Abstract
Background
A major complication associated with cardiac surgery is excessive and prolonged bleeding in the perioperative period. Improving coagulation by inhibiting fibrinolysis, primarily through inhibition of plasmin activity (PLact) with antifibrinolytics such as tranexamic acid (TXA) has been a pharmacological mainstay in cardiac surgical patients. Despite its almost ubiquitous use, the temporal and regional modulation of PLact profiles by TXA remain unexplored. Accordingly, the present study developed a fluorogenic-microdialysis system to measure in-vivo dynamic changes in PLact following TXA administration in a large animal model.
Methods
Pigs (25–35kg) were randomly assigned to receive TXA (30mg/kg, diluted into 50cc normal saline; n=9) or vehicle (50cc normal saline; n=7) in which microdialysis probes were placed in the liver, myocardium, kidney and quadricep muscle compartments. The microdialysate infusion contained a validated plasmin specific fluorogenic peptide. The fluorescence emission (Standard Fluorogenic Units; SFU) of the interstitial fluid collected from the microdialysis probes, which directly reflects PLact, was determined at steady-state baseline, 30, 60, 90 and 120 minutes following TXA/vehicle infusion. Plasma PLact was determined at the same time points using the same fluorogenic substrate approach.
Results
With respect to vehicle values, TXA reduced plasma PLact at 30 minutes post infusion by more than 110 SFU (p<0.05). Specifically, there was a decrease in liver PLact at 90 and 120 minutes post TXA infusion of greater than 150 (p<0.05) and 175 (p<0.05) SFU, respectively. The decline in liver PLact occurred 60 minutes after the maximal decline in plasma PLact. In contrast, kidney, heart, and quadriceps PLact transiently increased followed by an overall decrease at 120 minutes.
Conclusions
Using a large animal model and in-vivo microdialysis measurements of PLact, the unique findings from this study were 2-fold. First, TXA induced temporally distinct PLact profiles within the plasma and selected interstitial compartments. Second, TXA caused region specific changes in PLact profiles. These temporal and regional differences in the effects of TXA may have important therapeutic considerations when managing fibrinolysis in the perioperative period.
Keywords: tranexamic acid, microdialysis, interstitial plasmin, regional activity
Introduction
Excessive perioperative bleeding is a major complication associated with cardiothoracic, major vascular, liver transplantation, orthopedic spine and trauma surgeries. Blood products and antifibrinolytics have been effectively utilized to achieve needed hemostasis in these clinical scenarios. 1, 2, 3, 4, 5, 6 Antifibrinolytics have been the pharmacological mainstay with proven efficacy in reducing blood loss and blood product transfusion requirements, particularly in relation to cardiac surgery. 1, 3 Common clinically utilized antifibrinolytics affect plasmin activity (PLact) primarily by inhibiting the enzymatic interaction of plasminogen/plasmin with fibrinogen/fibrin and can be classified as either serine protease inhibitors or lysine analogues. 7 The serine protease inhibitor aprotinin significantly inhibits fibrinolysis, although this drug has been removed from clinical use. 7 As a consequence, lysine analogues, such as tranexamic acid (TXA), have now become the major class of pharmacological intervention in which antifibrinolytic therapy is indicated for the management of excessive perioperative bleeding and has likely resulted in an increase use of TXA for this purpose. However, the basic regional and temporal PLact profiles following TXA administration remain unexplored. Accordingly, the primary goal of this study was to characterize the effects of TXA on the regional and temporal PLact profiles in plasma and selected tissue compartments.
Common clinically implemented weight based TXA dosing regimens are largely empirically derived and, as such, no consensus exists as to appropriate dosing to provide optimal perioperative control of fibrinolysis. 8 This lack of established clinical dosing parameters suggests that the modulation of fibrinolysis by TXA may be enhanced by regional and temporal measurements of PLact. Accordingly, this study used a common weight based TXA dosing scheme to investigate the effects of TXA on regional and temporal PLact profiles. 9 In order to explore the regional dynamics of PLact, a large animal model utilizing established microdialysis techniques was employed. 10, 11 Such microdialysis techniques, utilizing a fluorogenic substrate, allowed the detection of interstitial enzymatic activity, such as plasmin. 12 Accordingly, the objectives of this study were two-fold. One, the validation and calibration of a fluorogenic peptide that could be used to assess PLact in-vivo. Second, the development of a porcine model to measure PLact in plasma and interstitial regions of clinical relevance utilizing this validated fluorogenic approach.
Methods
The present study was conducted in two stages. First, in-vitro validation studies were performed to develop a PLact measurement system using a plasmin specific fluorogenic substrate. 12 This validated PLact measurement system was utilized to perform in-vivo PLact measurements, via microdialysis probes, within targeted regions. Then, TXA was infused intravenously and PLact was continuously monitored within these regions. Finally, TXA plasma and D-dimer concentrations were measured.
