Abstract
We examined the effects of chronic metals ingestion on social behavior in the normally highly social prairie vole to test the hypothesis that metals may interact with central dopamine systems to produce the social withdrawal characteristic of autism. Relative to water-treated controls, ten weeks of chronic ingestion of either Hg++ or Cd++ via drinking water significantly reduced social contact by male voles when they were given a choice between isolation or contact with an unfamiliar same-sex conspecific. The effects of metals ingestion were specific to males: no effects of metals exposure were seen in females. Metals ingestion did not alter behavior of males allowed to choose between isolation or their familiar cagemates, rather than strangers. We also examined the possibility that metals ingestion affects central dopamine functioning by testing the voles’ locomotor responses to peripheral administration of amphetamine. As with the social behavior, we found a sex-specific effect of metals on amphetamine responses. Males that consumed Hg++ did not increase their locomotor activity in response to amphetamine, whereas similarly-treated females and males that ingested only water significantly increased their locomotor activities. Thus, an ecologically relevant stimulus, metals ingestion, produced two of the hallmark characteristics of autism – social avoidance and a male-oriented bias. These results suggest that metals exposure may contribute to the development of autism, possibly by interacting with central dopamine function, and support the use of prairie voles as a model organism in which to study autism.
Keywords: microtus, autism, dopamine, toxicology, metal, mercury, social behavior, prairie vole
Introduction
The autism spectrum disorders are widespread in the developed world and the incidence of autism may be increasing. It is clear from several decades of study that autism is a complex (of) disorder(s) involving both genetic and environmental factors, but there is far from consensus as to the relative influence of each, or even if the same factors affect all autism sufferers in the same fashion [23, 27, 55, 58, 69]. Significant effort has been expended toward identifying genetic underpinnings of autism. However, observations such as the lack of total concordance of autism in monozygotic twins [27], suggest that genetic causes cannot account for all aspects of autism. Thus, it may be just as important to identify environmental triggers as it is to identify genetic vulnerabilities (see also [58]).
One reason for the slow progress in gaining an understanding of autism is the relative lack of ecologically relevant animal models in which to study potential causes of the disorder (but see [73, 74, 78]). Any animal model that is intended as a platform on which to base studies of autism must, at minimum, address the broad aspects and symptoms of autism: social dysfunction, restricted or repetitive behaviors and/or perseveration, language impairments, and the fact that sex differences are apparent in the incidence of autism [76, 87, 91]. Unfortunately, many of the current animal models are limited in their ability to address the broader aspects of autism [83]. Consequently, rather than modeling autism as a disorder, many animal studies focus on specific symptoms of autism. For example, lesion studies typically are targeted toward brain regions that may logically be implicated in autism. When successful, the brain lesions produce behaviors characteristic of autism, and thereby provide important evidence supporting involvement of specific brain regions in autism [7, 21]. However, such techniques do not model the disorder itself, which makes determination of causal factors and effective treatments problematic. As an alternative to the currently used animal models, we propose a unique animal model in which to study autism and a novel mechanism by which autism may be manifested.
Although there often are cognitive deficits associated with the disorder, at its core autism may best be described as a socialization disorder, and the social impairments may persist throughout life. In a comparison of autism symptoms in adults and adolescents, Seltzer et al. [76] found that deficits in social behaviors were least likely to improve with age. Thus, examining social behavior may be the most direct route to understanding autism. For the past two decades, prairie voles (Microtus ochrogaster) have been the dominant animal model in which to study the formation and maintenance of social affiliations [6, 14] and, more recently, have gained the attention of researchers interested in autism [44, 93]. In contrast to more traditional laboratory animals, prairie voles display social behaviors remarkably similar to those of humans, even displaying characteristics of monogamy such as long-term pair-bonding, care of offspring by both parents, and sharing of a nest even beyond the breeding season [15]. Further, autonomic responses in voles are more similar to those of humans than they are to other rodent species [37]. These parallels have led to the extensive use of voles to study quantitatively the behavioral, neural, and physiological bases of social attachment [5, 16, 20, 24, 29, 36, 38, 39, 45, 52, 88, 89]. As a result, both the behavioral repertoire and the physiology of voles are well documented, so there is a strong literature base upon which future studies can rest. Moreover, voles likely possess much greater genetic variability than do the inbred strains of rats and mice typically used as rodent models. For a disorder such as autism that may have multiple genetic vulnerabilities, an animal model with more genetic variability may confer significant advantages, including more closely paralleling the genetic variability of humans and serving to reduce the possibility of false negatives.
A number of studies have implicated exposure to various metals in the development of autism [1, 26, 32, 62, 71, 90] but, to date, mechanisms by which metals exposure may cause autism have been difficult to ascertain. Recently, two independent lines of research have converged to provide a possible link between metals exposure and autism. First, there is evidence that low-level exposure to metals (mercury, lead, copper and other multivalent cations) can alter dopamine transporter (DAT) functioning [51, 54] including that in cells derived from the ventral striatum [42]. In vivo, one outcome of altered DAT functioning could be altered synaptic dopamine concentrations, which may, in turn, result in abnormal dopamine receptor activation patterns in people with metals exposure. A second line of research has shown that changes in patterns of dopamine receptor activation in the ventral striatum can fundamentally alter the behavioral responses of prairie voles [4], such that exposure to a novel individual produces an aversive response rather than an affiliative response during social interactions [5]. These findings have led us to hypothesize that changes in dopamine functioning due to metals exposure may contribute substantially to the symptoms associated with autism. As a first test of this hypothesis, we provided sexually naïve adult prairie voles of both sexes with solutions containing metals as their sole drinking water sources, while a second group of voles was treated identically except that they received unadulterated de-ionized water to drink. After several weeks of metals consumption, group comparisons of a variety of social and non-social measures were performed.
METHODS
Subjects
Animals were housed in USDA approved facilities with general animal care provided by Laboratory Animal Resources personnel. Animals were monitored daily and veterinary staff was available for consultation regarding animal health and welfare. All experimental manipulations, animal handling procedures, and behavioral testing were approved by the Oklahoma State University Center for Health Sciences Institutional Animal Care and Use Committee.
Subjects were sexually-naïve adult prairie voles of both sexes from a laboratory breeding-colony descended from an Illinois population and were of the F3 and F4 generations relative to the most recent out-crossing with wild stock. Breeding pairs (F2 and F3 generations) were housed in plastic cages (20×25×45 cm) containing corncob bedding with hay as nesting material. Ad libitum food (rabbit chow supplemented with sunflower seeds) and water were available. Offspring were weaned at 19–21 days of age and were housed in same-sex pairs in plastic cages (10×17×28 cm) maintained at 21°C with a 14:10 light:dark cycle. After weaning, males were maintained in a separate room from females and the breeder stock until used in experiments. However, during experimental manipulations, subjects and control animals of both sexes were housed in a single room.
