Abstract
Hypertension is both a cause and consequence of chronic kidney disease, but the prevalence of chronic kidney disease throughout the diagnostic spectrum of blood pressure has not been established. We determined the prevalence of chronic kidney disease within blood pressure categories in 17,794 adults surveyed by the National Health and Nutrition Examination Survey during 1999–2006. Diagnosed hypertension was defined as self-reported provider diagnosis (n=5,832); undiagnosed hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, without report of provider diagnosis (n=3,046); pre-hypertension was defined as systolic blood pressure ≥120 and < 140 mmHg or diastolic blood pressure ≥80 and < 90 mmHg (n=3,719); and normal was defined as systolic blood pressure < 120 mmHg and diastolic blood pressure < 80 mmHg (n=5,197). Chronic kidney disease was defined as estimated glomerular filtration rate 15–60 ml/min/1.73m2 or urinary albumin-creatinine ratio > 30 mg/g. Prevalence of chronic kidney disease among those with pre- and undiagnosed hypertension was 17.3% and 22.0%, respectively, compared to 27.5% with diagnosed hypertension and 13.4% with normal blood pressure, after adjustment for age, gender and race in multivariable logistic regression. This pattern persisted with varying definitions of kidney disease; macro-albuminuria (urinary albumin-creatinine ratio > 300 mg/g) had the strongest association with increasing blood pressure category [odds ratio 2.37 (95% confidence interval, 2.00–2.81)]. Chronic kidney disease is prevalent in undiagnosed and pre-hypertension. Earlier identification and treatment of both these conditions may prevent or delay morbidity and mortality from chronic kidney disease.
Keywords: epidemiology, albuminuria, renal, prevention, awareness, surveillance
INTRODUCTION
Hypertension is the second leading cause of end-stage renal disease (ESRD) in the United States (U.S.). 1 and is well-established as both a cause and consequence of chronic kidney disease (CKD) 2. An estimated 26 million adults in the U.S. (13%) had CKD in 1999 to 2004, representing a 3% increase from the previous ten years 3. Hypertension has been estimated to affect 29% of U.S. adults.4 However, many adults with hypertension are not aware they have this condition. Between 1999 and 2004, approximately 28% of those with hypertension were not aware of their diagnosis 4. What is unknown is how many adults in the U.S. who have undiagnosed hypertension also have CKD. Understanding the burden of CKD among adults with undiagnosed hypertension could assist public health and health care professionals in prevention and screening efforts for both of these conditions.
In addition to the well-known risk of cardiovascular and renal disease posed by hypertension, extensive literature is emerging regarding the risks associated with elevated blood pressure throughout its diagnostic continuum. Several prospective studies have shown that even modestly elevated blood pressures place individuals at increased risk of ESRD, relative to those with normal blood pressures 5–7. Recently published prospective data have shown that, even in the absence of diabetes and atherosclerosis, “high-normal” blood pressure (defined as systolic 130–139 mmHg or diastolic 85–89 mmHg) is associated with an almost 3-fold greater risk of future development of ESRD 8.
Given the evidence that risk of ESRD is increased throughout the diagnostic spectrum of elevated blood pressure, it is important to understand the prevalence of pre-ESRD CKD among persons with undiagnosed or pre-hypertension in the U.S. Such data has not been previously reported, and could serve as a platform for future studies of the efficacy of screening such persons for CKD. Therefore, we sought to determine the prevalence of CKD stages 1–49 in individuals with undiagnosed or pre-hypertension using data from the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2006. Additionally, we sought to describe the demographic, socioeconomic and clinical indicators of individuals with undiagnosed or pre-hypertension and CKD, in order to provide a foundation for targeted studies of individuals who may be at increased risk of the consequences of both conditions.
METHODS
Study Design
The NHANES surveys are currently conducted every 2 years by the National Center for Health Statistics to examine disease prevalence and trends over time in representative samples of non-institutionalized U.S. civilian residents10. The survey consists of a standardized in-home interview and a physical examination and blood and urine collection at a mobile examination center (MEC). Participants gave informed consent. The protocol was approved by an institutional review board.
We examined combined data from the 1999–2000, 2001–2002, 2003–2004, and 2005–2006 NHANES surveys. Although the total number of participants for these study years was 41,474, our study was limited to NHANES participants from 1999–2006 who provided self-reported information on hypertension and had blood pressure measured (n= 31,266), underwent a MEC exam (n= 29,815), were at least 20 years old (n= 18,938), had estimated glomerular filtration rate (eGFR) ≥ 15 ml/min/1.73 m2 (n= 18,885) and were not pregnant (final N=17,794).
Measurements
Blood pressure was measured during the MEC visit using a standardized protocol11. Each participant was in a seated position with at least 5 minutes of rest prior to the first measurement. Up to three brachial systolic and diastolic blood pressures (separated by 30 seconds after a 5-minute rest period) were taken by trained physicians using appropriate cuff sizes and a mercury sphygmomanometer. The averages of all available measurements for systolic and diastolic blood pressure were used.
Self-reported information on demographics (age, gender, race/ethnicity), socioeconomic status (education, insurance, income), and health conditions (diabetes) were obtained during the interview portions of the surveys. Income was assessed using the poverty income ratio, which is a ratio of household income to household poverty level12. Additionally, prescription medication information was obtained during the interview, with the interviewer recording the names of medications from medication containers provided by the participant. Height and weight, used in the calculation of body mass index (BMI), were measured during the MEC exam. Serum creatinine was measured by the modified kinetic method of Jaffe using different analyzers in different survey years. Random spot urine samples were obtained, and urine albumin and creatinine were measured using frozen specimens. Urine albumin was measured using a solid-phase fluorescence immunoassay; urine creatinine was measured using the modified Jaffe kinetic method in the same laboratory.