In-vitro Validations
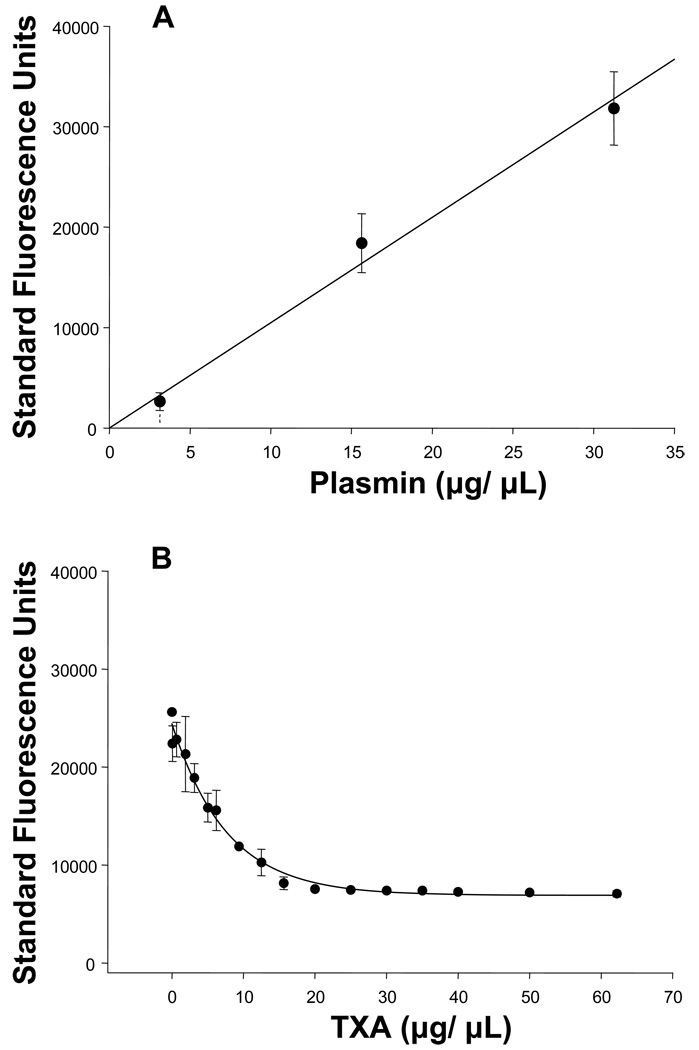
Several in-vitro validation studies were performed using a plasmin specific fluorogenic substrate 12 (Sigma-Aldrich, St. Louis, MO, Cat. # A8171). In particular, this substrate contained a validated fluorogenic peptide which, when specifically cleaved by plasmin, yielded a coumarin fluorescent moiety with excitation/emission wavelengths of 365/440nm respectively. 12 The first in-vitro validation study determined the response of the fluorogenic substrate to increasing concentrations of plasmin. Briefly, 6.25µM of plasmin substrate was injected into a 96-well polystyrene plate (Nalge Nunc) with increasing concentrations of plasmin (0–31.25 µg/mL, Sigma-Aldrich, Cat #P1867). Following 5 minute incubation at 37°C, the plate was placed into a fluorescence microplate reader (FLUOstar Galaxy, BMG LABTECH Inc., Offenburg/Germany) and the fluorescence emission was recorded. Fluorescence emission, reflective of PLact, increased with increasing concentrations of plasmin (Figure 1A).
Figure 1.
(A) Fluorescence emission of the plasmin specific substrate (6.25µg/mL), reflective of PLact, increased with increasing concentrations of plasmin (0–31.25 µg/mL) in a linear concentration dependent manner (n=3, plotted values are mean±SEM; linear regression, y(x) = 1048.8*x, r2 = 0.996, p = 0.002).
(B) Fluorescence emission of the plasmin specific substrate (6.25µg/mL), reflective of PLact, in the presence of plasmin (31.25µg/mL) and control porcine plasma (1:32) decreased in response to increasing concentrations of TXA (0–62.2mg/mL)in a classic logarithmic concentration dependent manner 13 (n=3, plotted values are mean±SEM, regression, y(x) = 23280*e−0.063*x, r2=0.964, p<0.001).
Next, a series of in-vitro experiments were performed using a solution of reference normal porcine plasma which determined the TXA plasma concentration inhibition curve. Specifically, plasmin (31.25µg/mL) and diluted control porcine plasma (1:32) were incubated with increasing concentrations of TXA (0–62.2mg/mL) and subjected to the same fluorescence measurement procedure previously described. In Figure 1B, the fluorescence emission, reflective of PLact, decreased in response to increasing concentrations of TXA in a classic, logarithmic, concentration-dependent manner. 13 A logarithmic equation was matched to this data using regression analysis.
Therefore, these in-vitro studies established the optimal substrate concentration, demonstrated specificity of the substrate for plasmin and determined the fluorescence emission inhibition curve for TXA in porcine plasma. The development of this PLact measurement system was then translated to the in-vivo PLact studies described below.
Animal and Surgical Preparation
Yorkshire pigs (n= 16, male, 25–30 kg, Hambone Farms, Reevesville, SC) were instrumented to measure plasma and interstitial PLact. All animals were treated and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Institutes of Health, 1996). Approval of all animal care and use protocols was obtained from the Medical University of South Carolina Institutional Animal Care and Use Committee, AR# 2786.
After sedation with diazepam (100 mg po, Elkins-Sinn Inc (ESI), Cherry Hill, NJ), general inhalational anesthesia was induced using isoflurane (3%, Baxter Healthcare Corp., Deerfield, IL) mixed with oxygen and nitrous oxide (67:33%) and peripheral intravenous access obtained. A stable surgical plane of anesthesia was established and maintained throughout the protocol using sufentanyl (2 µg/kg IV, ESI), etomidate (0.1mg/kg IV, ESI), vecuronium (10mg IV bolus, 0.5mg/kg/hr IV infusion, Ben Venue Laboratories Inc, Bedford, OH), morphine sulfate(3 mg/kg/hr IV, ESI) and isoflurane (1%, Baxter Healthcare Corp.) Tracheal intubation was achieved via tracheostomy and mechanical ventilation established (Narkomed 2B, North American Drager, Telford, PA). Intravenous fluids (lactated Ringer's) were administered per established weight based protocols for maintenance fluids and estimated blood loss replacement. A single lumen catheter (8 Fr) was placed into the right external jugular vein for fluid and drug administration. An arterial line catheter (7 Fr) was placed into the right carotid artery to continuously monitor systemic blood pressures and obtain blood samples. Following a 60 minute baseline and stabilization period, each pig was assigned to receive TXA (Pharmacia & Upjohn Co., New York, NY ) (30mg/kg, diluted into 50cc normal saline) or vehicle (50cc normal saline) over a 10 minute period using a pre-specified randomization protocol. This anesthesia regimen and surgical preparation provided a physiologically and hemodynamically stable experimental model for up to 6 hours as previously reported. 11
Microdialysis Techniques
Microdialysis probes (CMA/Microdialysis, North Chelmsford, MA) with a molecular weight cutoff of 20 kDa and an outer diameter of 0.5 mm were surgically placed interstitially in the anterior myocardium of the left ventricle, right lobe of the liver, lower pole of the right kidney and left quadriceps muscle compartments. Placement of the microdialysis probes required a median sternotomy, a subxyphoid intra-abdominal incision, a subcostal flank incision and a medial mid-thigh incision with associated tissue dissections respectively.