Metals exposure
Same-sex pairs were randomly assigned to treatment groups that received dilute (60 ppm) mercuric chloride (HgCl2) or cadmium chloride (CdCl2) solutions as their sole drinking water sources. These concentrations are near the low end of the range of concentrations found during a survey of the toxicology literature. Control pairs received unadulterated drinking water. Groups otherwise were treated identically. Fresh solutions were supplied as part of normal cage maintenance, although solutions were replaced if they became contaminated with bedding outside of the normal cage maintenance regimen. Fresh metals solutions were prepared weekly. In sub-groups of animals of each sex, water and metal ingestion by sibling pairs were monitored by weighing bottles each time solutions were changed. Since animals required multiple housing, ingestion was assumed to be equally distributed between the two individuals in each same-sex pair. For most animals, behavioral testing took place around the tenth week of exposure. A separate group of males was subjected to behavioral testing after about 4 weeks of Hg ingestion to examine whether treatment effects would be present after shorter metals exposure.
Behavioral testing
Social interactions were examined using an apparatus consisting of two parallel cages (10×17×28 cm) connected by a tube (7.5×16 cm). One cage was always empty and, depending on the goal of the test, the other cage contained either an unfamiliar vole of the same sex and of similar age and size as the subject, or the subject’s familiar same-sex cage-mate, as a stimulus animal. The stimulus animals were tethered loosely to confine them to one cage while the subject had unfettered access to both cages. Subjects were released into the empty cage and their behavior was monitored for 3 hours. Movements between cages were counted using a customized computer program (R. Henderson, Florida State University) that monitored light-beams across the tube connecting the cages. All social interaction testing was digitally recorded for later detailed analysis of social behavior. The primary dependent measure was time spent by the subject in quiet direct contact (with or without auto- or allo-grooming) with the stimulus animal. The number of crossings between cages was used as an index of locomotor activity. In two cases, unusual aggression on the part of the stimulus animal necessitated excluding subjects from the final behavioral analysis.
Statistical analyses consisted of two-way ANOVA (sex × metal) to examine sex effects of metals exposure, or two-way ANOVA (stimulus animal × metal) when males were tested for responses toward a stranger vs. toward a familiar partner. Significant (p < 0.05) main effects or interactions were examined further using Student-Neuman-Keuls (SNK) analyses.
Additional groups of 6–8 animals of each sex were assigned to groups that received either untreated drinking water or drinking water containing 60 ppm Hg++. After 10 weeks, locomotor behaviors by these animals in response to amphetamine were tested. Locomotor activity was measured twice for each animal, once after being injected with a 0.5mg/kg dose of d-amphetamine (in 0.1 ml/40g of body weight of isotonic saline vehicle), and once after receiving an equivalent volume of the saline vehicle alone. Testing occurred on two consecutive days with the order of treatments counter-balanced. Immediately after being injected, each vole was placed into an open-field arena (floor area 43 × 43 cm, Noldus) for ten minutes and locomotor behavior was digitally recorded using a video camera mounted approximately 2 m directly overhead. The total distance traveled by each animal during the test was quantified using Noldus EthoVision XT6. The performance of each animal was defined as the difference between the distances traveled after the vehicle and amphetamine injections.
Tissue collection
After behavioral testing, most voles were weighed and sacrificed; the brains were harvested and divided into several portions and stored at −80°C until used for tissue analyses.
Brain mercury concentration
A subsample of brainstems (n = 6/treatment/sex) was assayed for tissue mercury concentration at Texas Tech University, Lubbock, Texas, using established procedures [17]. Each brainstem was digested with HNO3 at elevated temperatures, treated with peroxide, and diluted to 10 ml with de-ionized water, as described in EPA protocol 3050B. Digested samples then were analyzed using a cold vapor mercury procedure. Solutions of known concentration were used to create standard curves. No mercury was detected in blanks and recovery from spiked samples was between 85 and 106%. The detection limit was 0.04 mg/kg of tissue. All control samples were below reliable detection limits for tissue Hg++ content. For these samples, the actual values generated in the analyses were used for statistical purposes, but the results are reported as below detection limits.
RESULTS
Animal demographics and metals exposure
All animals were at least 59 days of age at the onset of any treatments or testing. At the time of the behavioral testing, the mean age of animals that received ten week metals exposure was 147.9 ± 3.5 days (n = 86) and there were no sex differences in age at testing (F1,85 = 1.85, p = 0.18; males – 144.4 ± 3.7 days, females - 154.0 ± 6.8 days). There also were no differences in the duration of metals exposure for sex (F1,42 = 1.76, p = 0.19; males – 72 ± 1.4 days, females - 75 ± 1.6 days) or for metal (F1,42 = 1.84, p = 0.18; Hg++ - 71 ± 1.7 days, Cd++ – 76 ± 0.4 days). Males tested after shorter metals exposure received Hg++ in their drinking water for 30.7 ± 1.1 days prior to testing. Due to the shorter treatment duration, males in this experiment were tested at an earlier age (101.5 ± 1.4 days of age).
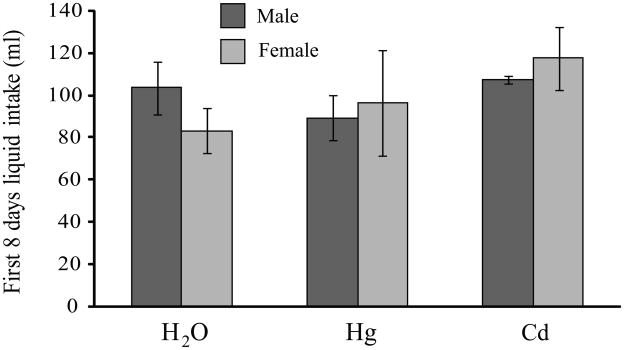
Groups that received only water and groups that received water containing Hg++ or Cd++ did not differ in the total amount of liquid ingested during the first week of exposure (Fig 1). Total liquid ingestion by one group of animals that received Hg++ was monitored for the entire 10 week exposure period. There were no sex differences (n = 6 pairs of each sex) in total amount of mercury ingested, either in terms of absolute amounts ingested (F1,11 = 1.66, p = 0.22; males – 41.3 ± 3.1 mg, females - 50.8 ± 7.7 mg) or when intakes were expressed as amount per unit body weight (F1,11 = 2.51, p = 0.14; males - 1.1 ± 0.1 mg/g of body weight, females – 1.4 ± 0.2 mg/g of body weight). There were significant sex differences in body weight at termination (F1,52 = 16.91, p < 0.001), with males being somewhat heavier than females (males- 42.9 ± 1.1 g, females – 38.0 ± 0.8 g). In addition, Cd++-treated males were heavier (47.5 ± 1.3 g) than either Hg++-treated (40.5 ± 1.8 g, p < 0.004) or control males (41.5 ± 1.5 g, p < 0.02). There were higher levels of Hg++ in the brains of animals that received Hg++ (0.39 ± 0.09 μg/g tissue) than in the animals that consumed only water (< 0.04 μg/g tissue; F2,20 = 17.42, p < 0.001). When brain tissue from only those animals that received Hg++ was compared, there were no sex differences (F1,10 = 0.23, p = 0.64; males – 0.34 ± 0.05 μg/g of tissue, females – 0.43 ± 0.10 μg/g of tissue).
Fig 1.
The presence of 60ppm HgCl2 or CdCl2 in drinking water did not affect the amount of liquid ingested by prairie voles. Cumulative liquid intakes during the first 8 days of access did not differ between animals that received water only or a metals solution as their sole drinking water source, nor were there any sex differences in total liquid ingested. Dark bars indicate male totals, lighter bars indicate female totals.