Definitions
Diagnosed hypertension was defined by an answer of “Yes” to the question “Have you ever been told by a doctor or other health professional that you have hypertension, also called high blood pressure?” Among those who answered “No”, undiagnosed hypertension was defined by a measured average systolic blood pressure of ≥140 mmHg or by a measured average diastolic blood pressure of ≥90 mmHg and pre-hypertension was defined by an average systolic blood pressure of ≥120 mmHg and <140 mmHg (with diastolic <90 mmHg), or by a measured average diastolic blood pressure of ≥80 and <90 mmHg (with systolic <140 mmHg)13. Normal blood pressure was defined by an average systolic blood pressure of <120 mmHg and a measured average diastolic blood pressure of <80 mmHg and an answer of “No” to the same question.
CKD was defined using eGFR and the presence of albuminuria, according to the Kidney Disease Outcomes Quality Initiative (KDOQI) staging guidelines9. Estimated GFR was calculated according to the modified Modification of Diet in Renal Disease Study equation for calibrated creatinine: eGFR = 175 × [(calibrated serum creatinine in milligrams/deciliter)−1.154] × age−0.203 × (0.742 if female) × (1.210 if African-American)14,15. Serum creatinine was calibrated to adhere to the Cleveland Clinic protocol for survey years 1999–2000 and 2005–2006 using regression formulas provided by NHANES; no correction was required for calibrated serum creatinine in participants in the 2001–2002 or 2003–2004 surveys. 16 Albuminuria was considered to be present at urinary albumin-to-creatinine ratios ≥30mg/g. Micro-albuminuria was defined as 30–300 mg/g creatinine and macro-albuminuria as >300 mg/g creatinine. Because urinary albumin measurements in NHANES are cross-sectional, we did not have data on persistent albuminuria, and the definitions of stages in our study were therefore modified as: stage 1, eGFR > 90 ml/min per 1.73 m2 and presence of albuminuria at a single measurement; stage 2, eGFR 60–89 ml/min per 1.73 m2 and presence of albuminuria at a single measurement; stage 3, eGFR 30–59 ml/min per 1.73 m2; and stage 4, 15–29 ml/min per 1.73 m2.
Self-reported diabetes was defined by an answer of “Yes” to the question “Have you ever been told by a doctor that you have diabetes or sugar diabetes?” The use of hypertension medications was defined as any prescription for diuretics, ACE inhibitors, alpha- and/or beta-blockers, calcium channel blockers, angiotensin II receptor blockers, central alpha-2 agonists, aldosterone receptor blockers, or direct vasodilators, either prescribed alone or in combination. CKD awareness was defined by an answer of “Yes” to the question “Have you ever been told by a doctor or other health professional that you have weak or failing kidneys?”
Statistical Methods
Participant characteristics were compared by hypertension status, as were the unadjusted proportions with CKD both overall and by patient characteristics. Variance of proportions was estimated with Taylor series linearization. These characteristics were examined in logistic models predicting CKD, with adjustment for age, gender, and race/ethnicity. Odds ratios and adjusted percentages were obtained from these models; only adjusted percentages are shown since the odds ratios are likely to overestimate the relative risk in the setting of a condition as common as uncontrolled blood pressure. Sensitivity analyses, in which diagnosed hypertension was also defined by use of hypertension medications, were also performed, to estimate the effect of possible misclassification of medication-controlled hypertension. Additional sensitivity analyses wherein various cutoffs of both reduced kidney function and albuminuria were also performed, as was an analysis using the CKD-EPI equation17 to estimate GFR.
To estimate nationally representative population results, all analyses were performed using the SVY commands in Stata v. 10.0 to account for NHANES study design weights, strata, and pseudostrata. Appropriate NHANES 8-year MEC weights were used; 8-year weights were calculated as: 8-year weight=1/2 × 4-year weight (if survey year = 1999–2002); and 8-year weight=1/4 × 2-year weight (if survey year = 2003–2004 or 2005–2006)18. Estimates of the proportion of U.S. adults with undiagnosed or pre-hypertension and CKD were calculated using 2006 U.S. Census estimates of the number of individuals > 20 years of age (111,440,340) and the burden of undiagnosed, pre-hypertension and CKD found in the present study.
RESULTS
There were 17,794 NHANES participants from the 1999–2006 surveys who were at least 20 years old, underwent mobile examination center (MEC) examinations (including measurement of serum creatinine and urinary albumin-to-creatinine ratio), provided self-reported information on hypertension, and had measured blood pressures; excluding pregnant individuals and those with estimated glomerular filtration rate (eGFR) <15 ml/min/1.73 m2. Among these participants, 5,832 had diagnosed hypertension, 3,046 had undiagnosed hypertension, 3,719 had pre-hypertension, and 5,197 had normal blood pressure. Participant characteristics by hypertension status are shown in Table 1. Mean systolic and diastolic blood pressures were highest in those with undiagnosed hypertension. Females comprised the majority of those with diagnosed hypertension, while males were the majority in pre-hypertension. The proportion of individuals who were Mexican-American was larger in undiagnosed and pre-hypertension than in diagnosed hypertension. High school graduates comprised a greater percentage of participants with pre-hypertension and normal blood pressure than they did diagnosed and undiagnosed hypertension. Those participants whose annual household income (relative to household size) fell in the highest quartile were more likely to have pre-hypertension or normal blood pressure rather than diagnosed or undiagnosed hypertension. The presence of health insurance increased across the diagnostic spectrum of blood pressure, and individuals with health insurance comprised the vast majority of those with diagnosed hypertension. The overwhelming majority of participants in all diagnostic groups reported having a regular site for health care, including 84% of those with undiagnosed hypertension. Those individuals with diagnosed hypertension were the least likely to report current or past cigarette smoking. Obese participants (BMI ≥30 kg/m2) comprised a greater percentage of those with diagnosed hypertension than they did undiagnosed and pre-hypertension. Finally, self-reported diabetes mellitus increased across the diagnostic spectrum of blood pressure, with persons with diabetes comprising 16.7% of those with diagnosed hypertension.