The microdialysis probes were connected to precision infusion pumps and controller system (BASi, West Lafayette, IN). A flow rate of 6.0 µL/min was established and an iso-osmotic dialysis performed. Dialysate was infused for 30 minutes to allow for equilibration with each of the respective tissue compartments. The microdialysate infusion contained the validated fluorogenic peptide (Sigma A-8171, 10µM). Preliminary studies demonstrated this microdialysate concentration yielded a steady state fluorescence emission within 30 minutes of the initiation of dialysis, indicative of equilibration with the interstitial space of the target tissue. The fluorescence emission of the interstitial fluid collected from each of the microdialysis probes, which directly reflected PLact, was determined at steady-state baseline, 30, 60, 90 and 120 minutes following TXA/vehicle infusion, using fluorescence measurement techniques previously described.
Plasma Sampling
Arterial blood samples (50cc) were collected immediately following a 30 minute stabilization period. The plasma from these blood samples was used to develop a reference normal porcine plasma solution for in-vitro validations previously described. At baselines and at 30 minute intervals throughout the protocol, coinciding with the microdialysis samples, arterial blood samples (10mL) were collected. All blood samples were collected in EDTA tubes, centrifuged and the plasma decanted and frozen for subsequent measurement of PLact using the previously described fluorescence measurement system.
TXA Plasma Concentration Measurements
An Acquity UPLC coupled to a Quattro Premier XE mass spectrometer (Waters, Milford, MA) was used to measure TXA plasma concentrations. Chromatographic separation was performed on an Acquity UPLC HSS C18 2.1×100mm (1.8µm) column preceded by an Acquity UPLC HSS C18 (1.8µm) pre-column. Samples were eluted isocratically over 5 min and the mobile phase consisted of 10% acetonitrile in 2mM ammonium acetate (pH 3.5) with a flow rate of 0.15 ml/min. The mass spectrometer was operated in positive ion mode with capillary voltage 3.1 kV, source temperature 120°C, desolvation temperature 400°C and nitrogen gas flow at 700 L/Hr. Data acquisition was performed using MassLynx 4.1 and quantification using QuanLynx 4.1 (Waters, Milford, MA). TXA plasma concentrations were determined from precalibrated TXA standards (0.5–40 µg/mL).
D-dimer Measurements
D-dimer measurements were made on plasma collected at baseline (time 0) and 120 minute time intervals for vehicle and TXA treatment groups using an enzyme-linked immunosorbent assay (American Diagnostics Inc, Stamford, CT, Cat. 602).
Data Analysis
Comparisons for baseline steady state as well as for net change in fluorescence for all time points within each region were made using an analysis of variance (ANOVA) followed by pair-wise tests of individual time points means using Bonferroni bounds. Net change in fluorescence compared to baseline for all time points within each region were made using a 2-sample t-test. Comparison of D-dimer concentrations at baseline (time 0) and 120 minute intervals were performed using a 2-sample t-test. All statistical procedures were performed using STATA statistical software (Intercooled STATA 8.0). Results are presented as mean±standard error of the mean (SEM) with p values <0.05 considered to be statistically significant.
Results
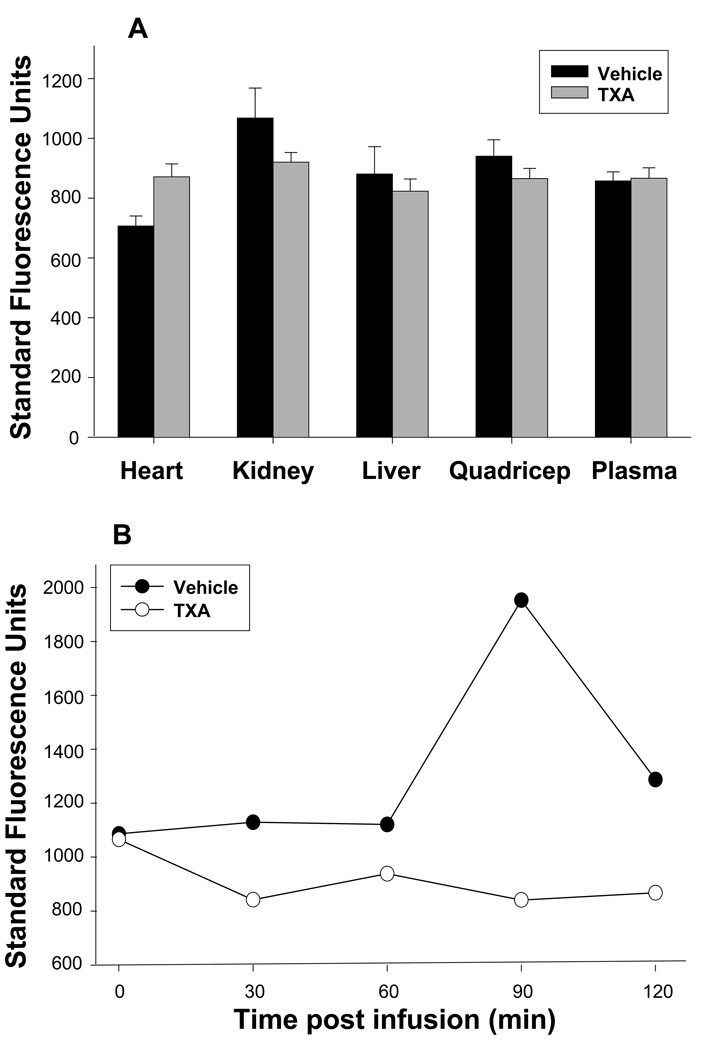
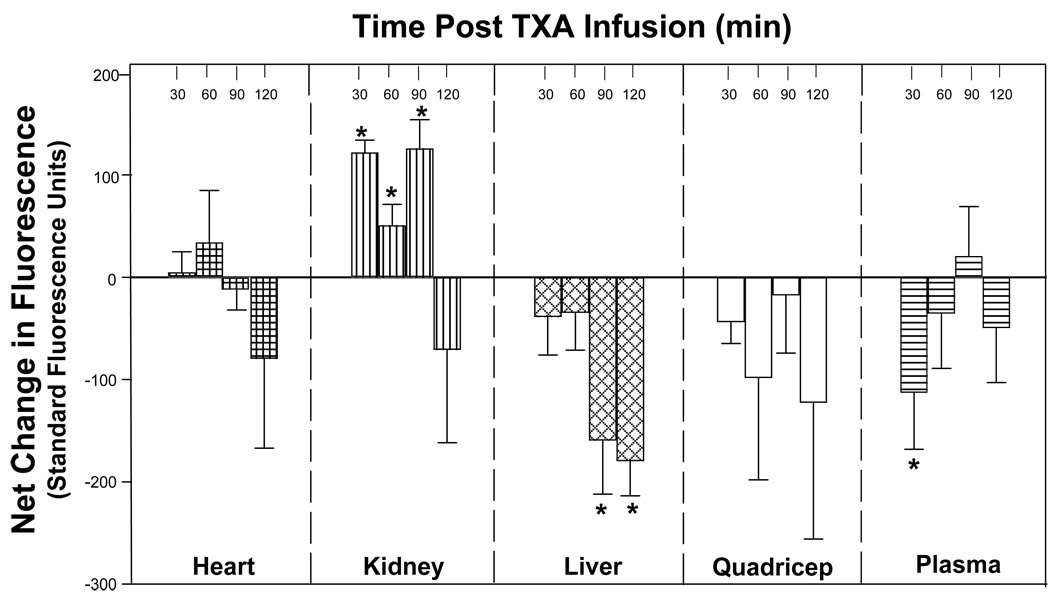
