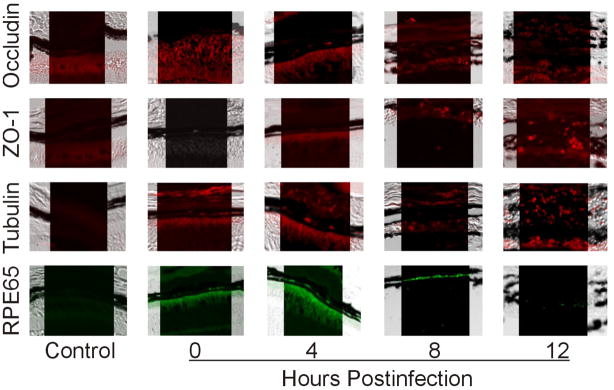
To understand the mechanism of BRB permeability during endophthalmitis, we analyzed the integrity of tight junctions occludin and ZO-1 in the retina, focusing on the RPE of the outer BRB (Figure 3). Both ZO-1 and occludin are expressed in the retina, but control eyes had little visible immunofluorescent signal due to quenching of fluorescent signal by the RPE pigment. However, by 12 h postinfection, the RPE became disrupted. Dispersed, fragmented ZO-1 and occludin signal was visible due to disruption at the level of the RPE, indicating abnormal distribution of tight junction proteins. Tubulin is highly concentrated at the apical border of epithelium25 and has also been found to play a role in barrier function of epithelial cells.26 At 0 h, tubulin was detected in the photoreceptor cell layer (Figure 3), ganglion cell layer and inner plexiform layers (data not shown). Pigment may have quenched the fluorescent signal of tubulin in the intact RPE. During B. cereus infection, tubulin condensed and fragments of intense fluorescence were scattered in the disrupted RPE layer. This is consistent with in vitro studies in which B. cereus induced tubulin condensation around ARPE-19 nuclei (data not shown). RPE65, an RPE-specific 65 kDa protein, is involved in conversion of all-trans retinol to 11-cis retinal, which is then used in visual pigment regeneration in photoreceptor cells. This protein is involved in RPE cell function, and therefore was analyzed to assess RPE function indirectly. RPE65 was detected in the RPE at 0, 4, and 8 h postinfection. Complete loss of RPE65 by 12 h (Figure 3) correlated with reported disruption of retinal layers, retinal detachment, and loss of visual function by 12 h.14
Figure 3. Tight junction protein redistribution, tubulin condensation, and RPE65 degradation at the level of the RPE during experimental B. cereus endophthalmitis.
Retinas of B. cereus-infected eyes were harvested and paraffin-embedded sections were used for histological analysis and immunohistochemistry. Intact retinas had little fluorescent signal in the RPE due to quenching by the pigment. However, at 12 h ZO-1 and occludin expression is intermittent and scattered. Additionally, tubulin condensed and RPE-65 expression was not detected by 12 h postinfection. Control samples were developed with secondary antibody alone. Images represent three or more individual experiments. Images were acquired at 100× magnification.
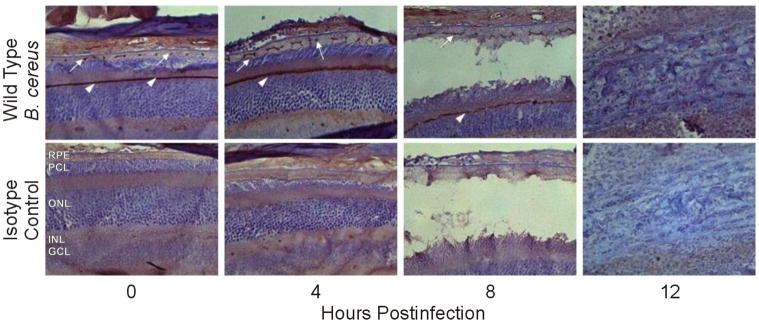
Due to the quenching of fluorescent signal by RPE pigment, a second immunohistochemical technique using polymer detection was utilized to visualize tight junction alterations during infection (Figure 4). Immunohistochemistry was preceded by bleaching the RPE pigment in eye sections, allowing for improved visualization of ZO-1 (but not occludin). In control eyes, a brown punctuate signal indicated the presence of ZO-1 between the RPE. The signal was continuous in the adherens junctions of the outer limiting membrane. By 4 h ZO-1 signal was laterally elongated. Abnormal distribution of ZO-1 in the RPE layer continued at 8 h postinfection. Retinal detachment was evident, and ZO-1 signal in the RPE continued to elongate. By 12 h postinfection, retinal destruction was severe and no signal was detected. Throughout the course of infection, B. cereus induced abnormal redistribution of ZO-1 away from the cell-cell junctions.
Figure 4. Lateral redistribution of ZO-1 in the retina during B. cereus endophthalmitis.
Retinas of B. cereus infected eyes were harvested and paraffin-embedded sections were used for histological analysis and immunohistochemistry. Bleaching RPE pigment prior to immunohistochemistry resulted in a visible brown punctuate ZO-1 signal in the RPE (white arrows) as well as a continuous signal along the outer limiting membrane (white arrow heads). ZO-1 lateral redistribution was evident at 4 and 8 h postinfection (white arrows). By 12 h severe damage to retinal layers had occurred and ZO-1 signal was not detectable (top). Isotype controls confirm lack of nonspecific antibody (bottom). Images were acquired at 40× magnification.