Table 1.
Characteristics of adults age 20 years and older by hypertension status, NHANES 1999–2006.
| Characteristic |
Hypertension Status |
|||
|---|---|---|---|---|
| Diagnosed hypertension (self-reported diagnosis) | Undiagnosed hypertension (SBP ≥140 mmHg or DBP ≥90 mmHg, and no self-reported diagnosis) | Pre-hypertension (SBP ≥120 and <140 mmHg or DBP ≥80 and <90 mmHg) | Normal (SBP <120 mmHg and DBP <80 mmHg) | |
| N | 5,832 | 3,046 | 3,719 | 5,197 |
| Mean SBP, mmHg | 135.0 (.39) | 149.3 (.62) | 125.4 (.17) | 108.4 (.15) |
| Mean DBP, mmHg | 73.5 (.31) | 80.6 (.67) | 74.4 (.23) | 66.6 (.18) |
| Mean age, years | 56.9 (.44) | 49.4 (.52) | 44.6 (.35) | 38.2 (.27) |
| Gender | ||||
| % female | 54.4 (52.5–56.3) | 50.9 (48.6–53.1) | 37.4 (35.6–39.3) | 57.8 (56.1–59.4) |
| Race/Ethnicity | ||||
| % non-Hispanic white | 74.0 (70.7–77.0) | 68.4 (64.8–71.9) | 72.9 (69.7–75.8) | 71.1 (67.8–74.2) |
| % non-Hispanic black | 13.9 (11.6–16.5) | 12.4 (10.4–14.7) | 9.9 (8.3–11.9) | 8.5 (7.1–10.1) |
| % Mexican-American | 4.3 (3.2–5.7) | 7.7 (6.3–9.3) | 7.4 (6.0–9.1) | 9.4 (8.0–11.1) |
| Education | ||||
| % high school or more | 76.2 (74.3–78.1) | 77.4 (75.0–79.6) | 81.7 (80.0–83.4) | 83.4 (81.7–85.1) |
| Income Quartiles* | ||||
| Q1 (low) | 11.6 (10.5–12.8) | 13.0 (11.0–15.2) | 10.5 (9.3–11.9) | 13.1 (11.6–14.7) |
| Q2 | 20.5 (18.8–22.2) | 19.2 (17.4–21.3) | 16.1 (14.3–18.2) | 18.3 (16.7–20.1) |
| Q3 | 29.6 (27.6–31.6) | 29.1 (26.6–31.8) | 28.4 (26.3–30.7) | 26.3 (24.8–28.0) |
| Q4 (high) | 38.4 (35.7–41.1) | 38.7 (35.8–41.6) | 44.9 (41.8–48.0) | 42.3 (39.4–45.3) |
| Insurance | ||||
| % insured | 89.0 (87.3–90.5) | 82.3 (80.3–84.2) | 79.6 (77.2–81.8) | 77.8 (76.0–79.4) |
| Routine site for healthcare | ||||
| % yes | 94.3 (93.2–95.1) | 84.0 (81.5–86.3) | 80.8 (79.2–82.4) | 80.8 (78.9–82.6) |
| Smoking | ||||
| % current | 31.9 (29.6–34.2) | 40.1 (37.0–43.3) | 45.3 (42.6–48.1) | 49.8 (46.6–53.0) |
| % past | 5.5 (4.4–6.8) | 7.2 (5.7–9.1) | 7.9 (6.7–9.1) | 9.7 (8.2–11.5) |
| % never | 62.6 (60.3–64.9) | 52.7 (49.5–55.9) | 46.8 (44.4–49.3) | 40.5 (37.4–43.6) |
| Body mass index | ||||
| % ≥30 kg/m2 | 46.2 (44.6–47.9) | 30.4 (28.1–32.9) | 31.1 (29.0–33.4) | 20.8 (19.3–22.4) |
| Self-reported diabetes | ||||
| % yes | 16.7 (15.6–17.8) | 6.1 (5.4–6.9) | 4.4 (3.7–5.4) | 2.5 (2.0–3.1) |
Note: Standard errors are reported in parentheses after continuous variables, and 95% confidence intervals are reported for categorical variables. P <0.001 for each comparison of characteristics across hypertension categories (using ANOVA).
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure.
Income was assessed using the poverty income ratio, which is a ratio of household income to household poverty level.