Following successfully placement of microdialysis probes in all tissue compartments, respective steady state baseline fluorescence emission measurements, reflective of PLact within each compartment, were obtained (Figure 2A). There was no significant difference in baseline fluorescence emissions between groups, randomized to either vehicle or TXA treatment, for each tissue compartment, reflective of equivalent PLact prior to initiation of treatment. Figure 2B illustrates the representative fluorescence emission for a selected tissue compartment (i.e. the liver) for both a representative vehicle and TXA pig preparation. Respective fluorescence emission measurements were obtained at baseline (time 0) and 30, 60, 90 and 120 minutes following either vehicle (saline) or TXA (30mg/kg) infusion. The differences in fluorescence emission values between the vehicle and TXA groups at each of the respective time intervals are reflective of changes in PLact induced by the administration of TXA. Therefore, in order to directly examine the effects of TXA on PLact, the absolute fluorescence emission values were transformed to yield a net change in mean fluorescence emission with respect to mean vehicle values for each of the selected compartments at the specified times intervals (Figure 3). With respect to vehicle values, TXA significantly reduced plasma PLact at 30 minutes post infusion. However, in the interstitial compartments, temporal and regional differences in PLact were observed following TXA administration. Specifically, there was a significant decrease in liver PLact at 90 and 120 minutes. This reduction in liver PLact occurred 60 minutes after the maximal decline in plasma PLact. In contrast, kidney PLact was significantly increased at 30, 60, and 90 minutes. Within the myocardium, PLact remained virtually unchanged. In the quadriceps muscle, PLact fell post TXA infusion but did not reach statistical significance at any time point (p>0.5).
Figure 2.
(A) Steady state baseline fluorescence emission, reflective of PLact within each of the target tissue compartments was equivalent in pigs randomized to either vehicle (saline) or TXA (30mg/kg). Thus, the baseline fluorescence emissions between the two groups was comparable prior to initiation of treatment (plotted values are mean±SEM, *p < 0.05).
(B) Representative fluorescence emission measurements within the liver tissue compartment were obtained at baseline (time 0) and 30, 60, 90 and 120 minutes following either vehicle (saline) or TXA (30mg/kg) infusion. There was a notable increase in absolute fluorescence emission over time following vehicle (saline) infusion. In contrast, there was an overall decrease in fluorescence emission over time, reflective of reduced PLact within the liver following TXA administration. The summary data reflective of PLact across each target compartment and all time intervals are shown in figure 3.
Figure 3.
The computed net change in mean fluorescence emission, reflective of changes in PLact, with respect to time matched vehicle values following TXA (30mg/kg) infusion for selected compartments demonstrates the unique temporal and regional differences in the affects of TXA on PLact. Specifically, TXA significantly reduced plasma PLact at 30 minutes. In addition, there was a significant decrease in liver PLact at 90 and 120 minutes. In contrast, kidney PLact was significantly increased at 30, 60, and 90 minutes. There was no significant change in heart PLact for all time points. The PLact within the quadriceps muscle fell post TXA infusion but did not reach statistical significance at any time point (plotted values are mean±SEM, *p < 0.05 verses baseline).