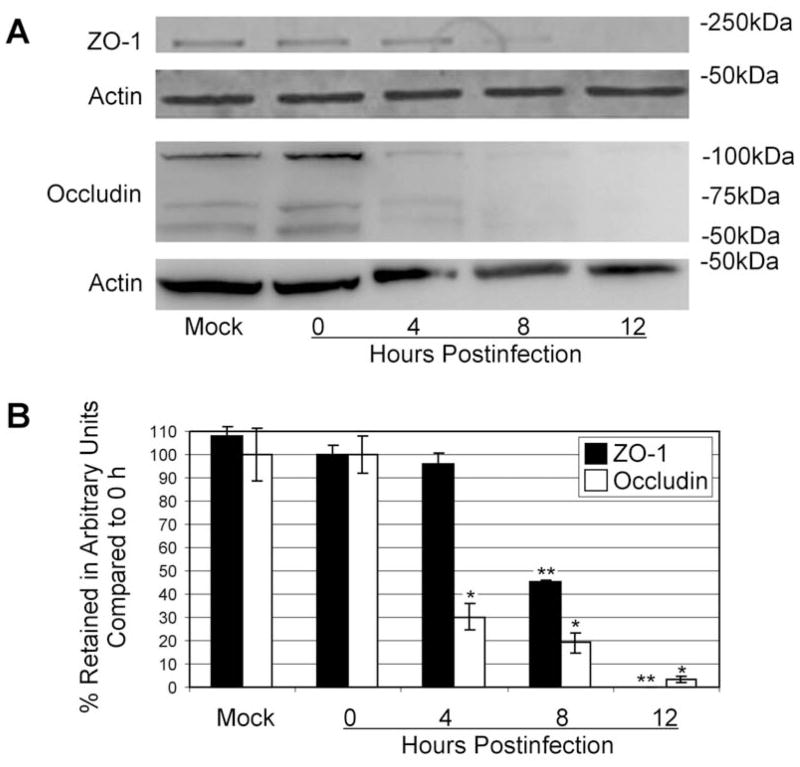
To quantify alterations in tight junction proteins, retinal lysates were analyzed by immunoblot (Figure 5a). The occludin signal was detectable in mock, 0h, and 4 h samples. The occludin signal significantly decreased (P=0.001) at 8h and was almost undetectable by 12 h. ZO-1 was detected in retinal lysates at 0, 4, and 8 h, but not at 12 h postinfection. Expression was further quantified by densitometry (Figure 5b), demonstrating that ZO-1 and occludin expression were decreased during the course of infection. This significant loss of ZO-1 (P<0.0001) and occludin (P<0.0003) by 12 h postinfection indicated retinal tight junction disruption which could contribute to barrier permeability during infection.
Figure 5. Decreases in retinal occludin and ZO-1 expression during experimental B. cereus endophthalmitis.
Expression of occludin and ZO-1 in retinas was analyzed by immunoblot and densitometry during B. cereus infection. Retinal occludin and ZO-1 expression was not detectable by 8 and 12 h postinfection, respectively (A). Densitometry indicated decreased ZO-1 and occludin expression over the infection course, with complete loss of detectable ZO-1 (**compared to 0 h ZO-1, P=0.00006) and >95% loss of occludin (*compared to 0 h occludin, P=0.0003) by 12 h (B).
Proinflammatory Cytokines and Chemokines
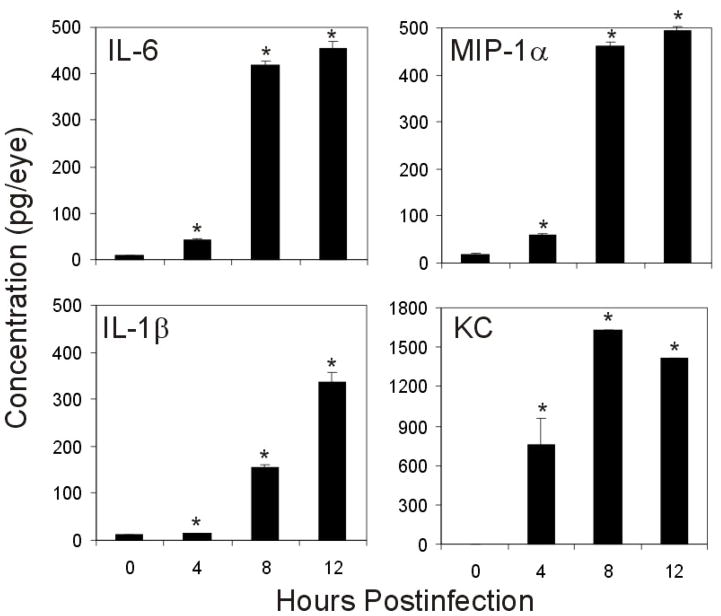
Proinflammatory cytokine and chemokine synthesis in a whole eyes during experimental B. cereus endophthalmitis is summarized in Figure 6. Production of TNFα was previously described, reaching 49.05 ± 4.9 pg/eye by 10 h postinfection.14 Similarly, other proinflammatory cytokines were significantly elevated during B. cereus infection. IL-6 increased 52.57-fold over 0 h controls, reaching 455.18 ± 15.83 pg/eye by 12 h postinfection (P<0.0001). Similarly IL-1β and MIP-1α expression increased 31.00- (P=0.0001) and 29.04-fold (P<0.0001) over 0 h controls by 12 h, reaching 335.50 ± 22.32 and 494.90 ± 9.10 pg/eye, respectively. KC expression was not detectable at 0h, but expression drastically increased to 751.23 ± 204.9 by 4 h (P=0.02). KC concentrations continued to increase, peaking at 1627.09 ± 15.37 pg/eye by 12 h postinfection (P=0.03).
Figure 6. Proinflammatory cytokines and chemokines related to barrier permeability are produced during experimental B. cereus endophthalmitis.
C57BL/6J mouse eyes were intravitreally injected with 100 CFU of B. cereus, injected with BHI (mock), or were not injected, and production of proinflammatory cytokines and chemokines were analyzed by ELISA (data represents mean ± SEM of ≥ 8 eyes per group). Significant increases in cytokine production, above 0 h controls, occurred at 4, 8, and 12 h postinfection for KC (P=0.02, P<0.0001, P=0.03), IL-6 (P=0.001, P<0.0001, P<0.0001), IL-1β (P=0.03, P<0.0001, P=0.0001), and MIP-1α (P<0.0001, P<0.0001, P<0.0001).
plcR-deficient B. cereus-Induced BOB Permeability
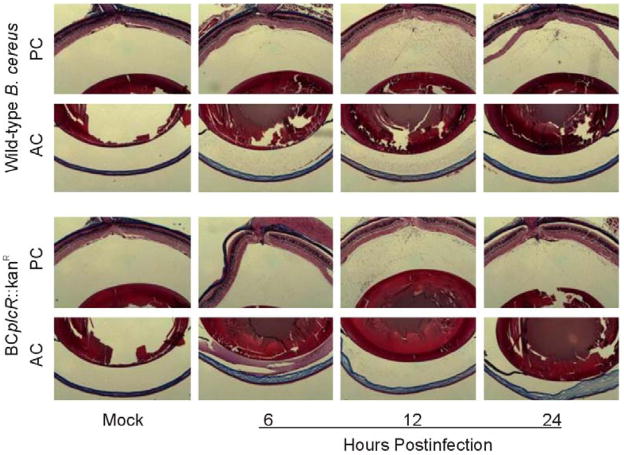
Because in vitro data suggested that plcR-deficient B. cereus induced permeability of polarized RPE monolayers similarly to that of wild-type B. cereus,13 we analyzed whether plcR-deficient B. cereus induced leakage of fibrin across the BOB during experimental B. cereus endophthalmitis. Histological analysis by trichrome staining demonstrated fibrin leakage into the anterior and poster chambers by 8 h, with similar dispersal throughout the eye by 12 h postinfection (Figure 7). plcR-deficient B. cereus appeared to induce BOB permeability comparable to that induced by wild-type B. cereus (Figure 7 and Figure 1), suggesting that a non-plcR-regulated toxin, or group of toxins, may contribute to loss of barrier function during experimental B. cereus endophthalmitis.