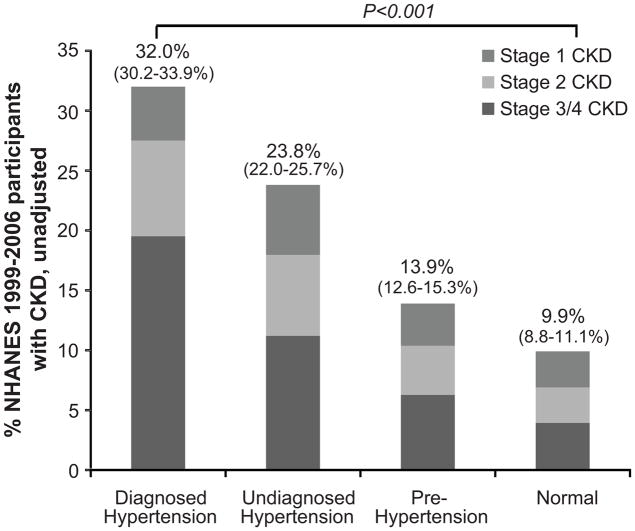
Figure 1 reveals that the unadjusted prevalence of CKD increases throughout the diagnostic spectrum of blood pressure, with 9.9%, 13.9%, 23.8% and 32.0% having CKD in the normal blood pressure, pre-, undiagnosed and diagnosed hypertension groups, respectively. In the same figure, examination of the stages of CKD revealed that the unadjusted prevalence of CKD stages 3/4 (eGFR 15 to 60 ml/min/1.73m2)was 3.9%, 6.3%, 11.2% and 19.6% for the normal blood pressure, pre-hypertension, undiagnosed and diagnosed hypertension groups, respectively. Notably, a greater proportion of CKD stage 3/4 was found among those with diagnosed hypertension than among the other groups (comprising 61.0% of those with CKD and diagnosed hypertension).
Figure 1. Population prevalence (%) of CKD stages 1–4, by hypertension status, NHANES 1999–2006.
Diagnosed hypertension is defined as self-report of provider diagnosis; undiagnosed hypertension is defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, without a report of provider diagnosis; pre-hypertension is defined as systolic blood pressure ≥120 and <140 mmHg or diastolic blood pressure ≥80 and <90 mmHg; and normal is defined as systolic blood pressure <120 mmHg and diastolic blood pressure <80 mmHg. CKD defined by eGFR 15–60 ml/min/1.73 m2 or a single determination of albuminuria measurement. CKD stages defined as stage 1, eGFR > 90 ml/min per 1.73 m2 and presence of albuminuria at a single measurement; stage 2, eGFR 60–89 ml/min per 1.73 m2 and presence of albuminuria at a single measurement; stage 3, eGFR 30–59 ml/min per 1.73 m2; stage 4, 15–29 ml/min per 1.73 m2). Values in parentheses represent 95% confidence intervals.
Table 2 shows that even after adjustment for age, race/ethnicity and gender the prevalence of CKD increases throughout blood pressure categories, with 13.4%, 17.3%, 22.0% and 27.5% having CKD in the normal blood pressure, pre-, undiagnosed and diagnosed hypertension groups, respectively. Additionally, persons with either pre- or undiagnosed hypertension accounted for 35.0% of all cases of CKD, while those with diagnosed hypertension accounted for 52.2% of cases (data not shown). These findings suggest that there are as many as 8 million U.S. adults with undiagnosed or pre-hypertension who also have CKD (data not shown).
Table 2.
* Adjusted prevalence of CKD by participant characteristics and hypertension status, NHANES 1999–2006.
| Characteristic | % participants with CKD stages 1–4 |
|||
|---|---|---|---|---|
| Diagnosed hypertension (self-reported diagnosis) | Undiagnosed hypertension (SBP ≥140 mmHg or DBP ≥90 mmHg, and no self-reported diagnosis) | Pre-hypertension (SBP ≥120 and <140 mmHg or DBP ≥80 and <90 mmHg) | Normal (SBP <120 mmHg and DBP <80 mmHg) | |
| (n= 5,832) % (95% CI) | (n=3,046) % (95% CI) | (n=3,719) % (95% CI) | (n=5,197) % (95% CI) | |
| Overall | 27.5 (25.7–29.4) | 22.0 (20.8–23.2) | 17.3 (16.3–18.3) | 13.4 (12.3–14.6) |
| Age | ||||
| 20–39 | 13.3 (11.1–15.8) | 13.5 (11.3–16.0) | 9.0 (7.7–10.5) | 7.0 (5.9–8.3) |
| 40–59 | 23.8 (21.6–26.1) | 21.1 (19.3–23.2) | 14.3 (13.0–15.6) | 12.5 (11.1–14.0) |
| 60–69 | 38.9 (37.1–40.1) | 31.5 (29.4–33.7) | 21.8 (19.8–24.0) | 21.3 (18.5–24.4) |
| 70+ | 56.4 (53.7–59.1) | 44.1 (39.9–48.5) | 32.0 (27.9–36.4) | 33.8 (27.9–40.3) |
| Ptrend | <0.001 | <0.001 | <0.001 | <0.001 |
| Gender | ||||
| Male | 33.6 (31.3–36.0) | 22.7 (20.6–25.0) | 10.5 (9.3–11.9) | 8.4 (7.1–10.0) |
| Female | 37.3 (34.9–39.8) | 27.7 (24.9–30.8) | 14.3 (12.1–16.9) | 11.0 (9.5–12.7) |
| P | 0.015 | 0.010 | 0.001 | 0.008 |
| Race/Ethnicity | ||||
| Non-Hispanic White | 33.0 (31.0–35.0) | 24.0 (21.5–26.7) | 13.3 (11.7–15.1) | 8.7 (7.5–10.2) |
| Non-Hispanic Black | 36.3 (34.3–38.4) | 25.2 (23.5–27.1) | 14.5 (13.2–15.9) | 9.9 (8.7–11.2) |