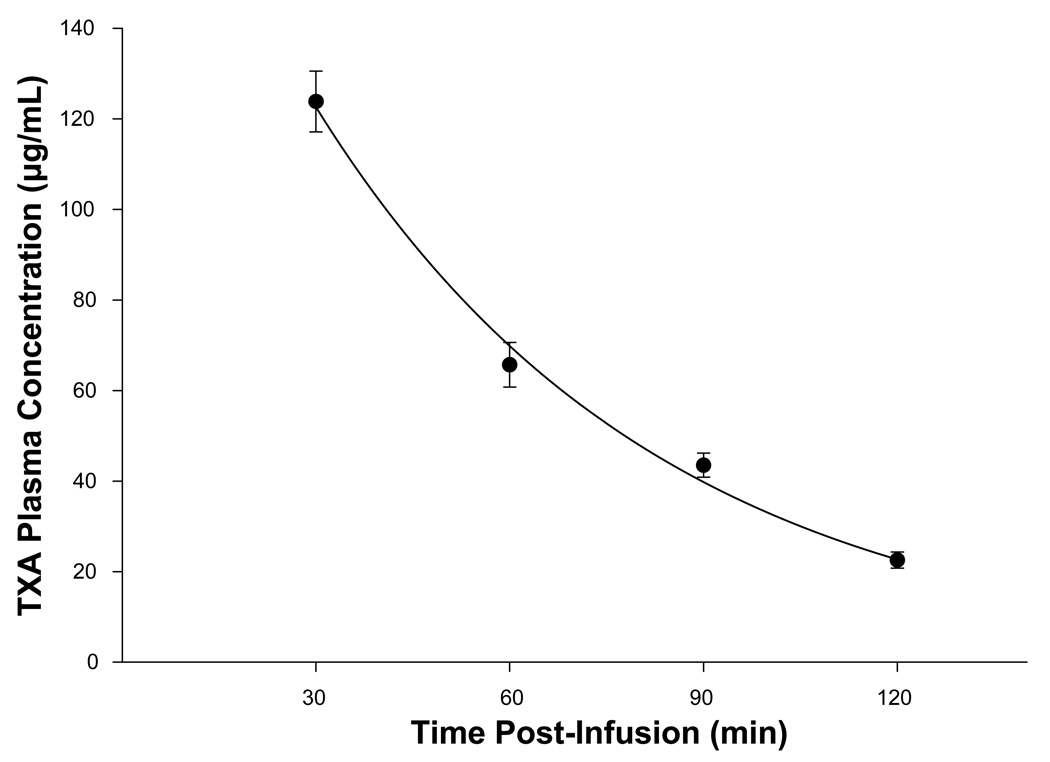
The TXA plasma concentrations for time intervals 30, 60, 90 and 120 minutes post TXA infusion are shown in Figure 4. The peak TXA plasma concentration occurred at 30 minutes post TXA infusion and subsequently decreased in a negative logarithmic time dependent manner consistent with first order elimination pharmacokinetics. 13 Plasma from baseline (time 0) and 120 minute time intervals for vehicle and TXA treatment groups was subjected to D-dimer analysis. The baseline, steady state plasma D-dimer concentration was 20±8 µg/mL with no difference between vehicle or TXA groups at randomization (p=0.67). Plasma D-dimer concentration at 120 minutes post infusion fell slightly, but not significantly, from baseline (13±3 µg/mL, p=0.49) with no difference between vehicle or TXA (p=0.77)
Figure 4.
TXA plasma concentrations determined by high performance liquid chromatography / mass spectrometry techniques obtained at time intervals 30, 60, 90 and 120 minutes post TXA infusion decreased in a negative logarithmic time dependent manner consistent with first order elimination pharmacokinetics 13 (plotted values are mean±SEM, regression, y(x) = 219.37*e−0.019*x, r2 = 0.994, p = 0.003).
Discussion
Perioperative hemorrhage constitutes an important risk factor for morbidity and mortality in most major surgical procedures; notably cardiovascular surgery. 14, 15, 16, 17 Accordingly, blood transfusions, blood product and coagulation factor delivery, as well as pharmacological modalities targeted at the coagulation/fibrinolytic mechanisms are important clinical maneuvers in the perioperative setting. 3, 14 However these interventional strategies, such as pharmacological approaches, can be associated with adverse outcomes which may be due to differences in dosing regimens as well as off-target effects. 14, 15, 16, 17, 18 One commonly utilized antifibrinolytic is tranexamic acid (TXA), which can modulate the fibrinolytic pathway by inhibiting local plasmin activity (Plact). 19 However, current TXA dosing schedules are largely empirical and the regional and temporal effects with respect to changes in PLact remained unknown. 8 The present study addressed this issue through the use of a validated fluorogenic-microdialysis approach in a large animal model, in order to provide serial assessment of PLact on a regional basis, following a standardized dose of TXA. 9 The unique finding from this study is that interstitial PLact is differentially affected following TXA infusion in both a region and time dependent manner. For example, TXA induced temporally distinct PLact profiles within the plasma and selected interstitial compartments such as the kidney and the liver. These temporal and regional differences in the effects of TXA on PLact may have important therapeutic considerations when managing fibrinolysis in the perioperative period. The prophylactic use of lysine analogue antifibrinolytics during cardiac surgery has the potential to induce a hypercoagulable prethrombotic state. 20 As such, thrombosis (DVT, pulmonary artery, renal pelvic and artery, bladder and cerebral vascular) with respective concomitant organ injury and dysfunction have been associated with the use of antifibrinolytics such as TXA. 17, 21, 22, 23, 24, 25, 26 The primary mechanism of elimination of TXA is via renal excretion. As such, acute temporal alterations in renal function associated with cardiac surgery further compound the complexity of maintaining a safe hemostatic state in such clinical scenarios in which TXA is indicated.27 Thus, there are several temporal and regional parameters that must be considered when attempting to balance the extensively dynamic and sensitive coagulation/fibrinolytic state(s) of cardiac surgical patients in the perioperative period.