Choice test – sex comparisons
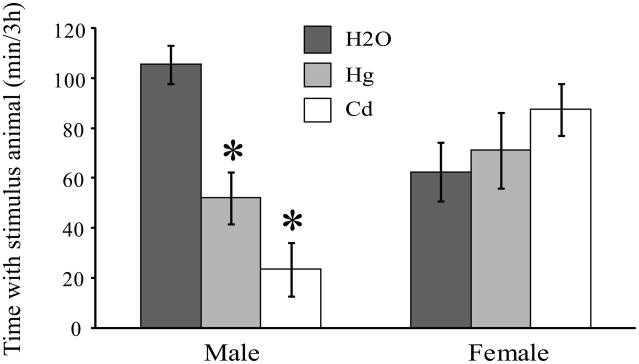
When the amount of time that the subjects (n = 10/group) spent engaged in quiet, direct contact with an unfamiliar stranger was compared, a strong sex by treatment interaction was found (F1,36 = 10.53, p < 0.01). Male voles that consumed water containing Hg++ or Cd++ spent half as much time, or less, in contact with the stranger compared to control males (Fig 2; H2O-treated v. Hg++ -treated, p < 0.002; H2O-treated v. Cd-treated, p < 0.001), while contact by Hg++- and Cd++-treated males did not differ (p = 0.09). In contrast to the effects seen in males, female voles that ingested metals in their drinking water did not differ from control females in the amount of time spent in direct contact with a stranger (Fig 2; H2O-treated v. Hg++ -treated, p = 0.60; H2O-treated v. Cd-treated, p = 0.15).
Fig 2.
Chronic ingestion of metals altered social behavior in male prairie voles. Male prairie voles that consumed either Hg++ or Cd++ in their drinking water spent significantly less time in contact with an unfamiliar, same-sex conspecific than did males that received only unadulterated water. Social contact did not differ between males that received Hg++ or Cd++. In contrast, metals ingestion did not alter the amounts of time females spent in contact with a same-sex stranger. * p < 0.002.
Choice test – stranger v. familiar stimulus animal
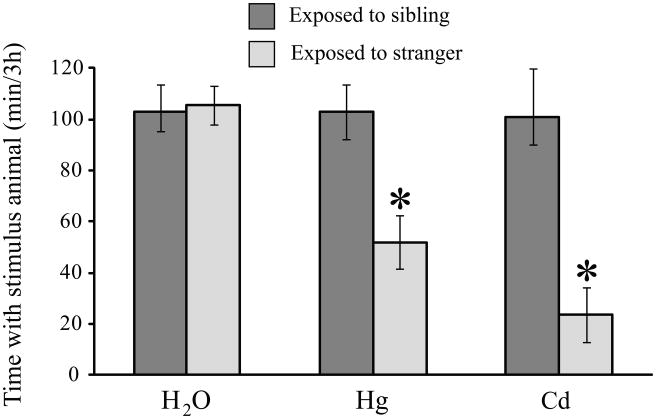
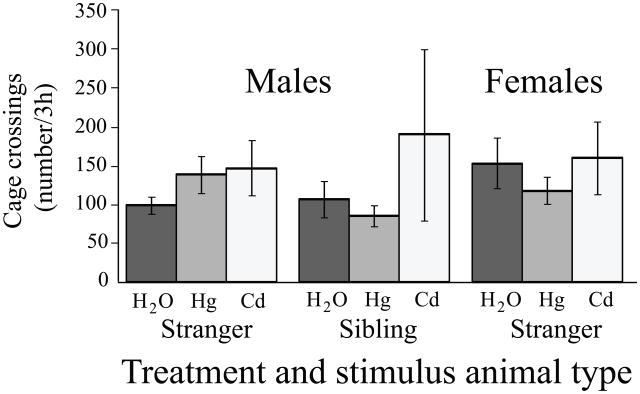
Males that consumed metals in their drinking water were given the choice between an empty cage and a cage containing either their familiar cage-mates or unfamiliar strangers (Fig 3). There were significant effects of metal exposure (F2,49 = 5.53, p < 0.01) and the type of stimulus animal used in the choice test (F1,49 = 16.64, p < 0.001). Males that consumed only water did not differ in the amounts of time spent with either a stranger or their familiar cage-mates. In contrast, males that ingested Hg++ or Cd++ in their drinking water avoided strangers, but not their familiar cage-mates.
Fig 3.
Male prairie voles only displayed reduced social contact when the stimulus animal was an unfamiliar animal. Males that were given a choice between isolation and contact with another vole displayed no effects of metals ingestion if the stimulus animal was the subject’s same-sex cagemate. In contrast, metals-treated males that were exposed to strangers displayed reduced amounts of social contact relative to water-treated controls. Data for stranger-exposed males are the same data as were used in Fig 2 and are presented here to provide a point of comparison. * p < 0.002.
Choice test – shorter time course
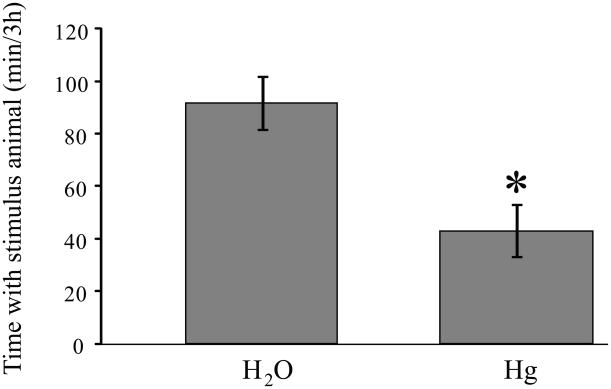
There was a significant treatment effect for males tested after 4 weeks of Hg++ exposure (F1,35 = 11.9, p < 0.002). As was seen with the longer exposure time, males that consumed Hg++ for 4 weeks spent less time in contact with the unfamiliar stimulus animal (Fig. 4) than did control males.
Fig 4.
Male prairie voles that received Hg++ in their drinking water for four weeks displayed reduced time spent with the unfamiliar same-sex conspecific, relative to males that received water only. The magnitude of the decrease was similar to that seen from males that received metals for 10 weeks. * p < 0.002.
Non-social behaviors
There were no effects of sex (F1,60 = 0.41, p = 0.53) or metals consumption (F2,60 = 0.49, p = 0.62) on locomotor activity during the exposure to the stranger (Fig 5). Similarly, there were no effects of metal treatment (F1,38 = 0.15, p = 0.70) or stimulus animal type (F1,38 = 1.10, p = 0.30) on locomotor activity when males were tested for responses to a stranger v. to a familiar cage-mate (Fig 5).
Fig 5.
Unstimulated locomotor activity was unchanged by metals treatment. Regardless of animals’ sex, metal ingested, or stimulus animal used in the behavioral testing, animals that received metals in their drinking water did not differ from water-treated controls in their locomotor activity during the social choice testing.