| Mexican-American | 39.8 (36.5–43.2) | 26.5 (23.5–29.7) | 15.8 (13.7–18.2) | 11.2 (9.5–13.2) |
| P | <0.001 | 0.280 | 0.083 | 0.014 |
| Education | ||||
| <High school | 42.8 (39.7–46.0) | 30.7 (26.6–35.0) | 16.8 (14.4–19.5) | 10.4 (8.3–13.0) |
| High school+ | 32.6 (30.4–35.0) | 23.1 (20.9–25.5) | 13.7 (12.2–15.3) | 9.7 (8.4–11.1) |
| P | <0.001 | 0.005 | 0.030 | 0.546 |
| Income Quartiles† | ||||
| Q1 (low) | 45.4 (41.9–48.9) | 30.7 (25.6–36.5) | 20.1 (16.5–24.3) | 12.2 (10.1–14.6) |
| Q2 | 39.6 (37.6–41.6) | 26.9 (24.1–29.8) | 16.6 (14.7–18.5) | 10.5 (9.2–11.9) |
| Q3 | 34.1 (32.0–36.3) | 23.3 (21.4–25.4) | 13.5 (12.2–15.0) | 9.0 (7.9–10.3) |
| Q4 (high) | 29.0 (25.7–32.5) | 20.1 (17.1–23.5) | 11.0 (9.0–13.3) | 7.7 (6.3–9.4) |
| Ptrend | <0.001 | 0.007 | 0.001 | 0.003 |
| Insurance | ||||
| Yes | 35.6 (33.7–37.6) | 24.2 (22.1–26.5) | 14.5 (13.0–16.1) | 9.6 (8.2–11.1) |
| No | 32.6 (27.4–38.3) | 27.0 (22.0–32.7) | 13.0 (10.3–16.3) | 10.1 (8.2–12.4) |
| P | 0.304 | 0.365 | 0.414 | 0.661 |
| Smoking | ||||
| % current | 36.5 (32.0–41.2) | 29.9 (24.2–36.3) | 14.4 (11.8–17.4) | 9.1 (7.3–11.1) |
| % past | 35.1 (32.9–37.4) | 28.2 (25.2–31.4) | 14.3 (12.6–16.2) | 8.6 (7.3–10.1) |
| % never | 33.8 (30.3–37.3) | 26.6 (22.5–31.0) | 14.3 (11.6–17.5) | 8.2 (6.3–10.7) |
| Ptrend | 0.424 | 0.419 | 0.970 | 0.583 |
| Routine site for healthcare | ||||
| % yes | 36.6 (34.3–38.9) | 25.6 (23.1–28.2) | 14.4 (12.4–16.7) | 9.6 (8.0–11.4) |
| % no | 34.3 (25.3–44.6) | 30.2 (24.0–37.1) | 15.1 (11.5–19.6) | 9.1 (6.9–11.7) |
| P | 0.657 | 0.242 | 0.798 | 0.727 |
| BMI | ||||
| <30 kg/m2 | 33.2 (30.9–35.6) | 23.2 (21.0–25.5) | 13.2 (11.7–14.8) | 9.4 (8.2–10.8) |
| ≥30 kg/m2 | 37.2 (34.5–40.0) | 27.0 (23.5–30.7) | 16.2 (13.4–19.4) | 10.4 (8.3–12.9) |
| P | 0.018 | 0.094 | 0.074 | 0.387 |
| Self-reported diabetes | ||||
| Yes | 55.1 (51.5–58.6) | 43.4 (34.1–53.2) | 21.0 (15.3–28.1) | 22.7 (15.5–32.0) |
| No | 31.0 (28.9–33.2) | 23.6 (21.8–25.5) | 13.8 (12.5–15.2) | 9.4 (8.2–10.7) |
| P | <0.001 | <0.001 | 0.014 | <0.001 |
Note: CKD defined by eGFR 15–60 ml/min/1.73 m2 or a single albuminuria measurement (stage 1, eGFR > 90 ml/min per 1.73 m2 and presence of albuminuria at a single measurement; stage 2, eGFR 60–89 ml/min per 1.73 m2 and presence of albuminuria at a single measurement; stage 3, eGFR 30–59 ml/min per 1.73 m2; stage 4, 15–29 ml/min per 1.73 m2).
Prevalence estimates for each covariate were adjusted for age, gender, and race/ethnicity in individual logistic regression models. Ptrend values represent statistical significance across ordinal categorical variables within blood pressure categories.
Income was assessed using the poverty income ratio, which is a ratio of household income to household poverty level.
Increasing age and female gender were associated with greater prevalence of CKD in all hypertension groups (Table 2). Mexican-Americans had the highest adjusted prevalence of CKD (39.8%) among those with diagnosed hypertension, and a similar, but non-significant, trend was present among those with undiagnosed and pre-hypertension. Lack of high school diploma was associated with a greater prevalence of CKD among those with pre-, undiagnosed and diagnosed hypertension; and decreasing household income was associated with greater prevalence of CKD in all blood pressure groups. Obesity was associated with a greater prevalence of CKD among those with diagnosed hypertension, and a similar (but non-significant) trend was seen among those with undiagnosed and pre-hypertension. Self-reported diabetes was associated with very high adjusted prevalence of CKD in all hypertension groups, including 43.4% of those with diabetes and undiagnosed hypertension and 21.0% of those with diabetes and pre-hypertension. Smoking history, insurance status, and having a routine healthcare site had no relationship with CKD in any category of blood pressure.