While the pharmacology of TXA has been rigorously described previously with respect to mechanisms of action,19 there have been no studies which have precisely quantified the effects of TXA on interstitial PLact in-vivo - the primary target for TXA with respect to modulating fibrinolysis. Tissue plasminogen activator is synthesized and secreted by endothelial cells intraluminally and abluminaly into the vascular and interstitial spaces respectively where it catalyzes the conversion of plasminogen to plasmin and thus facilitating fibrinolysis. 28
This microdialysis approach provides for interstitial interrogation of PLact and thus a means to directly measure a key determinant of fibrinolysis and avoids the interference of intraluminal dynamics. Furthermore, while past basic and clinical studies have described the utility of TXA in the context of cardiovascular surgery, such as that associated with cardiopulmonary bypass, optimal dosing strategies remain a subject of debate. 8 The present study is the first to develop an approach to continuously measure the major biological response variable relevant to TXA administration, PLact, within the plasma as well as interstitial space of critical target tissues. This study utilized a microdialysis approach to interrogate the interstitial compartment, an approach that has been well described previously in both animal and clinical studies. 10, 11 This microdialysis method was coupled with a fluorogenic substrate specific for plasmin, and therefore provided a means to quantify PLact within the interstitial space. This methodology may provide a useful analytical approach to assess PLact with varying TXA dosing regimens, and thereby provide a basis for optimal TXA administration. The present study provided the fundamental temporal and regional information necessary to move forward studies aimed at TXA dosing optimization. Moreover, the present study identified differences in PLact following TXA administration in critical target organs such as the liver and kidney, which may hold relevance in the clinical context of hepatic or renal dysfunction. 17, 18, 29 The continuous PLact profiling which is described in the current study, may provide a means by which to address these issues and further optimize current and future antifibrinolytic therapies. 30
The present study utilized TXA to investigate the effects of a commonly used antifibrinolytic agent on plasma and interstitial PLact profiles. The rationale for focusing this study on TXA with respect to PLact profiles was two-fold. First, the objective of this study was to demonstrate the proof of concept that regional and temporal heterogeneity exists with respect to one computed dose of an antifibrinolytic, and TXA was chosen as a prototypical example. Second, the serine protease inhibitor, aprotinin, while historically considered the first line agent for modulating PLact, has been withdrawn from clinical use, thus leaving lysine analogues such as TXA as the pharmacological mainstay for antifibrinolytic therapy. Lysine analogues such as TXA affect PLact primarily by inhibiting the enzymatic interaction of plasminogen and plasmin with fibrinogen and fibrin, which is key to the enzymatic induction of fibrinolysis. 19 Thus, TXA served as a reasonable first step, with respect to clinical relevance, in determining the fundamental mechanistic underpinnings of the regional and temporal effects of lysine analogues on PLact profiles. Comparative studies of specific antifibrinolytic agents hold significant clinical relevance and warrant future investigation. Nevertheless, it is likely that the results from the present study can be extrapolated, to some degree to other lysine analogues (i.e. epsilon aminocaproic acid) as well as aprotinin, with respect to the regional and temporal heterogeneity observed. For example, following a single bolus dose of TXA transient effects on PLact were observed in the heart and kidney, whereas there were persistent effects in the liver. While this acute study could not address this issue directly, the disparate effects on PLact may in turn affect hepatic and renal function, which the latter has been identified as a potential risk factor for the adverse effects of antifibrinolytics such as aprotinin. 17, 18, 29
The peak TXA plasma concentrations obtained in the present study are consistent with those typically reported in prior clinical investigations. 8, 31, 32 As such, the TXA dosing regimen used in the present study represents a clinically relevant dosing approach. The TXA plasma elimination profile obtained is congruent with classic first order pharmacokinetics, 13 indicating that the large animal model used in the present study holds pharmacological relevance. The time of the peak TXA plasma at 30 minutes coincides with the occurrence of peak plasma PLact inhibition, demonstrating the pharmacological efficacy of the TXA within the vascular compartment. Thus, the large animal preparation and TXA dosing paradigm utilized in the present study is likely to be a clinically relevant simulation.
Study Limitations and Conclusions
One potential limitation of the present study was that the TXA regimen implemented involved a loading dose only without a subsequent continuous infusion of TXA. In addition, the in-vivo investigations did not include the context of cardiopulmonary bypass which is a typical clinical scenario in which TXA is commonly utilized. The primary objective of the current study was to quantify the regional and temporal effects of TXA on relevant compartment PLact profiles. Accordingly, the TXA regimen involved a loading dose only in order to examine the compartment specific temporal dynamics of TXA on PLact profiles which would have been potentially obscured by the subsequent administration of a continuous infusion of TXA. Furthermore, the context of cardiopulmonary bypass would have included requisite systemic heparinization which could have added coagulation interactions that potentially impacted de novo fibrinolytic processes. Indeed, the present study demonstrated that static measurements to quantify fibrinolysis (i.e. D-dimers) were stable and not different between vehicle and TXA groups. This suggests that the experimental design did not evoke a substantial fibrinolytic response. Nevertheless, using a continuous interstitial monitoring approach, the present study demonstrated that heterogeneity in steady state PLact existed in specific tissue compartments which were differentially effected by TXA. These observations suggest that continuous PLact monitoring would be of much greater importance in the context of a heightened fibrinolytic state such as cardiopulmonary bypass. The primary focus of this preliminary study was to determine the fundamental mechanistic underpinnings of the regional and temporal effects of TXA on PLact profiles in a de novo, non-pathological, fibrinolytic state. Logically, one may anticipate an even greater magnitude of affect by TXA in a pathological fibrinolytic state such as that induced by cardiopulmonary bypass. The extension of the current study findings will provide a basis for the pursuit of similar PLact investigations involving a clinically relevant cardiopulmonary bypass model. Nevertheless the present study demonstrated in a clinically relevant large animal model that regional and temporal heterogeneity in PLact exists following a single computed dose of TXA, a prototypical antifibrinolytic. Although commonly used, TXA and similar antifibrinolytics are not FDA approved for prophylactic use to reduce blood loss and blood component transfusions in patients undergoing coronary bypass surgery. Coupled with recent concerns for the adverse effects of aprotinin, the findings of the present study underscore the need for more rigorous monitoring and dosing of antifibrinolytics.