Figure 7. plcR-deficient B. cereus induces barrier permeability similarly to wild-type B. cereus during experimental endophthalmitis.
100 CFU of plcR-deficient (BCplcR::kanR) Bacillus was injected into C57BL/6J mouse eyes. Eyes were analyzed by histology and trichrome stained to detect fibrin in the aqueous and vitreous of the anterior (AC) and posterior chambers (PC). Fibrin leaked across the BOB, filling the aqueous and vitreous humor by 8 h postinfection. Images represent three or more individual experiments. Images were aquired at 5× magnification.
Non-plcR-Regulated Secreted Factor(s)-Induced BOB Permeability
To determine if B. cereus secreted factors were responsible for dysfunction of the BOB, cell-free sterile supernatants prepared from wild-type or plcR-deficient B. cereus cultures in early stationary phase were injected into the vitreous. Injection of these supernatants resulted in leakage of fibrin into the anterior and posterior chambers (Figure 8). Upon injection of wild-type supernatant, fibrin began to accumulate in the anterior chamber by 6 h. Fibrin was detectable in the posterior chamber, and this signal intensified by 12 h postinfection. Fibrin accumulation was less dense after injection of sterile cell-free toxins (Figure 8) than after injection of viable bacteria (Figure 1 and Figure 7). A significant amount of fibrin that had leaked into the eye was cleared by 24 h postinfection. This data demonstrates that leakage of fibrin across the BOB occurs in response to treatment with B. cereus secreted factors that are not under the regulation of plcR, indicating that these factors contribute to barrier permeability.
Figure 8. Sterile cell-free wild-type and plcR-deficient B. cereus supernatants are sufficient to induce BOB permeability.
C57BL/6J mouse eyes were intravitreally injected with 0.5 μl sterile B. cereus supernatant prepared from either wild-type (BCWT) or plcR-deficient (BCplcR::kanR) cultures. Eyes were analyzed by histology. Fibrin (A) and albumin (B) leakage into the anterior (AC) and posterior (PC) chambers was analyzed by trichrome staining. Both wild-type and plcR-deficient Bacillus sterile supernatants caused BOB permeability to fibrin, with wild-type inducing fibrin leakage more rapidly than the mutant strain. Images represent three or more individual experiments. Images were aquired at 5× magnification.
DISCUSSION
B. cereus endophthalmitis is a uniquely severe ocular inflammatory disease, which often has devastating consequences such as rapid loss of vision or of the eye itself despite treatment. During experimental B. cereus endophthalmitis, PMN infiltrate into the posterior segment as early as 4 h postinfection, indicative of BOB permeability. As with uveitis and other ocular inflammatory diseases, this barrier breach prevents proper separation of blood constituents from the neural retina, which can allow an unregulated influx of blood constituents into eye. Due to parallels between inflammation in uveitis and endophthalmitis, as well as the lack of information about mechanisms of barrier changes during endophthalmitis, we sought to determine to what extent the BOB is compromised during B. cereus endophthalmitis and to examine whether disruption of tight junctions comprising the BRB contributed to this dysfunction.
To globally examine changes in the BOB, we analyzed whole globes by histology, using trichrome staining and immunohistochemistry to identify infiltrating fibrin or blood albumin, respectively. Fibrin and albumin penetrated the blood-retina and blood-aqueous barriers by 8 h postinfection. Leakage of albumin appeared to occur more rapidly from the BRB than from the BAB. Fibrin and albumin moved from the blood into the ocular tissues and filled the aqueous and vitreous humor, contributing the occlusion of the visual axis. These data are consistent with reports of BRB disruption in conjunction with ocular inflammation16–19 and infection.27–28 In human cases of endophthalmitis, serum albumin was observed in the retina, but was not quantified.29 To further analyze changes in the BRB during experimental B. cereus endophthalmitis, we quantified albumin accumulation in the retina. The robust leakage of albumin into the retinal tissues from 4–8 h corroborated the histology showing fibrin and albumin leakage in the vitreous at 8 h postinfection. Decreasing albumin in the retina after 8 h may have been due to leakage of eye constituents during dissection, due to dissolution of retinal tissues late in infection. Taken together, these data suggest that early loss of BRB integrity may have permitted infiltration of PMN into the vitreous as early as 4 h postinfection.14 BRB permeability, infiltration of PMN, and loss of over 60% of A- and B- wave function occurred in parallel through 8 h postinfection.14 These events also correlate with the course of inflammation during infection, which was reported to be mild at 6 h and moderate to severe by 10–12 h postinfection. Early BRB permeability may allow influx of fluids into the posterior segment, contributing in part to edema, retinal detachment, and ultimately loss of vision.14 However, the contribution of BRB breakdown to vision loss has yet to be determined. Breakdown of the BRB during endophthalmitis may be due, in part, to the production of bacterial toxins or other secreted factors during infection. B. cereus produces a plethora of toxins that could contribute to permeability, including phospholipases, metalloproteases, enterotoxins, cytolysins, and proteins of unknown function.2, 30
Prior to investigating the role of secreted factors in barrier permeability, integrity of RPE tight junctions was analyzed by immunohistochemistry and Western blot. By Western blot, occludin appeared as a set of 110 kDa, 65 kDa, and 60 kDa bands. The 65 kDa and 60 kDa bands are consistent with reports of occludin at various molecular weights.31–35 The 110 kDa band may represent a dimerization of occludin, similar to that reported by McCaffrey et al.36,37 Degradation of RPE tight junctions occurred during experimental B. cereus endophthalmitis. Retinal ZO-1 and occludin expression decreased throughout the course of infection when compared to actin controls, a decrease that paralleled increasing barrier leakage through 8 h postinfection. When analyzed by Western, ZO-1 expression was not detected and occludin expression was <5% by 12 h postinfection. Retinal occludin signal was lost more rapidly than ZO-1 during early infection, corroborating similar findings in RPE monolayers in vitro.13 ZO-1 and occludin were detected at 12 h postinfection via immunohistochemistry, but signals were intermittent. This discrepancy may have been due to superior sensitivity of fluorescence detection compared to that of Western blotting. Redistribution of tight junction proteins in the RPE could have been sufficient to cause disruption of the tight junction, rendering the junction and the RPE barrier permeable, similar to that observed during EAU.19 These data correlated with in vitro data demonstrating degradation of ZO-1 and occludin in polarized RPE monolayers after B. cereus infection.13, 20 Changes in the RPE tight junctions could be the result of toxins or other factors secreted by B. cereus. B. cereus is traditionally a gastrointestinal pathogen that can opportunistically infect the eye. Some gastrointestinal pathogens synthesize toxins that can alter tight junctions and compromise epithelial barriers during infection. These tight junction-damaging toxins include Pseudomonas elastase and alkaline protease,38 Vibro cholera haemagglutinin/protease,39 Bacteroides fragilis metalloproteases,40 and Clostridium difficile exotoxins.40 Proteases produced by nonpathogenic organisms have also been shown to cause break down of tight junctions.41 In the present study, ZO-1 was redistributed laterally in the RPE cell layer by 4 h postinfection. ZO-1 and occludin have been reported to be redistributed and dissociated from the cellular junction of primary human gastric epithelial cells as early as 2 h after infection with Helicobacter pylori.42 However, alterations in ZO-1 structure of RPE during active infection in vivo have not previously been reported. In the present study, alterations in retinal ZO-1 were visible by 4 h postinfection, correlating with BRB permeability. Disruption of ZO-1 may contribute to the tight junction dysfunction that renders the BRB permeable during B. cereus endophthalmitis.