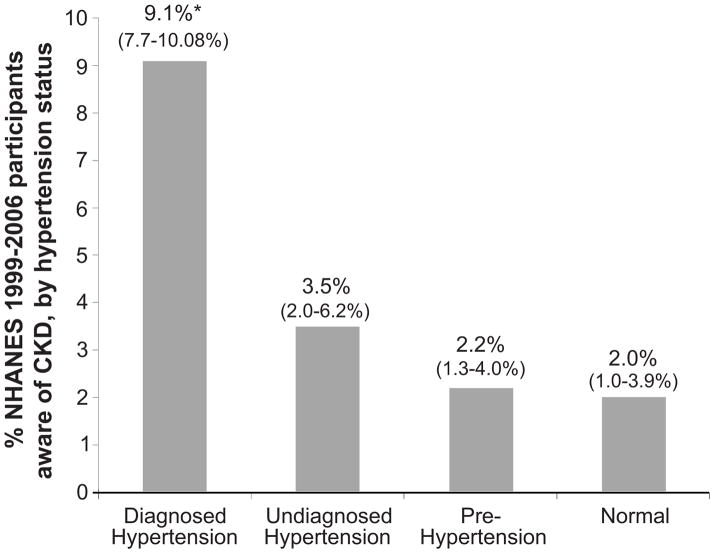
CKD awareness was assessed across blood pressure groups. Awareness was 2.0%, 2.2%, 3.5%, and 9.1%, among those with CKD and normal blood pressure, pre-, undiagnosed and diagnosed hypertension, respectively (Figure 2).
Figure 2. Prevalence of CKD awareness (%), by hypertension status, NHANES 1999–2006.
Diagnosed hypertension is defined as self-report of provider diagnosis; undiagnosed hypertension is defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, without a report of provider diagnosis; pre-hypertension is defined as systolic blood pressure ≥120 and <140 mmHg or diastolic blood pressure ≥80 and <90 mmHg; and normal is defined as systolic blood pressure <120 mmHg and diastolic blood pressure <80 mmHg. CKD defined by eGFR 15–60 ml/min/1.73 m2 or a single determination of albuminuria measurement. Values in parentheses represent 95% confidence intervals. *Statistically different from other awareness estimates (P<0.001).
A sensitivity analysis examining the inclusion of reported anti-hypertensive medication use in the definition of diagnosed hypertension (n=17,794), regardless of measured blood pressure or self-report, revealed similar results to the primary definition used above (data not shown).
Sensitivity analyses of various measures of kidney disease revealed that the presence of micro-albuminuria was more common across the diagnostic continuum of hypertension than was the presence of decreased eGFR (Table 3). Micro- or macro-albuminuria were more common among younger persons (mean age 42.5 years) while eGFR <60 ml/min/1.73m2 was more common among older persons (mean age 72.4 years). Stricter definitions of kidney disease (eGFR < 45 ml/min/1.73m2 or macro-albuminuria) resulted in much lower, but still substantial, CKD prevalence, with similar patterns across blood pressure categories (Table 3). Compared to other measures of kidney disease, macro-albuminuria had the strongest association with increasing blood pressure category. The use of the CKD-EPI equation yielded similar, but slightly attenuated estimates to our primary analysis (CKD prevalence of 26.0%, 20.5%, 16.0% and 12.3 % for the diagnosed, undiagnosed, pre-hypertension, and normal blood pressure groups, respectively). Adjustment for estimated persistence of albuminuria19 also resulted in slightly lower CKD prevalence across categories (24.5%, 14.8%, 14.9%, and 4.9%, respectively).
Table 3.
* Adjusted prevalence of various measures of kidney disease by hypertension status, NHANES 1999–2006.
| Measure of Kidney Disease |
% participants with each measure of kidney disease |
||||
|---|---|---|---|---|---|
| Diagnosed hypertension (self-reported diagnosis) | Undiagnosed hypertension (SBP ≥140 mmHg or DBP ≥90 mmHg, and no self-reported diagnosis) | Pre-hypertension (SBP ≥120 and <140 mmHg or DBP ≥80 and <90 mmHg) | Normal (SBP <120 mmHg and DBP <80 mmHg) | Odds of kidney disease as hypertension category increases (normal to diagnosed) | |
| (n= 5,832) % (95% CI) | (n=3,046) % (95% CI) | (n=3,719) % (95% CI) | (n=5,197) % (95% CI) | Odds Ratio (95% Confidence Interval) | |
| Micro-albuminuria† | 12.5 (11.5–13.6) | 9.5 (8.8–10.2) | 7.1 (6.6–7.7) | 5.3 (4.7–5.9) | 1.37 (1.30–1.44) |
| Macro-albuminuria□ | 3.3 (2.7–4.0) | 1.4 (1.1–1.7) | 0.6 (0.4–0.8) | 0.2 (0.2–0.4) | 2.37 (2.00–2.81) |
| Estimated GFR <60§ | 7.8 (6.6–9.2) | 5.6 (4.9–6.4) | 4.0 (3.4–4.6) | 2.8 (2.3–3.4) | 1.43 (1.33–1.53) |
| Estimated GFR <45§ | 1.4 (1.0–2.0) | 0.8 (0.6–1.1) | 0.5 (3.7–6.8) | 0.3 (0.2–0.4) | 1.69 (1.43–2.00) |
Prevalence estimates for each measure of kidney disease were adjusted for age, gender, and race/ethnicity in individual logistic regression models.