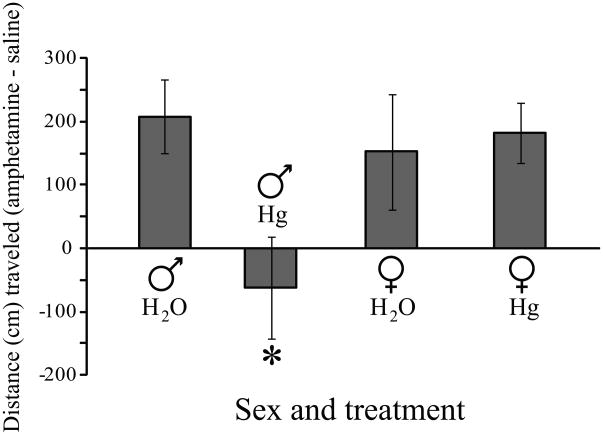
A separate group of Hg++-treated voles was tested for locomotor responses to amphetamine administration. There was a significant interaction between sex and treatment (F1,22 = 4.66, p < 0.05). Male voles that received Hg++ (Fig 6) displayed significantly blunted responses relative to similarly treated females (p < 0.02) or to males that received only water (p < 0.02). Hg++ treatment had no effect on amphetamine responses in females (H2O v. Hg++, p = 0.76). Counter-balancing the order of injections did not affect the animals’ responses as there were no significant differences in locomotor activity after saline injections on the first or second days of treatment (F1,24 = 1.21, p = 0.28).
Fig 6.
Metals ingestion altered locomotor activity in response to amphetamine administration. Male prairie voles that experienced chronic exposure to Hg++ in their drinking water did not display an increase in locomotor activity when given an intraperitoneal injection of 0.5mg/kg of amphetamine over that seen after control injections. In contrast, males that received only water traveled a greater distance in an open-field arena after amphetamine than they did after control injections. Locomotor responses of Hg++-treated males to amphetamine also differed from those of similarly treated females. There were no treatment effects between water-treated and metals-treated females. * p < 0.02 relative to control males or to Hg++-treated females.
Discussion
We have used ecologically relevant stimuli in a novel animal model to produce changes in ethologically valid measures of behavior that parallel two of the defining characteristics of autism. Our primary finding is that chronic ingestion of metals by a normally very social animal, the prairie vole, produces decreases in social contact which may parallel the social deficits observed in autistic patients. Further, we found that the effects of metals ingestion on social behavior are confined to males, which may parallel the male-bias characteristic of autism. In addition, we found that metals ingestion also alters a non-social behavior that is widely known to be dopamine-mediated, increased locomotor activity after amphetamine administration, and this change also was restricted to males. To our knowledge, this is the first use of prairie voles to test specific hypotheses regarding the processes underlying autism.
A variety of studies have provided evidence, either direct or indirect, that central dopamine plays a role in autism. For example, dopamine is involved in many of the behavioral and cognitive functions that are impaired in autistic patients [8, 25, 33, 40, 50] and in many cases, the effects of dopamine manipulations are sex-specific [2, 19, 60, 66]. In addition, drugs that target central dopamine systems can have significant effects (positive or negative) on autism symptoms [22, 40, 50]. Further, the stereotyped dyskinesias associated with autism have been shown to derive from hyperdopaminergic activity rather than from decreases in opposing neurochemical actions[8, 33]. Finally, polymorphisms of several proteins involved in dopamine metabolism and in dopamine receptor genes have been correlated with various aspects of autism [30, 41, 49], and knockout mice that lack the dopamine transporter are more likely to display aversive responses in a social encounter [68]. Thus, genetic differences in dopamine systems could contribute to the variability in susceptibility to autism, particularly in the face of a diverse array of environmental triggers that can interact with central dopamine functions.
The responses to unfamiliar animals vs. to familiar animals in our metals-treated voles are analogous to the responses to strangers vs. to mates by prairie voles after pair-bonding [5]. Pair-bond formation in this species involves changes in D1-type dopamine receptor function in the ventral striatum. As a result of these changes, novelty-induced dopamine release that occurs during a subsequent encounter with an unfamiliar vole elicits an aversive rather than an affiliative response as would occur in a non-pair-bonded vole [5]. We propose that similar processes might account for the social aversion often seen in autistic patients and hypothesize that changes in dopamine functioning which alter D1 receptor activation contribute to the deficits in social behavior seen in autistic patients. More specifically, in the case of metals exposure, decreased density or activity of dopamine clearance mechanisms may lead to abnormal synaptic levels of dopamine, resulting in increased D1 activation. Note that the changes associated with pair bonding are, to some extent, permanent. Pair-bonded voles that lose their mates rarely form a second pair bond, despite the fact that there may still be some social interaction with conspecifics [65]. This may be analogous to the difficulties exhibited by some autistic patients in forming social attachments [61]. Since the mechanism for adult pair-bonding likely shares common features with infant-parent bonds [57], the occasional lack of strong bonds between autistic patients and their parents [72] may be explained by alterations of dopamine systems that preclude the formation of appropriate social bonds. Thus, the competing hypotheses regarding processes underlying autism outlined by Buitelaar (impaired attachment v. approach-avoidance conflicts [13]) instead may reflect two aspects of the same fundamental processes in the brain (also see [92]).
One of the more striking results in the present study was the robust and consistent difference in the effects of metals ingestion between male and female voles. The sex differences in the aversive responses to a novel individual after metals treatment resemble the known differences in the incidences of autism in boys and girls. These behavioral differences were not due to sex differences in metal ingestion or to differences in accumulation of mercury in brain tissue, nor did they appear to be the result of generalized effects of long-term exposure to mercury since there were no differences in total water consumption or in animal weights at the end of the experiment. Sex-specific changes in locomotor activity associated with metals exposure such as those we found in the present study have previously been observed [70] and Aragona et al. [3] reported sex differences in both the rewarding aspects of amphetamine administration, and dopamine mediated changes in social affiliation. In each of these studies, the sex differences were attributed to sex differences in dopamine functioning, in the latter two cases, via differences in sensitivity to dopamine. It also should be noted that central dopamine interacts with the neuropeptides oxytocin (through co-localization of receptors in the nucleus accumbens), and vasopressin (via projections from the nucleus accumbens to the ventral pallidum) to modulate vole social interactions [94]. An intriguing possibility is that sex differences in these neuromodulatory systems serve either to “protect” females from the effects of metals (oxytocin) or to make males more susceptible to metals effects (vasopressin).
But can altered dopamine function account for any of the other symptoms/behaviors associated with autism? We believe that the answer is at least a qualified “Yes”. Dopaminergic projections to the frontal cortex likely play a role in executive function and abnormal patterns of dopamine receptor activation can interfere with such function [81]. Thus, altered dopamine functioning could contribute to the repetitive behaviors typically associated with autism. In fact, mice lacking the dopamine transporter display enhanced perseverative responses and have difficulty suppressing inappropriate responses [31], and take much longer to adapt to a novel situation [34]. The possibility for dopamine involvement in autism is further supported by the observation that autistic patients displayed reduced activity in the frontal, striatal, and parietal regions when confronted with a task that required a shift in cognitive set [77]. Finally, it should again be noted that the metals-induced social avoidance was limited to that toward strangers, while interactions with familiar animals were unimpaired. This may be an important parallel to the observation that autistic children performed on a par with control children in tasks with a predictable pattern, but showed deficits when no pattern was present [46]. This latter observation may reflect the differences in mesolimbic dopamine system functioning when reward is predictable as opposed to unpredictable [75]. In fact, changes in dopamine release associated with differences in predictability could account for the importance of a structured environment in treating autistic children [43].