Tubulin and RPE65 localization in RPE during endophthalmitis was also analyzed. The eukaryotic cytoskeleton is comprised of actin microfilaments, intermediate filaments, and microtubules consisting of tubulin. Microtubule integrity is vital for epithelial barrier function.26 During endophthalmitis, the tubulin signal in the retina became intensified, indicating condensation, and was intermittent in the disrupted retinal layers. Several pathogens, including B. cereus, can target cytoskeletal elements during infection. Although we did not analyze the effect of B. cereus infection on actin in the retina, B. cereus has been shown to disrupt actin in intestinal epithelium.43 B. anthracis disrupts actin in neutrophils44 and H. pylori and enteropathogenic E. coli (EPEC) target host cell actin.45 B. cereus, or its toxins or other secreted factors, may disrupt the cytoskeleton of RPE by targeting tubulin. However, alterations in tubulin may also be an indirect result of other cellular changes. The direct effects of B. cereus and its products on cytoskeletal elements in barrier cells have yet to be defined. RPE65 expression is an indicator of epithelial function. Because RPE65 is essential for the regeneration of 11-cis-retinol, loss of RPE65 has been reported to directly correlate with loss of vision. Patients with mutations in RPE65 lose vision during adolescence and mice with a knock-in R91W mutation of RPE65 experience loss of retinal function.46 In the present study, RPE65 expression was not detectable by 12 h postinfection, which is consistent with a loss of retinal function by 12 h during experimental B. cereus endophthalmitis.14 However, whether the loss of RPE65 contributes to loss of vision during endophthalmitis is an open question.
BRB permeability during B. cereus endophthalmitis may also be a consequence of proinflammatory cytokine production during infection. The host response during endophthalmitis has not been well-defined. Examining the proinflammatory mediators produced in other models of ocular inflammation (i.e., uveitis) may provide insight into the contributions of these molecules during endophthalmitis.15–17, 47, 48 In the present study, expression of IL-1β, MIP-1α, KC, and IL-6 were analyzed by ELISA. These candidate cytokines were selected because they are detected in the eye during uveitis and are thought to be directly linked to BRB permeability.15–17, 47, 48
Specifically, IL-1β is associated with BRB permeability and induction of inflammation involving PMN and mononuclear cell infiltration when this cytokine is intravitreally injected into Lewis rats,49, 50 produced during EAU,16, 48 or produced in the vitreous of uveitis patients.16, 51 In the present study, intraocular IL-1β increased over the course of infection with B. cereus. This IL-1β could be derived predominantly from vascular endothelium and may contribute to PMN infiltration seen during B. cereus endophthalmitis. IL-6, MIP-1α, and IL-8 have been detected in eyes of uveitis patients and in experimental uveitis models. Each mediator has the capacity to increase neutrophil recruitment. IL-6 levels were elevated in mouse eyes during experimental B. cereus endophthalmitis. Expression of IL-6 by polarized ARPE-19 monolayers was increased upon exposure to B. cereus,13 but production of IL-6 by other cell types has not been examined. KC (an IL-8 homolog) may significantly modulate the immune response during B. cereus endophthalmitis by recruiting PMN to the site of infection. KC and its homologs have been detected in both experimental and human ocular infections, including keratitis caused by fungi,52 adenovirus,53 Pseudomonas,54, 55 and Staphylococcus,56 and in acute bacterial conjunctivitis57 and uveitis58. Several of these studies noted that increases in PMN likely resulted from KC production. The increase in PMN infiltration and MPO activity between 4 and 8 h during experimental B. cereus endophthalmitis may be the result of the robust production of KC at the respective time points.14 In experimental B. cereus endophthalmitis, KC increased to a greater concentration more rapidly than the other cytokines quantified, especially in eyes of TNFα knockout mice.59
MIP-1α was also synthesized during experimental B. cereus endophthalmitis. During uveitis, MIP-1α increased prior to onset of EAU, suggesting that this mediator contributes to initial cellular recruitment.60 MIP-1α has been reported to play a role in recruitment of circulating monocytes. However, during experimental B. cereus endophthalmitis, monocytes and macrophages detected in small numbers in the eye (<5%), whereas >70% of the infiltrating cells were identified as PMN.14 It is possible that the PMN are synthesizing MIP-1α61 or local cells in the retina, such as astrocytes, may produce MIP-1α,62 but the infection may reaches an experimental endpoint before monocyte recruitment occurs. MIP-1α may also be produced to recruit neutrophils. MIP-1α mediates lung leukocyte recruitment in mice challenged with LPS.63 Injection of MIP-1 into the footpad of mice results in a local influx of PMN followed by a monocyte infiltrate.64 MIP-1α may play a similar role in PMN recruitment during B. cereus endophthalmitis.
TNFα has also been detected in ocular fluids of uveitis patients,15 during EAU,16 and in mouse eyes infected with B. cereus and may induce neutrophil proliferation during inflammation in both models. During experimental B. cereus endophthalmitis in TNFα knockout mice, decreased intraocular PMN infiltration was reported.59 In addition, the increase in intraocular B. cereus growth and decrease in retinal function occurred more rapidly in eyes of TNFα knockout mice than in control mice.59 These data suggested that TNFα played an important role in the inflammatory response which attempts to control B. cereus intraocular infection.