Micro-albuminuria is defined as 30–300 mg/g creatinine
Macro-albuminuria is defined as >300 mg/g creatinine.
Estimated GFR= glomerular filtration rate, measured in ml/min/1.73 m2.
DISCUSSION
We found the prevalence of CKD to be high among individuals with undiagnosed or pre-hypertension in the U.S. Although CKD was most prevalent among those with diagnosed hypertension, persons with either undiagnosed or pre-hypertension accounted for more than one-third of all cases of CKD, representing an estimated 8 million U.S adults. Additionally, we found that such individuals are largely unaware of their kidney disease.
Prevalence of CKD was found to be increased across the spectrum of blood pressure, with those with normal blood pressure having the lowest prevalence of CKD, and those with diagnosed hypertension having the greatest prevalence. Micro-albuminuria was the most commonly noted marker of kidney disease across all blood pressure categories, far more common than reduced eGFR or macro-albuminuria. Importantly, our study provides evidence that the risk factors for CKD are similar across blood pressure categories. We noted that certain demographic (increasing age, female gender and Mexican-American ethnicity), socioeconomic (limited education and low income) and clinical (diabetes and obesity) factors were each associated with an increased prevalence of CKD irrespective of blood pressure category.
Our finding that the prevalence of CKD was lower among the undiagnosed as compared to those with diagnosed hypertension may be due to a number of patient, physician and health care system factors. For example, patients with greater kidney disease severity may be more likely to have received blood pressure measurements by their physician, potentially leading to a diagnosis of hypertension. This idea is supported by our finding of a greater proportion of CKD stage 3/4 among those with diagnosed hypertension than among other blood pressure groups.
Individuals with both undiagnosed hypertension and CKD represent a population uniquely in need of improved screening efforts. The Seventh Report of the Joint National Committee (JNC-7) recommends screening persons on antihypertensive therapy with creatinine measures at least one to two times annually. Also, the report recommends aggressive blood pressure control to less than 130/80 mmHg in persons with hypertension and CKD or diabetes.13 Individuals with undiagnosed hypertension may not be receiving these recommended evaluation and treatment measures. Further, in the absence of traditional risk factors for CKD (e.g. younger persons, non-obese, without diabetes) providers who see these persons may not be screening them for CKD or aiming for treatment targets appropriate for CKD patients. Our analysis demonstrated that 17.7% of adults with undiagnosed hypertension lack health insurance and 16.0% do not have a routine site for healthcare. Additionally, we found that the greatest burden of CKD in the setting of undiagnosed hypertension is among those with very low income, again, suggesting that health care resources may be very limited in this population. Also, CKD awareness was extremely low in this population, as has been shown in the general population20, underscoring the need for improved education both in the community and among health care providers, especially since awareness of hypertension has also been shown to be suboptimal but improving4.
We found that the prevalence of CKD among persons with pre-hypertension is higher than that of those with normal blood pressure. This finding complements previous studies recognizing the risk of ESRD throughout the continuum of hypertension5. Thus, persons with pre-hypertension may warrant increased screening and preventive efforts towards identifying and reducing risk of CKD. Such efforts have been encouraged in the Kidney Disease Improving Global Outcomes position statement, wherein it is recommended that CKD screening be targeted at those who would derive the most benefit 21. However, screening for CKD using urinary albumin testing has only been estimated to be cost-effective in high-risk patient populations, including individuals with hypertension 22. Little is known about the cost-effectiveness of screening those with pre-hypertension for CKD. Such individuals may have other well-established indications for CKD screening, such as diabetes, which may lead their physician to evaluate them for CKD. In our study, we found that 21% of individuals with pre-hypertension and CKD also had diabetes. Further study of the most appropriate patient populations to undergo CKD screening is needed.
To our knowledge, this is the first report of population-based CKD prevalence estimates across the diagnostic spectrum of blood pressure classification in the U.S. Our study does have certain limitations, however. First, given the cross-sectional design of NHANES, we were not able to follow the relationship between hypertension status and CKD over time and blood pressures were measured on a single day as opposed to on at least two separate days as guidelines for the diagnosis of hypertension recommend13 CKD was also defined based on a single laboratory measure, as opposed to measures separated by at least 3 months, as the KDOQI guidelines recommend9. We attempted to address this by estimating persistence of albuminuria, and found slightly attenuated prevalence estimates. Third, NHANES participants are a representative sample of the non-institutionalized civilian U.S. population; therefore, we may have missed some individuals with greater disease severity, such as nursing home residents. We also were limited to those participants in NHANES who completed the MEC exam, which may represent a more highly motivated population than those who only completed the survey. Fourth, some cases of hypertension may have been misclassified because of the definitions that were used in our study. For example, persons with well-controlled hypertension may have been classified as having pre-hypertension or normal blood pressure if they did not report knowledge of their diagnosis. We attempted to address this issue by performing a sensitivity analysis evaluating the use of anti-hypertensive medications as the defining factor for diagnosed hypertension, and found largely similar results to our primary definition Fifth, because our blood pressure cut-off for diagnosed hypertension was that recommended for the general adult population by the JNC-7, we may have misclassified persons with diabetes whose physicians may have “diagnosed” them with hypertension when their blood pressure reached >130/80 mmHg, which may have resulted in an overestimate of the proportion with diagnosed hypertension. Finally, our measure of CKD awareness may not have captured all participants who were aware of their kidney disease, as many physicians may not communicate CKD as being the presence of “weak or failing kidneys”.