This interpretation suggests that metals exposure should produce changes in other dopamine-mediated responses. To test this possibility, we examined another, non-social, dopamine-mediated behavior - locomotor responses to peripheral administration of amphetamine. Changes in locomotor responses to amphetamine have been reported after chronic exposure to manganese [56] and cadmium in rats, although the directions of the changes are not always consistent [86]. We found that locomotor responses to amphetamine administration were reduced after mercury ingestion, which is consistent with other reports of effects of metals on dopamine mediated behaviors [56, 86] or dopamine metabolism [82], particularly within the mesolimbic dopamine system [48]. The fact that the sex differences we observed in amphetamine responses after metals exposure paralleled the altered social behaviors strengthens the argument that the changes in social behavior may be dopamine-mediated. Together, these results further support our working hypothesis that changes in central dopamine function after metals exposure might contribute to the etiology of autism, and suggest involvement of the ventral striatum.
Many of the altered behaviors in the present study result from exposure to mercury. We chose to use mercury largely because more is known regarding the toxic effects of mercury than about any other metal save possibly lead. However, we recognize that any discussion of mercury in an autism context requires acknowledgement of the vaccine controversy [63, 64]. Although widely discredited [12, 59, 80, 85], the idea that thimerasol, a compound containing mercury and formerly used as a preservative in childhood vaccines, is responsible for autism, gained a life of its own [28]. Parents, desperate for a solution, gravitated toward this idea, and many are reluctant to accept the scientific community’s assurances that preservatives in vaccines likely play little role in the development of autism. Unfortunately, for many in the autism field, this controversy has resulted in the mercury being thrown out with the thimerasol. In fact, mercury remains at least a potential contributing factor for autism [18], and should continue to be studied in that context. That having been said, however, it is important to note that a variety of metals such as lead, aluminum, cadmium, some of the lanthanides, copper and manganese (all multivalent cations) can negatively impact central dopamine metabolism[11, 53, 84, 95]. The fact that we were able to replicate the effects of mercury exposure by treating the animals with cadmium provides important support for the idea that it might be important to examine classes of environmental influences on the development of autism rather than looking for a specific cause. This suggestion is supported further by observations that environmental exposure to multiple toxins simultaneously is not only possible, but may in fact be likely [90]. The existence of multiple insults, all of which can produce the same or similar symptoms, may account for the observed inconsistencies between individual exposure to specific stimuli and the incidence of the disorder. In this context, it should be noted that among a group of autistic patients [79], stimulated increases in excretion levels for three different metals were seen in different individuals (cadmium, lead, and mercury, respectively). Similarly, hair analysis revealed elevated mercury, lead, and uranium, although the data presentation does not allow comparison between individual subjects [26]. Finally, it is important to note that, although we have concentrated on metals effects on dopamine-mediated behaviors, other environmental factors such as endocrine disruptors and pesticides also can alter dopamine function (reviewed by [47]). Together, these results suggest that future correlative and retrospective studies might find it valuable to test for exposure to a range of toxins instead of focusing on a single cause of autism.
One obvious caveat inherent in the present study is the fact that metals exposure occurred during adulthood while autism is a childhood-onset disorder. It is unknown at this point whether metals exposure during the perinatal period would produce similar changes in behavior. However, prenatal exposure to mercury produces sex specific changes in locomotor activity [70], and perinatal exposure to toxins has been shown to alter DAT function, perhaps permanently [67]. More to the point, altered dopamine function during the perinatal period can be tied, at least indirectly, to autism. For example, Caesarian birth is a risk factor for autism [35] and can cause changes in dopamine receptor binding in rats [9]. Importantly, in rats, these changes are not apparent until three months after birth [9], which may be an important parallel to the onset of autism typically occurring at 2–3 years of age in children. Further, some obstetrical complications can permanently alter the very dopamine systems [10] that we propose are responsible for the behavioral effects of metals that we observed in our voles. The present study establishes social interactions by prairie voles as an ecologically valid measure by which the effects of autism risk factors may be tested. Future studies may extend the model by exposing vole pups to metals during defined portions of the perinatal period and then examining their responses during social interactions as adults.
Significant strides have been made in understanding autism and animal studies have contributed to these advances. However, ultimate understanding of this disorder will require more than modeling the symptoms. To be of maximum utility, an animal model of autism must address three fundamental questions. First - What causes autism? Addressing this question will allow hypotheses regarding specific causative agents to be tested and will provide insights into potential environmental insults that may trigger the disorder. The second question is - How does it cause autism? Being able to address this question will allow not only hypothesis testing of the mechanism(s) by which a causative agent produces autism symptoms, but also will provide a framework for assessing different classes of causative agents. Answering the third question - Why does it cause autism? - will allow important insights into why two children in what seem to be identical circumstances do not both suffer from autism. When the ability to answer these questions can be combined in the same animal model, the rate of progress in understanding autism should increase significantly. Our results show that prairie vole social responses are sensitive to environmental influences at ecologically relevant doses and change in ways that are consistent with what already is known about autism. Here, a putative risk factor for autism (metals exposure) produced a symptom of autism (aversion to social novelty), possibly via changes in a neurotransmitter system known to affect social behavior (dopamine) in brain regions known to influence social behavior (mesolimbic areas) in a sex-specific fashion (autism is more prevalent in males). As a result, we have potential insight into two of three questions outlined above (What? and How?). We currently are examining how polymorphisms in genes associated with dopamine function may convey resistance or susceptibility to autism risks. The results of those studies may allow us to add an answer to the Why? question as well. In fact, we feel that the prairie vole model may well allow these questions to be addressed simultaneously.