Production of proinflammatory cytokines during experimental B. cereus endophthalmitis may contribute to permeability of the BRB. The complex synthesis and interaction of proinflammatory mediators during ocular inflammation makes identification of their specific roles difficult. While proinflammatory cytokines have been shown to contribute to BRB breakdown in uveitis, their activities in directly altering tight junctions is an open question. In a rabbit model of EAU, treatment of ex vivo retinas (with RPE) with proinflammatory cytokines (including TNFα, IL-1β, and MIP-1α) did not result in downregulation of tight junction proteins.19 The role of individual proinflammatory mediators in endophthalmitis could be further analyzed by establishing infections in mice deficient in individual cytokines of interest. However, compensation for absence of an individual cytokine59 and the production of other cytokines by multiple pathways could confound such studies. For example, IL-1β and TNFα act on similar cell types during inflammation and both signal through the NFκB pathway. The absence of one cytokine may induce synthesis of compensating cytokines, as has been reported during B. cereus endophthalmitis in TNFα knockout mice59 and in experimental EAU where cytokine receptors were deleted.65 The ability for one cytokine to compensate for the absence of a different cytokine makes it difficult to analyze the contribution of individual cytokines to barrier permeability and tight junction disruption.
Though the contribution of proinflammatory cytokines must be further investigated, understanding the role of B. cereus toxins and other secreted factors is equally important. Previous studies indicate that intraocular injection of B. cereus cell wall alone causes transient inflammation, but not loss of vision,4 whereas injection of B. cereus toxins and other secreted factors results in rapid loss of vision.2 This highlights the importance of identifying the B. cereus secreted factors that contribute to tight junction disruption and BRB permeability. The role of plcR-regulated secreted factors in BOB permeability was analyzed by histology. plcR-deficient B. cereus caused RPE toxicity and in vitro BRB permeability to the same extent as the wild-type strain by 8 h.13 To determine if plcR-deficient B. cereus induced BOB permeability during experimental endophthalmitis, we analyzed fibrin leakage in the aqueous and vitreous humor. Infection with plcR-deficient B. cereus resulted in seepage of fibrin in both the anterior and posterior chambers by 8 h postinfection, similar to that seen during infection with wild-type B. cereus, suggesting that plcR-regulated toxins were not required for BOB permeability to fibrin. We also examined the ability of sterile cell-free supernatants from wild-type or plcR-deficient B. cereus to induce BOB permeability. Eyes injected with these sterile cell-free supernatants also exhibited permeability to fibrin, but fibrin accumulation was less dense that that induced by viable bacteria, and was cleared by 24 h postinfection. This may have been due to the absence of replicating bacteria and continual replenishing of toxic factors. Fibrin accumulated in the posterior and anterior chambers of eyes injected with wild-type sterile supernatant more rapidly than in mice injected with supernatant from the plcR-deficient mutant strain. This difference in rate of leakage is consistent with reports in which viable wild-type B. cereus caused inflammation more rapidly than did the plcR-deficient mutant in rabbit2 or mouse eyes.20 In both studies, infection with either strain eventually caused significant inflammation, retinal detachment, and complete loss of retinal function. These data indicate a role for non-plcR-regulated secreted factors in BOB dysfunction during endophthalmitis.
B. cereus produces a plethora of factors that could contribute to permeability, including phospholipases, metalloproteases, enterotoxins, cytolysins, and proteins of unknown function.2, 30 Several proteins are not, or are only partially, regulated by plcR, including neutral protease, InhA2, enolase, oligopeptide permease A, penicillin-binding protein B, or other proteins of unknown function.30 Previous data indicated that barrier permeability of ARPE-19 monolayers in vitro was caused by B. cereus secreted factors. In that study, toxins and other secreted factors not regulated by plcR contributed to BRB disruption in vitro.13 In the present study, plcR-deficient B. cereus, as well as plcR-deficient B. cereus sterile cell-free supernatants, induced permeability of the BOB. This suggests that non-plcR-regulated secreted factors may contribute to loss of BRB integrity. Unpublished data indicates the production of a 20–30 kDa protease and a 160 kDa protease by both wild-type and plcR-deficient B. cereus. Of the B. cereus proteases in this size range, 94% do not encode a plcR box upstream the protease gene, indicating they are produced independently of plcR regulation.66 Such proteases may include camelysin or a zinc metalloprotease, whose activities include cleavage of extracellular matrix proteins.68–70 B. cereus proteases could similarly target components of the extracellular matrix, disrupting the RPE barrier and indirectly inducing tight junction dysfunction. The identities and roles of the secreted factors that facilitate barrier permeability in vivo are still under investigation.
This study is the first to characterize permeability of the BRB and demonstrate alterations in RPE tight junctions during bacterial endophthalmitis. The rapid production of toxic secreted factors by B. cereus may cause significant barrier dysfunction through the alteration of tight junctions at the level of the RPE, but proinflammatory mediators may also be indirectly involved in BRB permeability during infection. These findings are a platform for further studies to understand the mechanisms of BRB dysfunction, the role of bacterial products and inflammatory cytokines in this process, and the contribution of these factors to inflammation and vision loss during endophthalmitis. Understanding the mechanism of barrier dysfunction may facilitate identification of therapeutic targets designed to improve the stability of the BRB, obstruct cellular influx, and protect the retina from explosive inflammation during B. cereus endophthalmitis.
Acknowledgments
This work was funded by National Institutes of Health grant R01EY12985 (M.C.C.). This research was also supported in part by a Lew R. Wasserman Award from Research to Prevent Blindness, Inc. (M.C.C.); P30EY12190 (NIH CORE Grant to Robert E. Anderson, OUHSC), and an unrestricted award from Research to Prevent Blindness, Inc. (Dean McGee Eye Institute). Thanks are extended to Drs. Sarah X. Zhang and Jian-xing Ma (Department of Medicine, Section of Endocrinology and Diabetes, OUHSC) for their guidance and instruction of the Evan’s blue technique. We thank Mark Dittmar (DMEI Animal Facility) for technical assistance and Paula Peirce (Excalibur Pathology, Oklahoma City, OK) for histology assistance. The authors also thank Dr. John Ash (Ophthalmology, OUHSC) for the albumin antibody. Appreciation is extended to Drs. Anne Pereira (Pathology, OUHSC), Jimmy Ballard, Ira Blader, and Rebecca Blackstock (Microbiology and Immunology, OUHSC) for their helpful advisement of experimental design.