Acknowledgements
This study was supported in part by NIH grants HL059165, HL078650 and a Merit Award form the Veterans' Affairs Health Administration.
The authors would like to gratefully acknowledge the assistance of Danyelle M. Townsend, PhD and Joachim de Klerk Uys, PhD of the Medical University of South Carolina Hollings Cancer Center Drug Metabolism and Clinical Pharmacology Core Facility in the measurement of plasma tranexamic acid concentrations.
Footnotes
No conflict of interest.
Implications Statement
Using a large animal model and in-vivo microdialysis measurements of plasmin activity (PLact), tranexamic acid (TXA) induced temporally distinct and region specific changes in PLact profiles within the plasma and selected interstitial compartments.
References
- 1.Laupacis A, Fergusson D. Drugs to minimize perioperative blood loss in cardiac surgery: meta-analyses using perioperative blood transfusion as the outcome. Anesth Analg. 1997;85:1258–1267. doi: 10.1097/00000539-199712000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Hardy JF, Bélisle S. Natural and synthetic antifibrinolytics in adult cardiac surgery: efficacy, effectiveness and efficiency. Can J Anesth. 1994;41:1104–1112. doi: 10.1007/BF03015662. [DOI] [PubMed] [Google Scholar]
- 3.Society of Thoracic Surgeons Blood Conservation Guideline Task Force; Ferraris VA, Ferraris SP, Saha SP, Hessel EA, 2nd, Haan CK, Royston BD, Bridges CR, Higgins RS, Despotis G, Brown JR, Spiess BD, Shore-Lesserson L, Stafford-Smith M, Mazer CD, Bennett-Guerrero E, Hill SE, Body S Society of Cardiovascular Anesthesiologists Special Task Force on Blood Transfusion. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007 May;83(5 Suppl):S27–S86. doi: 10.1016/j.athoracsur.2007.02.099. [DOI] [PubMed] [Google Scholar]
- 4.Molenaar IQ, Warnaar N, Groen H, Tenvergert EM, Slooff MJ, Porte RJ. Efficacy and safety of antifibrinolytic drugs in liver transplantation: a systematic review and meta-analysis. Am J Transplant. 2007 Jan;7(1):185–194. doi: 10.1111/j.1600-6143.2006.01591.x. [DOI] [PubMed] [Google Scholar]
- 5.Gill JB, Chin Y, Levin A, Feng D. The use of antifibrinolytic agents in spine surgery. A meta-analysis. J Bone Joint Surg Am. 2008 Nov;90(11):2399–2407. doi: 10.2106/JBJS.G.01179. [DOI] [PubMed] [Google Scholar]
- 6.Coats T, Roberts I, Shakur H. Antifibrinolytic drugs for acute traumatic injury. Cochrane Database Syst Rev. 2004 Oct;18(4):CD004896. doi: 10.1002/14651858.CD004896.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Stensrud PE, Nuttal GA. Pharmacology of Antifibrinolytic Agents. Advances in Cardiovascular Pharmacology. 2008;Chapter 8:183–204. [Google Scholar]
- 8.Dowd NP, Karski JM, Cheng DC, Carroll JA, Lin Y, James RL, Butterworth J. Pharmacokinetics of Tranexamic Acid during Cardiopulmonary Bypass. Anesthesiology. 2002;97(2):390–399. doi: 10.1097/00000542-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Chauhan S, Bisoi A, Kumar N, Mittal D, Kale S, Kiran U, Venugopal P. Dose comparison of tranexamic acid in pediatric cardiac surgery. Asian Cardiovasc Thorac Ann. 2004 Jun;12(2):121–124. doi: 10.1177/021849230401200208. [DOI] [PubMed] [Google Scholar]
- 10.Spinale FG, Koval CN, Deschamps AM, Stroud RE, Ikonomidis JS. Dynamic changes in matrix metalloprotienase activity within the human myocardial interstitium during myocardial arrest and reperfusion. Circulation. 2008 Sep;30(14 Suppl)(118):S16–S23. doi: 10.1161/CIRCULATIONAHA.108.786640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deschamps AM, Zavadzkas J, Murphy RL, Koval CN, McLean JE, Jeffords L, Saunders SM, Sheats NJ, Stroud RE, Spinale FG. Interruption of endothelin signaling modifies membrane type 1 matrix metalloproteinase activity during ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2008 Feb;294(2):H875–H883. doi: 10.1152/ajpheart.00918.2007. Epub 2007 Dec 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith RE, Bissell ER, Mitchell AR, Pearson KW. Direct photometric or fluorometric assay of proteinases using substrates containing 7-amino-4-trifluoromethylcoumarin. Thromb Res. 1980 Feb 1–15;17(3–4):393–402. doi: 10.1016/0049-3848(80)90074-2. [DOI] [PubMed] [Google Scholar]
- 13.Buxton Iain L, Brunton LL, Lazo JS, Parker KL. "Chapter 1. Pharmacokinetics and Pharmacodynamics: The Dynamics of Drug Absorption, Distribution, Action, and Elimination" (Chapter) Goodman & Gilman's The Pharmacological Basis of Therapeutics. 11e http://www.accessmedicine.com/content.aspx?aID=935800.