Acknowledgments
Funding for this study was provided by National Institutes of Health grants to JTC (HD48462) and DRW (DA13137).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- 1.Adams JB, Romdalvik J, Ramanujam VMS, Legator MS. Mercury, lead, and zinc in baby teeth of children with autism versus controls. J Toxicol Env Heal A. 2007;70:1046–1051. doi: 10.1080/15287390601172080. [DOI] [PubMed] [Google Scholar]
- 2.Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neurosci Biobehav R. 2000;24:137–141. doi: 10.1016/s0149-7634(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 3.Aragona BJ, Detwiler JA, Wang ZX. Amphetamine reward in the monogamous prairie vole. Neurosci Lett. 2007;418:190–194. doi: 10.1016/j.neulet.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J Neurosci. 2003;23:3483–90. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–9. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- 6.Aragona BJ, Wang Z. The prairie vole (Microtus ochrogaster): an animal model for behavioral neuroendocrine research on pair bonding. ILAR Journal. 2004;45:35–45. doi: 10.1093/ilar.45.1.35. [DOI] [PubMed] [Google Scholar]
- 7.Bachevalier J, Loveland KA. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci Biobehav R. 2006;30:97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Berridge KC, Aldridge JW. Super-stereotypy I: Enhancement of a complex movement sequence by systemic dopamine D1 agonists. Synapse. 2000;37:194–204. doi: 10.1002/1098-2396(20000901)37:3<194::AID-SYN3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 9.Boksa P, Zhang Y, Bestawros A. Dopamine D1 receptor changes due to caesarean section birth: Effects of anesthesia, developmental time course, and functional consequences. Exp Neurol. 2002;175:388–397. doi: 10.1006/exnr.2002.7896. [DOI] [PubMed] [Google Scholar]
- 10.Brake WG, Noel MB, Boksa P, Gratton A. Influence of perinatal factors on the nucleus accumbens dopamine response to repeated stress during adulthood: An electrochemical study in the rat. Neuroscience. 1997;77:1067–1076. doi: 10.1016/s0306-4522(96)00543-x. [DOI] [PubMed] [Google Scholar]
- 11.Bryan-Lluka LJ, Bonisch H. Lanthanides inhibit the human noradrenaline, 5-hydroxytryptamine and dopamine transporters. N-S Archives Pharmacol. 1997;355:699–706. doi: 10.1007/pl00005002. [DOI] [PubMed] [Google Scholar]
- 12.Buescher JJ. Vaccinations containing thimerosal do not increase rates of autism. J Fam Practice. 2004;53:94–95. [PubMed] [Google Scholar]
- 13.Buitelaar JK. Attachment and social withdrawal in autism:- hypotheses and findings. Behaviour. 1995;132:319–350. [Google Scholar]
- 14.Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav R. 1995;19:1–12. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- 15.Carter CS, Getz LL. Monogamy and the prairie vole. Sci Am. 1993;268:100–106. doi: 10.1038/scientificamerican0693-100. [DOI] [PubMed] [Google Scholar]
- 16.Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- 17.Cobb GP, Moore AW, Rummel KT, Adair BM, McMurry ST, Hooper MJ. Mercury and methylmercury accumulation and excretion in prairie voles (Microtus ochrogaster) receiving chronic doses of methylmercury. Arch Environ Con Tox. 2007;52:441–449. doi: 10.1007/s00244-006-0006-6. [DOI] [PubMed] [Google Scholar]
- 18.Counter SA, Buchanan LH. Mercury exposure in children: a review. Toxicol Appl Pharm. 2004;198:209–230. doi: 10.1016/j.taap.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 19.Curtis JT, Liu Y, Aragona BJ, Wang ZX. Dopamine and monogamy. Brain Res. 2006;1126:76–90. doi: 10.1016/j.brainres.2006.07.126. [DOI] [PubMed] [Google Scholar]
- 20.Curtis JT, Wang Z. Glucocorticoid receptor involvement in pair bonding in female prairie voles: The effects of acute blockade and interactions with central dopamine reward systems. Neuroscience. 2005;134:369–76. doi: 10.1016/j.neuroscience.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Daenen EW, Wolterink G, Gerrits MA, Van Ree JM. The effects of neonatal lesions in the amygdala or ventral hippocampus on social behaviour later in life. Behav Brain Res. 2002;136:571–82. doi: 10.1016/s0166-4328(02)00223-1. [DOI] [PubMed] [Google Scholar]
- 22.Dalley JW, Thomas KL, Howes SR, Tsai TH, Aparicio-Legarza MI, Reynolds GP, Everitt BJ, Robbins TW. Effects of excitotoxic lesions of the rat prefrontal cortex on CREB regulation and presynaptic markers of dopamine and amino acid function in the nucleus accumbens. Eur J Neurosci. 1999;11:1265–74. doi: 10.1046/j.1460-9568.1999.00532.x. [DOI] [PubMed] [Google Scholar]
- 23.Deth R, Muratore C, Benzecry J, Power-Charnitsky VA, Waly M. How environmental and genetic factors combine to cause autism: A redox/methylation hypothesis. Neurotoxicology. 2008;29:190–201. doi: 10.1016/j.neuro.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 24.DeVries AC, Carter CS. Sex differences in temporal parameters of partner preference in prairie voles (Microtus ochrogaster) Can J Zool. 1999;77:885–889. [Google Scholar]
- 25.Drago F, Caldwell JD, Pedersen CA, Continella G, Scapagnini U, Prange AJ., Jr Dopamine neurotransmission in the nucleus accumbens may be involved in oxytocin-enhanced grooming behavior of the rat. Pharmacol Biochem Behav. 1986;24:1185–8. doi: 10.1016/0091-3057(86)90168-1. [DOI] [PubMed] [Google Scholar]
- 26.Fido A, Al-Saad S. Toxic trace elements in the hair of children with autism. Autism. 2005;9:290–298. doi: 10.1177/1362361305053255. [DOI] [PubMed] [Google Scholar]
- 27.Folstein SE, Rosen-Sheidley B. Genetics of autism: Complex aetiology for a heterogeneous disorder. Nat R Genet. 2001;2:943–955. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- 28.Fombonne E. Thimerosal disappears but autism remains. Arch Gen Psychiat. 2008;65:15–16. doi: 10.1001/archgenpsychiatry.2007.2. [DOI] [PubMed] [Google Scholar]
- 29.Fowler CD, Liu Y, Ouimet C, Wang Z. The effects of social environment on adult neurogenesis in the female prairie vole. J Neurobiol. 2002;51:115–28. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- 30.Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S. The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenetics J. 2001;1:152–156. doi: 10.1038/sj.tpj.6500026. [DOI] [PubMed] [Google Scholar]
- 31.Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- 32.Geier DA, Geier MR. A prospective assessment of porphyrins in autistic disorders: A potential marker for heavy metal exposure. Neurotox Res. 2006;10:57–63. doi: 10.1007/BF03033334. [DOI] [PubMed] [Google Scholar]
- 33.Gerlach J, Reisby N, Randrup A. Dopaminergic hypersensitivity and cholinergic hypofunction in pathophysiology of tardive-dyskinesia. Psychopharmacologia. 1974;34:21–35. doi: 10.1007/BF00421217. [DOI] [PubMed] [Google Scholar]
- 34.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 35.Glasson EJ, Bower C, Petterson B, de Klerk N, Chaney G, Hallmayer JF. Perinatal factors and the development of autism - A population study. Arch Gen Psych. 2004;61:618–627. doi: 10.1001/archpsyc.61.6.618. [DOI] [PubMed] [Google Scholar]