Footnotes
Propriety Interests: None
References
- 1.Callegan MC, Engelbert M, Parke DW, II, Jett BD, Gilmore MS. Bacterial endophthalmitis: Epidemiology, therapeutics, and bacterium-host interactions. Clin Microbiol Rev. 2002;15:111–124. doi: 10.1128/CMR.15.1.111-124.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callegan MC, Kane ST, Cochran DC, Gilmore MS, Gominet M, Lereclus D. Relationship of plcR-regulated factors to Bacillus endophthalmitis virulence. Infect Immun. 2003;71:3116–3124. doi: 10.1128/IAI.71.6.3116-3124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jepson MA, Schlecht HB, Collares-Buzato CB. Localization of dysfunctional tight junctions in Salmonella enterica serovar typhimurium-infected epithelial layers. Infect Immun. 2000;68:7202–7208. doi: 10.1128/iai.68.12.7202-7208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callegan MC, Booth MC, Jett BD, Gilmore MS. Pathogenesis of gram-positive bacterial endophthalmitis. Infect Immun. 1999;67:3348–3356. doi: 10.1128/iai.67.7.3348-3356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.David DB, Kirkby GR, Noble BA. Bacillus cereus endophthalmitis. Br J Ophthalmol. 1994;78:577–580. doi: 10.1136/bjo.78.7.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agaisse H, Gominet M, Okstad A, Kolsto AB, Lereclus D. PlcR is a pleiotropic regulator of extracellular virulence factor gene expression in Bacillus thuringiensis. Mol Microbiol. 1999;32:1043–1053. doi: 10.1046/j.1365-2958.1999.01419.x. [DOI] [PubMed] [Google Scholar]
- 7.Callegan MC, Kane ST, Cochran DC, Gilmore MS. Molecular mechanisms of Bacillus endophthalmitis pathogenesis. DNA Cell Biol. 2002;21:367–373. doi: 10.1089/10445490260099647. [DOI] [PubMed] [Google Scholar]
- 8.Cowan CL, Madden WM, Hatem GF, Merritt JC. Endogenous Bacillus cereus panophthalmitis. Ann Ophthalmology. 1987;19:65–68. [PubMed] [Google Scholar]
- 9.Davey RT, Tauber WB. Posttraumatic endophthalmitis: The emerging role of Bacillus cereus infection. Rev Infect Dis. 1987;9:110–123. doi: 10.1093/clinids/9.1.110. [DOI] [PubMed] [Google Scholar]
- 10.O’Day DM, Smith RS, Gregg CR, et al. The problem of Bacillus species infection with special emphasis on the virulence of Bacillus cereus. Ophthalmology. 1981;88:833–838. doi: 10.1016/s0161-6420(81)34960-4. [DOI] [PubMed] [Google Scholar]
- 11.Scott IU, Flynn HW, Feuer W, et al. Endophthalmitis associated with microbial keratitis. Ophthalmology. 1996;103:1864–1870. doi: 10.1016/s0161-6420(96)30415-6. [DOI] [PubMed] [Google Scholar]
- 12.Shamsuddin D, Tuazon CU, Levy C, Curtin J. Bacillus cereus panophthalmitis: Source of the organism. Rev Infect Dis. 1982;4:97–103. doi: 10.1093/clinids/4.1.97. [DOI] [PubMed] [Google Scholar]
- 13.Moyer AM, Ramadan RT, Thurman J, Burroughs A, Callegan MC. Bacillus cereus induces permeability of an in vitro blood retina barrier. Infect Immun. 2008;76:1358–1367. doi: 10.1128/IAI.01330-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramadan RT, Ramirez R, Novosad BD, Callegan MC. Acute inflammation and loss of retinal architecture and function during experimental Bacillus endophthalmitis. Curr Eye Res. 2006;31:1–11. doi: 10.1080/02713680600976925. [DOI] [PubMed] [Google Scholar]
- 15.Ooi KG-J, Galatowicz G, Calder VL, Lightman SL. Cytokines and chemokines in uveitis – Is there a correlation with clinical phenotype? Clin Med Res. 2006;4:294–309. doi: 10.3121/cmr.4.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luna JD, Chan C, Derevjanik NL, et al. Blood-retinal barrier (BRB) breakdown in experimental autoimmune uveoretinitis: Comparison with vascular endothelial growth factor, tumor necrosis factor α, and interleukin-1β-mediated breakdown. J Neurosci Res. 1997;49:268–280. doi: 10.1002/(sici)1097-4547(19970801)49:3<268::aid-jnr2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 17.Deeg CA, Altmann F, Hauck SM, et al. Down-regulation of pigment epithelium-derived factor in uveitic lesion associates with focal vascular endothelial growth factor expression and breakdown of the blood-retinal barrier. Proteomics. 2007;7:1540–1548. doi: 10.1002/pmic.200600795. [DOI] [PubMed] [Google Scholar]
- 18.Mangan BG, Al-Yahya K, Chen CT, et al. Retinal pigment epithelial damage, breakdown of the blood-retinal barrier, and retinal inflammation in dogs with primary glaucoma. Vet Ophthalmol. 2007;10:117–124. doi: 10.1111/j.1463-5224.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- 19.Xu H, Dawson R, Crane I, Liversidge J. Leukocyte diapedesis in vivo induces transient loss of tight junction protein at the blood-retinal barrier. Investig Ophthalmol Vis Sci. 2005;46:2487–2494. doi: 10.1167/iovs.04-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callegan MC, Gilmore MS, Gregory M, et al. Bacterial endophthalmitis: Therapeutic challenges and host-pathogen interactions. Prog Retin Eye Res. 2007;26:189–203. doi: 10.1016/j.preteyeres.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salamitou S, Ramisse F, Brehelin M, et al. The plcR regulon is involved in the opportunistic properties of Bacillus thuringiensis and Bacillus cereus in mice and insects. Microbiol. 2000;146:2825–2832. doi: 10.1099/00221287-146-11-2825. [DOI] [PubMed] [Google Scholar]
- 22.Zhang SX, Sima J, Shao C, et al. Plasminogen kringle 5 reduces vascular leakage in the retina in rat models of oxygen-induced retinopathy and diabetes. Diabetologia. 2004;47:124–131. doi: 10.1007/s00125-003-1276-4. [DOI] [PubMed] [Google Scholar]
- 23.Cunha-Vaz J. The Blood-Ocular Barriers. Surv of Ophthalmol. 1997;23:279–296. doi: 10.1016/0039-6257(79)90158-9. [DOI] [PubMed] [Google Scholar]