PERSPECTIVES
The prevalence of CKD is high among individuals with undiagnosed or pre-hypertension in the U.S. The presence of macro-albuminuria has the strongest relationship with increasing blood pressure category, however, the prevalence of CKD increases across the diagnostic spectrum of hypertension regardless of how kidney disease is defined. CKD awareness is low among individuals with undiagnosed or pre-hypertension and CKD. Risk factors for CKD are similar across blood pressure categories. Persons with undiagnosed or pre-hypertension warrant further study of appropriate CKD education, screening and prevention programs, as they are likely at high risk for the detrimental effects of both conditions.
Acknowledgments
We thank the participants and staff of the NHANES survey. Members of the CDC CKD Surveillance Team are listed in the online Appendix, please see http://hyper.ahajournals.org. This project was supported under a cooperative agreement from the Centers for Disease Control and Prevention through the Association of American Medical Colleges, grant number U36/CCU319276, AAMC ID numbers MM-0997-07/07 and MM-1143-10/10. Report contents are solely the responsibility of the authors and do not necessarily represent the official views of the AAMC or CDC.
Sources of Funding
Dr. Crews is supported by the Harold Amos Medical Faculty Development Program of the Robert Wood Johnson Foundation. Dr. Powe is partially supported by grant K24DK02643.
Appendix
The CDC CKD Surveillance Team consists of members from University of California, San Francisco [Neil Powe, Laura Plantinga, Kirsten Bibbins-Domingo, Josef Coresh, Alan Go, Chi-yuan Hsu, Lesley Stevens, Deidra Crews, Matthew Ong], University of Michigan [Rajiv Saran, Elizabeth Hedgeman, Brenda Gillespie, William Herman, Friedrich Port, Bruce Robinson, Vahakn Shahinian, Jerry Yee, Eric Young], and Centers for Disease Control and Prevention [Desmond Williams, Nilka Rios Burrows, Mark Eberhardt, Paul Eggers, Linda Geiss, Susan Hailpern, Regina Jordan, Juanita Mondeshire, Bernice Moore, Gary Myers, Meda Pavkov, Deborah Rolka, Sharon Saydah, Anton Schoolwerth, Rodolfo Valdez, Larry Waller].
Footnotes
Disclosures
None
References
- 1.US Renal Data System. USRDS 2008 annual data report: atlas of end-stage renal disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease; Bethesda, MD: 2008. [Google Scholar]
- 2.Bakris GL, Ritz E. The message for World Kidney Day 2009: hypertension and kidney disease--a marriage that should be prevented. J Hypertens. 2009;27:666–669. doi: 10.1097/HJH.0b013e328327706a. [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 4.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52:818–827. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 5.Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med. 2005;165:923–928. doi: 10.1001/archinte.165.8.923. [DOI] [PubMed] [Google Scholar]
- 6.Tozawa M, Iseki K, Iseki C, Kinjo K, Ikemiya Y, Takishita S. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension. 2003;41:1341–1345. doi: 10.1161/01.HYP.0000069699.92349.8C. [DOI] [PubMed] [Google Scholar]
- 7.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334:13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 8.Hsu CC, Brancati FL, Astor BC, Kao WH, Steffes MW, Folsom AR, Coresh J. Blood pressure, atherosclerosis, and albuminuria in 10,113 participants in the atherosclerosis risk in communities study. J Hypertens. 2009;27:397–409. doi: 10.1097/hjh.0b013e32831aede6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 10.National Center for Health Statistics. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–1994. Series 1: Program and collection procedures. Vital Health Stat. 1994;32:1–407. [PubMed] [Google Scholar]
- 11.National Health and Nutrition Examination Survey. Physician Examination Procedures Manual. Hyattsville, MD: National Center for Health Statistics; 2003. [Google Scholar]
- 12.National Health and Nutrition Examination Survey 1999–2000. Documentation, Codebook, and Frequencies. Hyattsville, MD: National Center for Health Statistics; 2009. [Google Scholar]
- 13.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ for the National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 14.Levey A, Coresh J, Greene T, Marsh J, Stevens LA, Kusek J, Van Lente F. Expressing the MDRD study equation for estimating GFR with IDMS traceable (gold standard) serum creatinine values. J Am Soc Nephrol. 2005;16:69A. [Google Scholar]
- 15.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Selvin E, Manzi J, Stevens LA, Van Lente F, Lacher DA, Levey AS, Coresh J. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50:918–926. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Center for Health Statistics. National Center for Health Statistics; 2005. [Accessed April 16, 2009]. Analytic and reporting guidelines: the National Health and Nutrition Examination Survey (NHANES) at http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf. [Google Scholar]
- 19.Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, Hostetter TH. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol. 2005;16:180–188. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]
- 20.Plantinga LC, Boulware LE, Coresh J, Stevens LA, Miller ER, 3rd, Saran R, Messer KL, Levey AS, Powe NR. Patient awareness of chronic kidney disease: trends and predictors. Arch Intern Med. 2008;168:2268–2275. doi: 10.1001/archinte.168.20.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, Powe NR, Rossert J, Wheeler DC, Lameire N, Eknoyan G. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 22.Boulware LE, Jaar BG, Tarver-Carr ME, Brancati FL, Powe NR. Screening for proteinuria in US adults: a cost-effectiveness analysis. JAMA. 2003;290:3101–3114. doi: 10.1001/jama.290.23.3101. [DOI] [PubMed] [Google Scholar]