- 36.Gobrogge KL, Liu Y, Jia XX, Wang ZX. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J Comp Neurol. 2007;502:1109–1122. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- 37.Grippo AJ, Lamb DG, Carter CS, Porges SW. Cardiac regulation in the socially monogamous prairie vole. Physiol Behav. 2007;90:386–393. doi: 10.1016/j.physbeh.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grippo AJ, Wu KD, Hassan BS, Carter CS. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occuring depression and anxiety. Depress Anxiety. 2007 doi: 10.1002/da.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammock EAD, Young LJ. Functional microsatellite polymorphism associated with divergent social structure in vole species. Mol Biol Evol. 2004;21:1057–1063. doi: 10.1093/molbev/msh104. [DOI] [PubMed] [Google Scholar]
- 40.Hammock RG, Schroeder SR, Levine WR. The effect of clozapine on self-injurious behavior. J Autism Dev Disord. 1995;25:611–626. doi: 10.1007/BF02178191. [DOI] [PubMed] [Google Scholar]
- 41.Hettinger JA, Liu XD, Schwartz CE, Michaelis RC, Holden JJA. A DRD1 haplotype is associated with risk for autism spectrum disorders in male-only affected sib-pair families. Am J Med Genet B. 2008;147B:628–636. doi: 10.1002/ajmg.b.30655. [DOI] [PubMed] [Google Scholar]
- 42.Hood AN, Little KY, Wallace DR. Combined effects of heavy metals and drugs of abuse on dopamine transporter function. J Neurochem. 2007;102:53–53. [Google Scholar]
- 43.Howlin P. Prognosis in autism: Do specialist treatments affect long-term outcome? Eur Child Adoles Psy. 1997;6:55–72. doi: 10.1007/BF00566668. [DOI] [PubMed] [Google Scholar]
- 44.Insel TR. A neurobiological basis of social attachment. Am J Psychiat. 1997;154:726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- 45.Insel TR, Wang Z-X, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J Neurosci. 1994;14:5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson KA, Robertson IH, Kelly SP, Silk TJ, Barry E, Daibhis A, Watchorn A, Keavey M, Fitzgerald M, Gallagher L, Gill M, Bellgrove MA. Dissociation in performance of children with ADHD and high-functioning autism on a task of sustained attention. Neuropsychologia. 2007;45:2234–2245. doi: 10.1016/j.neuropsychologia.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones DC, Miller GW. The effects of environmental neurotoxicants on the dopaminergic system: A possible role in drug addiction. Biochem Pharmacol. 2008;76:569–581. doi: 10.1016/j.bcp.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Jones GH, Robbins TW. Differential effects of mesocortical, mesolimbic, and mesostriatal dopamine depletion on spontaneous, conditioned, and drug-induced locomotor activity. Pharmacol Biochem Behav. 1992;43:887–895. doi: 10.1016/0091-3057(92)90422-c. [DOI] [PubMed] [Google Scholar]
- 49.Jones MB, Palmour RM, Zwaigenbaum L, Szatmari P. Modifier effects in autism at the MAO-A and DBH loci. Am J Med Genet B. 2004;126B:58–65. doi: 10.1002/ajmg.b.20172. [DOI] [PubMed] [Google Scholar]
- 50.Karler R, Calder LD, Thai DK, Bedingfield JB. The role of dopamine and GABA in the frontal cortex of mice in modulating a motor-stimulant effect of amphetamine and cocaine. Pharmacol Biochem Behav. 1998;60:237–44. doi: 10.1016/s0091-3057(97)00581-9. [DOI] [PubMed] [Google Scholar]
- 51.Kim Y, Kim J-M, Kim JW, Yoo CI, Lee CR, Lee JH, Kim HK, Yang SO, Chung HK, Lee DS, Jeon B. Dopamine transporter density is decreased in Parkinsonian patients with a history of manganese exposure: What does it mean? Movement Disord. 2002;17:568–575. doi: 10.1002/mds.10089. [DOI] [PubMed] [Google Scholar]
- 52.Klein SL, Hairston JE, Devries AC, Nelson RJ. Social environment and steroid hormones affect species and sex differences in immune function among voles. Horm Behav. 1997;32:30–9. doi: 10.1006/hbeh.1997.1402. [DOI] [PubMed] [Google Scholar]
- 53.Komulainen H, Anttonen P, Tuomisto J, Tuomisto L. Effect of transition or heavy metals on [3H] haloperidol binding in rat striatal membranes in vitro. Eur J Pharmacol. 1985;114:113–119. doi: 10.1016/0014-2999(85)90618-1. [DOI] [PubMed] [Google Scholar]
- 54.Komulainen H, Tuomisto J. Effect of heavy metals on dopamine, noradrenaline and serotonin uptake and release in rat brain synaptosomes. Acta Pharmacol Tox. 1981;48:199–204. [Google Scholar]
- 55.Lawler CP, Croen LA, Grether JK, Van de Water J. Identifying environmental contributions to autism: Provocative clues and false leads. Ment Retard Dev D R. 2004;10:292–302. doi: 10.1002/mrdd.20043. [DOI] [PubMed] [Google Scholar]
- 56.Leung TKC, Lai JCK, Tricklebank M, Davison AN, Lim L. Chronic Manganese Treatment of Rats Alters Synaptosomal Uptake of Dopamine and the Behavioral-Response to Amphetamine Administration. J Neurochem. 1982;39:1496–1499. doi: 10.1111/j.1471-4159.1982.tb12599.x. [DOI] [PubMed] [Google Scholar]
- 57.Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 58.London E, Etzel RA. The environment as an etiologic factor in autism: A new direction for research. Env Health Persp. 2000;108:401–404. doi: 10.1289/ehp.00108s3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madsen KM, Lauritsen MB, Pedersen CB, Thorsen P, Plesner AM, Andersen PH, Mortensen PB. Thimerosal and the occurrence of autism: Negative ecological evidence from Danish population-based data. Pediatrics. 2003;112:604–606. doi: 10.1542/peds.112.3.604. [DOI] [PubMed] [Google Scholar]
- 60.Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye WG, Wand GS. Sex differences in striatal dopamine release in healthy adults. Biol Psychiat. 2006;59:966–974. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 61.Naber FBA, Swinkels SHN, Buitelaar JK, Bakermans-Kranenburg MJ, van IJzendoorn MH, Dietz C, van Daalen E, van Engeland H. Attachment in toddlers with autism and other developmental disorders. J Autism Dev Disord. 2007;37:1123–1138. doi: 10.1007/s10803-006-0255-2. [DOI] [PubMed] [Google Scholar]
- 62.Nataf R, Skorupka C, Amet L, Lam A, Springbett A, Lathe R. Porphyrinuria in childhood autistic disorder: Implications for environmental toxicity. Toxicol Appl Pharm. 2006;214:99–108. doi: 10.1016/j.taap.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 63.Nelson KB, Bauman ML. Thimerosal and autism? Pediatrics. 2003;111:674–679. doi: 10.1542/peds.111.3.674. [DOI] [PubMed] [Google Scholar]
- 64.Parker SK, Schwartz B, Todd J, Pickering LK. Thimerosal-containing vaccines and autistic spectrum disorder: A critical review of published original data. Pediatrics. 2004;114:793–804. doi: 10.1542/peds.2004-0434. [DOI] [PubMed] [Google Scholar]
- 65.Pizzuto T, Getz LL. Female prairie voles (Microtus ochrogaster) fail to form a new pair after loss of mate. Behav Processes. 1998;43:79–86. doi: 10.1016/s0376-6357(97)00091-0. [DOI] [PubMed] [Google Scholar]