- 24.Magone MT, Whitcup SM. Mechanisms of Intraocular Inflammation. In: Streilein JW, Adorini L, Arai K, Berek C, Capra JD, Schmitt-Verhulst A-M, Waksman BH, editors. Immune Response and the Eye. S Karger AG; Basel (Switzerland): 1999. pp. 90–119. [Google Scholar]
- 25.Hussar P, Tserentsoodol N, Koyama H, et al. The glucose transporter GLUT1 and the tight junction protein occludin in nasal olfactory mucosa. Chem Senses. 2002;27:7–11. doi: 10.1093/chemse/27.1.7. [DOI] [PubMed] [Google Scholar]
- 26.Yap AS, Stevenson BR, Abel KC, Cragoe EJ, Manley SW. Microtubule integrity is necessary for the epithelial barrier function of cultured thyroid cell monolayers. Exp Cell Res. 1995;218:540–550. doi: 10.1006/excr.1995.1189. [DOI] [PubMed] [Google Scholar]
- 27.Chin MS, Nagineni CN, Hooper LC, Detrick B, Hooks JJ. Cyclooxygenase-2 Gene expression and regulation in human retinal pigment epithelial cells. Investig Ophthalmol Vis Sci. 2001;42:2338–2346. [PubMed] [Google Scholar]
- 28.Pepose JS, Holland GN, Nestor MS, Cochran AJ, Foos RY. Acquired immune deficiency syndrome: Pathogenic mechanisms of ocular disease. Ophthalmology. 1985;92:472–484. doi: 10.1016/s0161-6420(85)34008-3. [DOI] [PubMed] [Google Scholar]
- 29.Vinores SA, Amin A, Derevjanik NL, Green WR, Campochiaro PA. Immunohistochemical localization of blood-retinal barrier breakdown sites associated with post-surgical macular edema. Histochem J. 1994;26:655–665. doi: 10.1007/BF00158291. [DOI] [PubMed] [Google Scholar]
- 30.Gohar M, Økstad OA, Gilois N, Sanchis V, Kolstoø AB, Lereclus D. Two-dimensional electrophoresis analysis of the extracellular proteome of Bacillus cereus reveals the importance of the PlcR regulon. Proteomics. 2002;2:784–791. doi: 10.1002/1615-9861(200206)2:6<784::AID-PROT784>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 31.Koedel U, Winkler F, Angele B, Fontana A, Pfister H-W. Meningitis-associated central nervous system complications are mediated by the activation of poly(ADP-ribose) polymerase. J Cereb Blood Flow Metab. 2002;22:39–49. doi: 10.1097/00004647-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Mariscal L, Namorado MC, Martin D, et al. Tight junction proteins ZO-1, ZO-2, and occludin along isolated renal tubules. Kidney Int. 2000;57:2386–2402. doi: 10.1046/j.1523-1755.2000.00098.x. [DOI] [PubMed] [Google Scholar]
- 33.Furuse M, Hirase T, Itoh M, et al. Occludin: A novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirose T, Izumi Y, Nagashima Y, et al. Involvement of ASIP/PAR-3 in the promotion of epithelial tight junction formation. J Cell Sci. 2002;115:2485–2495. doi: 10.1242/jcs.115.12.2485. [DOI] [PubMed] [Google Scholar]
- 35.Bojarski C, Weiske J, Schöneberg T, et al. The specific fates of tight junction proteins in apoptotic epithelial cells. J Cell Sci. 2004;117:2097–2107. doi: 10.1242/jcs.01071. [DOI] [PubMed] [Google Scholar]
- 36.McCaffrey G, Seelbach MJ, Staatz WD, et al. Occludin oligomeric assembly at tight junctions of the blood-brain barrier is disrupted by peripheral inflammatory hyperalgesia. J Neurochem. 2008;106:2395–2409. doi: 10.1111/j.1471-4159.2008.05582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCaffrey G, Staatz WD, Quigley CA, et al. Tight junctions contain oligomeric protein assembly critical for maintaining blood-brain barrier integrity in vivo. J Neurochem. 2007;103:2540–2555. doi: 10.1111/j.1471-4159.2007.04943.x. [DOI] [PubMed] [Google Scholar]
- 38.Suter S. The role of bacterial protease in the pathogenesis of cystic fibrosis. Am J Respir Crit Care Med. 1994;150:S118–22. doi: 10.1164/ajrccm/150.6_Pt_2.S118. [DOI] [PubMed] [Google Scholar]
- 39.Wu Z, Nybom P, Magnusson KE. Distinct effects of Vibrio cholerae haemagglutinin/protease on the structure and localization of the tight junction-associated proteins occluding and ZO-1. Cell Microbiol. 2000;2:11–17. doi: 10.1046/j.1462-5822.2000.00025.x. [DOI] [PubMed] [Google Scholar]
- 40.Sears C. Molecular physiology and pathophysiololgy of tight Junctions V. Assault of the tight junction by enteric pathogens. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1129–34. doi: 10.1152/ajpgi.2000.279.6.G1129. [DOI] [PubMed] [Google Scholar]
- 41.Lewis SA, Berg JR, Kleine TJ. Modulation of epithelial permeability by extracellular macromolecules. Physiol Rev. 1995;75:561–589. doi: 10.1152/physrev.1995.75.3.561. [DOI] [PubMed] [Google Scholar]
- 42.Lai Y-P, Yang J-C, Lin T-Z, Lin J-T, Wang J-T. Helicobacter pylori infection and CagA protein translocation in human primary gastric epithelial cell culture. Helicobacter. 2006;11:451–459. doi: 10.1111/j.1523-5378.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- 43.Minnaard J, Lievin-Le MV, Coconnier MH, Servin AL, Perez PF. Disassembly of F-actin cytoskeleton after interaction of Bacillus cereus with fully differentiated human intestinal Caco-2 cells. Infect Immun. 2004;72:3106–1312. doi: 10.1128/IAI.72.6.3106-3112.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.During RL, Li W, Hao B, et al. Anthrax lethal toxin paralyzes neutrophils actin-based motility. J Infect Dis. 2005;192:837–845. doi: 10.1086/432516. [DOI] [PubMed] [Google Scholar]
- 45.Lommel S, Benesch S, Rohde M, Wehland J, Rottner K. Enterohaemorrhagic and enteropathogenic Escherichia coli use different mechanisms for actin pedestal formation that converge on N-WASP. Cell Microbiol. 2004;6:243–254. doi: 10.1111/j.1462-5822.2004.00364.x. [DOI] [PubMed] [Google Scholar]
- 46.Samardzija M, von Lintig J, Tanimoto N, et al. R91W mutation in RPE65 leads to milder early-onset retinal dystrophy due to the generation fo low levels of 11-cis-retinal. Hum Mol Genet. 2007;17:281–292. doi: 10.1093/hmg/ddm304. [DOI] [PubMed] [Google Scholar]