- 14.Nuttall GA, Brost BC, Connis RT, Gessner JS, Harrison CR, Miller RD, Nickinovich DG, Nussmeier NA, Rosenberg AD, Spence R. Practice Guidelines for Perioperative Blood Transfusion and Adjuvant Therapies. An Updated Report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006 Jul;105(1):198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 15.Marietta M, Facchini L, Pedrazzi P, Busani S, Torelli G. Pathophysiology of bleeding in surgery. Transplant Proc. 2006 Apr;38(3):812–814. doi: 10.1016/j.transproceed.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 16.Goodnough LT. Risks of blood transfusion. Anesthesiol Clin North America. 2005 Jun;23(2):241–252. v. doi: 10.1016/j.atc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Fraser IS, Porte RJ, Kouides PA, Lukes AS. A benefit-risk review of systemic haemostatic agents: part 1: in major surgery. Drug Saf. 2008;31(3):217–230. doi: 10.2165/00002018-200831030-00003. [DOI] [PubMed] [Google Scholar]
- 18.Fergusson DA, Hébert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM, Teoh K, Duke PC, Arellano R, Blajchman MA, Bussières JS, Côté D, Karski J, Martineau R, Robblee JA, Rodger M, Wells G, Clinch J, Pretorius R. BART Investigators. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008 May 29;358(22):2319–2331. doi: 10.1056/NEJMoa0802395. Epub 2008 May 14. [DOI] [PubMed] [Google Scholar]
- 19.Verstraete M. Clinical application of inhibitors of fibrinolysis. Drugs. 1985 Mar;29(3):236–261. doi: 10.2165/00003495-198529030-00003. [DOI] [PubMed] [Google Scholar]
- 20.Slaughter TF, Faghih F, Greenberg CS, Leslie JB, Sladen RN. The effects of epsilon-aminocaproic acid on fibrinolysis and thrombin generation during cardiac surgery. Anesth Analg. 1997 Dec;85(6):1221–1226. doi: 10.1097/00000539-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Mutter WP, Stillman IE, Dahl NK. Thrombotic microangiopathy and renal failure exacerbated by epsilon-aminocaproic acid. Am J Kidney Dis. 2009 Feb;53(2):346–350. doi: 10.1053/j.ajkd.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Wymenga LF, van der Boon WJ. Obstruction of the renal pelvis due to aninsoluble blood clot after epsilon-aminocaproic acid therapy: resolution with intraureteral streptokinase instillations. J Urol. 1998 Feb;159(2):490–492. doi: 10.1016/s0022-5347(01)63956-9. [DOI] [PubMed] [Google Scholar]
- 23.Hocker JR, Saving KL. Fatal aortic thrombosis in a neonate during infusion of epsilon-aminocaproic acid. J Pediatr Surg. 1995 Oct;30(10):1490–1492. doi: 10.1016/0022-3468(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 24.Dentz ME, Slaughter TF, Mark JB. Early thrombus formation on heparin-bonded pulmonary artery catheters in patients receiving epsilon aminocaproic acid. Anesthesiology. 1995 Feb;82(2):583–586. doi: 10.1097/00000542-199502000-00030. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman EP, Koo AH. Cerebral thrombosis associated with Amicar therapy. Radiology. 1979 Jun;131(3):687–689. doi: 10.1148/131.3.687. [DOI] [PubMed] [Google Scholar]
- 26.Seymour BD, Rubinger M. Rhabdomyolysis induced by epsilon-aminocaproic acid. Ann Pharmacother. 1997 Jan;31(1):56–58. doi: 10.1177/106002809703100109. [DOI] [PubMed] [Google Scholar]
- 27.Shaw A, Swaminathan M, Stafford-Smith M. Cardiac surgery-associated acute kidney injury: putting together the pieces of the puzzle. Nephron Physiol. 2008;109(4):55–60. doi: 10.1159/000142937. Epub 2008 Sep 18. [DOI] [PubMed] [Google Scholar]
- 28.Roelofs JJ, Rouschop KM, Leemans JC, Claessen N, de Boer AM, Frederiks WM, Lijnen HR, Weening JJ, Florquin S. Tissue-type plasminogen activator modulates inflammatory responses and renal function in ischemia reperfusion injury. J Am Soc Nephrol. 2006 Jan;17(1):131–140. doi: 10.1681/ASN.2005010089. Epub 2005 Nov 16. [DOI] [PubMed] [Google Scholar]
- 29.Kincaid EH, Ashburn DA, Hoyle JR, Reichert MG, Hammon JW, Kon ND. Does the combination of aprotinin and angiotensin-converting enzyme inhibitor cause renal failure after cardiac surgery? Ann Thorac Surg. 2005 Oct;80(4):1388–1393. doi: 10.1016/j.athoracsur.2005.03.136. discussion 1393. [DOI] [PubMed] [Google Scholar]
- 30.Dietrich W, Nicklisch S, Koster A, Spannagl M, Giersiefen H, van de Locht A. CU-2010: a novel small molecule protease inhibitor with antifibrinolytic and anticoagulant properties. Anesthesiology. 2009 Jan;110(1):123–130. doi: 10.1097/ALN.0b013e318191408c. [DOI] [PubMed] [Google Scholar]
- 31.Fiechtner BK, Nuttall GA, Johnson ME, Dong Y, Sujirattanawimol N, Oliver WC, Jr, Sarpal RS, Oyen LJ, Ereth MH. Plasma tranexamic acid concentrations during cardiopulmonary bypass. Anesth Analg. 2001 May;92(5):1131–1136. doi: 10.1097/00000539-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Nuttall GA, Gutierrez MC, Dewey JD, Johnson ME, Oyen LJ, Hanson AC, Oliver WC., Jr A preliminary study of a new tranexamic acid dosing schedule for cardiac surgery. J Cardiothorac Vasc Anesth. 2008 Apr;22(2):230–235. doi: 10.1053/j.jvca.2007.12.016. [DOI] [PubMed] [Google Scholar]