- 66.Pohjalainen T, Rinne JO, Nagren K, Syvalahti E, Hietala J. Sex differences in the striatal dopamine D-2 receptor binding characteristics in vivo. Am J Psychiat. 1998;155:768–773. doi: 10.1176/ajp.155.6.768. [DOI] [PubMed] [Google Scholar]
- 67.Purkerson-Parker S, McDaniel KL, Moser VC. Dopamine transporter binding in the rat striatum is increased by gestational, perinatal, and adolescent exposure to heptachlor. Toxicol Sci. 2001;64:216–223. doi: 10.1093/toxsci/64.2.216. [DOI] [PubMed] [Google Scholar]
- 68.Rodriguiz RM, Chu R, Caron MG, Wetsel WC. Aberrant responses in social interaction of dopamine transporter knockout mice. Behav Brain Res. 2004;148:185–198. doi: 10.1016/s0166-4328(03)00187-6. [DOI] [PubMed] [Google Scholar]
- 69.Ronald A, Happe F, Bolton P, Butcher LM, Price TS, Wheelwright S, Baron-Cohen S, Plomin R. Genetic heterogeneity between the three components of the autism spectrum: A twin study. J Am Acad Child Psy. 2006;45:691–699. doi: 10.1097/01.chi.0000215325.13058.9d. [DOI] [PubMed] [Google Scholar]
- 70.Rossi AD, Ahlbom E, Ogren SO, Nicotera P, Ceccatelli S. Prenatal exposure to methylmercury alters locomotor activity of male but not female rats. Exp Brain Res. 1997;117:428–436. doi: 10.1007/s002210050237. [DOI] [PubMed] [Google Scholar]
- 71.Russo AF. Anti-metallothionein IgG and levels of metallothionein in autistic families. Swiss Med Wkly. 2008;138:70–77. doi: 10.4414/smw.2008.12014. [DOI] [PubMed] [Google Scholar]
- 72.Rutgers AH, Bakermans-Kranenburg MJ, van Ijzendoorn MH, van Berckelaer-Onnes IA. Autism and attachment: a meta-analytic review. J Child Psychol Psyc. 2004;45:1123–1134. doi: 10.1111/j.1469-7610.2004.t01-1-00305.x. [DOI] [PubMed] [Google Scholar]
- 73.Sadamatsu M, Kanai H, Xu X, Liu Y, Kato N. Review of animal models for autism: implication of thyroid hormone. Congent Anom. 2006;46:1–9. doi: 10.1111/j.1741-4520.2006.00094.x. [DOI] [PubMed] [Google Scholar]
- 74.Schneider T, Roman A, Basta-Kaim A, Kubera M, Budziszewska B, Schneider K, Przewtocki R. Gender-specific behavioral and immunological alterations in an animal model of autism induced by prenatal exposure to valproic acid. Psychoneuroendocrinology. 2008;33:728–740. doi: 10.1016/j.psyneuen.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 75.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 76.Seltzer MM, Krauss MW, Shattuck PT, Orsmond G, Swe A, Lord C. The symptoms of autism spectrum disorders in adolescence and adulthood. J Autism Dev Disord. 2003;33:565–581. doi: 10.1023/b:jadd.0000005995.02453.0b. [DOI] [PubMed] [Google Scholar]
- 77.Shafritz KM, Dichter GS, Baranek GT, Belger A. The neural circuitry mediating shifts in behavioral response and cognitive set in autism. Biol Psychiat. 2008;63:974–980. doi: 10.1016/j.biopsych.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shultz SR, MacFabe DE, Ossenkopp KP, Scratch S, Whelan J, Taylor R, Cain DP. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: Implications for an animal model of autism. Neuropharmacology. 2008;54:901–911. doi: 10.1016/j.neuropharm.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 79.Soden SE, Lowry JA, Garrison CB, Wasserman GS. 24-Hour provoked urine excretion test for heavy metals in children with autism and typically developing controls, a pilot study. Clin Toxicol. 2007;45:476–481. doi: 10.1080/15563650701338195. [DOI] [PubMed] [Google Scholar]
- 80.Stehr-Green P, Tull P, Stellfeld M, Mortenson PB, Simpson D. Autism and thimerosal-containing vaccines: Lack of consistent evidence for an association. Am J Prev Med. 2003;25:101–106. doi: 10.1016/s0749-3797(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 81.Sullivan RM, Brake WG. What the rodent prefrontal cortex can teach us about attention-deficit/hyperactivity disorder: the critical role of early developmental events on prefrontal function. Behav Brain Res. 2003;146:43–55. doi: 10.1016/j.bbr.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 82.Szczerbak G, Nowak P, Kostrzewa RM, Brus R. Maternal lead exposure produces long-term enhancement of dopaminergic reactivity in rat offspring. Neurochem Res. 2007;32:1791–1798. doi: 10.1007/s11064-007-9306-0. [DOI] [PubMed] [Google Scholar]
- 83.Tordjman S, Drapier D, Bonnot O, Graignic R, Fortes S, Cohen D, Millet B, Laurent C, Roubertoux PL. Animal models relevant to schizophrenia and autism: Validity and limitations. Behav Genet. 2007;37:61–78. doi: 10.1007/s10519-006-9120-5. [DOI] [PubMed] [Google Scholar]
- 84.Tran TT, Chowanadisai W, Crinella FM, Chicz-DeMet A, Lonnerdal B. Effect of high dietary manganese intake of neonatal rats on tissue mineral accumulation, striatal dopamine levels, and neurodevelopmental status. Neurotoxicology. 2002;23:635–643. doi: 10.1016/s0161-813x(02)00091-8. [DOI] [PubMed] [Google Scholar]
- 85.Verstraeten T, Davis RL, DeStefano F, Lieu TA, Rhodes PH, Black SB, Shinefield H, Chen RT, Team VSD. Safety of thimerosal-containing vaccines: A two-phased study of computerized health maintenance organization databases. Pediatrics. 2003;112:1039–1048. [PubMed] [Google Scholar]
- 86.Vezer T, Kurunczi A, Naray M, Papp A, Nagymajtenyi L. Behavioral effects of subchronic inorganic manganese exposure in rats. Am J Ind Med. 2007;50:841–852. doi: 10.1002/ajim.20485. [DOI] [PubMed] [Google Scholar]
- 87.Volkmar FR, Klin A. Issues in the classification of autism and related conditions. In: Volkmar FR, Paul R, Klin A, Cohen D, editors. Handbook of Autism and Pervasive Developmental Disorders. John Wiley & Sons, Inc; Hoboken NJ: 2002. pp. 5–41. [Google Scholar]
- 88.Wang Z, Ferris CF, De Vries GJ. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proc Natl Acad Sci USA. 1994;91:400–4. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocrinol. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 90.Windham GC, Zhang LX, Gunier R, Croen LA, Grether JK. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco Bay area. Env Health Persp. 2006;114:1438–1444. doi: 10.1289/ehp.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wing L. Sex ratios in early childhood autism and related conditions. Psychiat Res. 1981;5:129–137. doi: 10.1016/0165-1781(81)90043-3. [DOI] [PubMed] [Google Scholar]
- 92.Young LJ. The neurobiology of social recognition, approach, and avoidance. Biol Psychiatry. 2002;51:18–26. doi: 10.1016/s0006-3223(01)01268-9. [DOI] [PubMed] [Google Scholar]
- 93.Young LJ, Pitkow L, Ferguson JN. Neuropeptides and social behavior: animal models relevant to autism. Mol Psychiat. 2002;7:S38–S39. doi: 10.1038/sj.mp.4001175. [DOI] [PubMed] [Google Scholar]
- 94.Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–54. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 95.Zhao F, Cai TJ, Liu MC, Zheng G, Luo WJ, Chen JY. Manganese induces dopaminergic neurodegeneration via microglial activation in a rat model of manganism. Toxicol Sci. 2009;107:156–164. doi: 10.1093/toxsci/kfn213. [DOI] [PubMed] [Google Scholar]