- 47.Curnow SJ, Murray PI. Inflammatory mediators of uveitis: Cytokines and chemokines. Curr Opin Ophthalmol. 2006;17:532–537. doi: 10.1097/ICU.0b013e32801094b5. [DOI] [PubMed] [Google Scholar]
- 48.Hylkema HA. The role of the immune system in uveitis induced in animals. Doc Ophthalmol. 1988;70:339–351. doi: 10.1007/BF00157064. [DOI] [PubMed] [Google Scholar]
- 49.Claudio L, Martiney JA, Brosnan CF. Ultrastructural studies of the blood-retina barrier after exposure to interleukin-1 beta or tumor necrosis factor-alpha. Lab Invest. 1994;70:850–861. [PubMed] [Google Scholar]
- 50.Bamforth SD, Lightman SL, Greenwood J. Ultrastructural analysis of interleukin-1 beta-induced leukocyte recruitment to the rat retina. Invest Ophthalmol Vis Sci. 1997;38:25–35. [PubMed] [Google Scholar]
- 51.Kuppner MC, McKillop-Smith S, Forrester JV. TGF-beta and IL-1 beta act in synergy to enhance IL-6 and IL-8 mRNA levels and IL-6 production by human retinal pigment epithelial cells. Immunology. 1995;84:265–271. [PMC free article] [PubMed] [Google Scholar]
- 52.Vasanthi M, Prajna NV, Lalitha P, Mahadevan K, Muthukkaruppan V. A pilot study on the infiltrating cells and cytokine levels in the tear of fungal keratitis patients. Indian J Ophthalmol. 2007;55(1):27–31. doi: 10.4103/0301-4738.29491. [DOI] [PubMed] [Google Scholar]
- 53.Chintakuntlawar AV, Astley R, Chodosh J. Adenovirus type 37 keratitis in the C57BL/6J mouse. Invest Ophthalmol Vis Sci. 2007;48:781–788. doi: 10.1167/iovs.06-1036. [DOI] [PubMed] [Google Scholar]
- 54.Xue ML, Thakur A, Willcox M. Gene expression of pro-inflammatory cytokines and chemokines in mouse eye infected with Pseudomonas aeruginosa. Clin Exp Ophthalmol. 2002;30:196–199. doi: 10.1046/j.1442-9071.2002.00510.x. [DOI] [PubMed] [Google Scholar]
- 55.Cole N, Bao S, Thakur A, Willcox M, Husband AJ. KC production in the cornea in response to Pseudomonas aeruginosa challenge. Immunol Cell Biol. 2000;78:1–4. doi: 10.1046/j.1440-1711.2000.00860.x. [DOI] [PubMed] [Google Scholar]
- 56.Hume EB, Cole N, Khan S, et al. A Staphylococcus aureus mouse keratitis topical infection model: Cytokine balance in different strains of mice. Immunol Cell Biol. 2005;83:294–300. doi: 10.1111/j.1440-1711.2005.01326.x. [DOI] [PubMed] [Google Scholar]
- 57.Fodor M, Facskó A, Rajnavölgyi E, et al. Enhanced release of IL-6 and IL-8 into tears in various anterior segment eye diseases. Ophthalmic Res. 2006;38:182–188. doi: 10.1159/000093068. [DOI] [PubMed] [Google Scholar]
- 58.Sijssens KM, Rijkers GT, Rothova A, Stilma JS, Schellekens PA, de Boer JH. Cytokines, chemokines and soluble adhesion molecules in aqueous humor of children with uveitis. Exp Eye Res. 2007;85:443–449. doi: 10.1016/j.exer.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 59.Ramadan RT, Moyer AL, Callegan MC. A role for tumor necrosis factor-alpha (TNFα) in experimental Bacillus cereus endophthalmitis pathogenesis. Investig Ophthalmol Vis Sci. 2008;49:4482–4489. doi: 10.1167/iovs.08-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang CH, Fang IM, Lin CP, Yang CM, Chen MS. Effects of the NF-kappaB inhibitor pyrrolidine dithiocarbamate on experimentally induced autoimmune anterior uveitis. Invest Ophthalmol Vis Sci. 2005;46:1339–1347. doi: 10.1167/iovs.04-0640. [DOI] [PubMed] [Google Scholar]
- 61.Kasama T, Miwa Y, Isozaki T, Odai T, Adachi M, Kunkel SL. Neutrophil-derived cytokines: Potential therapeutic targets in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:273–279. doi: 10.2174/1568010054022114. [DOI] [PubMed] [Google Scholar]
- 62.Miyamoto Y, Kim SU. Cytokines-induced production of macrophage inflammatory protein-1α (MIP-1α) in cultured human astrocytes. J Neurosci Res. 1999;55:245–251. doi: 10.1002/(SICI)1097-4547(19990115)55:2<245::AID-JNR12>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 63.Standiford TJ, Kunkel SL, Lukacs NW, et al. Macrophage inflammatory protein-1 alpha mediates lung leukocyte recruitment, lung capillary leak, and early mortality in murine endotoxemia. J Immunol. 1995;155(3):1515–1524. [PubMed] [Google Scholar]
- 64.Wolpe SD, Cerami A. Macrophage inflammatory proteins 1 and 2: Members of a novel superfamily of cytokines. FASEB J. 1989;3:2565–2573. doi: 10.1096/fasebj.3.14.2687068. [DOI] [PubMed] [Google Scholar]
- 65.Brito BE, O’Rourke LM, Pan Y, Anglin J, Planck SR, Rosenbaum JT. IL-1 and TNF receptor-deficient mice show decreased inflammation in an immune complex model of uveitis. Invest Ophthalmol Vis Sci. 1999;40:2583–2589. [PubMed] [Google Scholar]
- 66.Ivanova N, Sorokin A, Anderson I, et al. Genome sequence of Bacillus cereus and comparative analysis with Bacillus anthracis. Nature. 2003;423:87–91. doi: 10.1038/nature01582. [DOI] [PubMed] [Google Scholar]
- 67.Gohar M, Faegri K, Perchat S, et al. The PlcR virulence regulon of Bacillus cereus. PLoS ONE. 2008;3:e2793. doi: 10.1371/journal.pone.0002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fricke B, Drössler K, Willhardt I, Schierhorn A, Menge S, Rücknagel P. The cell envelope-bound metalloprotease (camelysin) from Bacillus cereus is a possible pathogenic factor. Biochim Biophys. 2001;1537:132–146. doi: 10.1016/s0925-4439(01)00066-7. [DOI] [PubMed] [Google Scholar]
- 69.Grass G, Schierhorn A, Sorkau E, et al. Camelysin is a novel surface metalloproteinase from Bacillus cereus. Infect Immun. 2004;72:219–228. doi: 10.1128/IAI.72.1.219-228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kooi C, Subsin B, Chen R, Pohorelic B, Sokol PA. Burkholderia cenocepacia ZmpB is a broad-specificity zinc metalloprotease involved in virulence. Infect Immun. 2006;74:4083–4093. doi: 10.1128/IAI.00297-06. [DOI] [PMC free article] [PubMed] [Google Scholar]