Abstract
Time-dependent increases in cue-induced reward seeking after forced abstinence were described in rats with a history of cocaine or sucrose self-administration, suggesting reward craving incubates over time. In the present study, we examined the effects of reduced training experience, or sucrose pre-loading just prior to testing, on the incubation of sucrose craving. Sucrose seeking (responding in extinction and then for a sucrose-paired cue) increased over time in groups of rats that self-administered sucrose 6 h/day for 10 days and were tested at 1, 7, or 30 days of forced abstinence. We found that groups of rats that had self-administered 2 instead of 6 h/day showed a similar profile of responding. Incubation of sucrose craving was attenuated by free access to sucrose in home cages for 17 h immediately prior to testing assessed as extinction responding on days 1 and 30. However, this sucrose pre-loading had no effect on the time-dependent increase in responding for the sucrose-paired cue. In summary, reducing the training experience had no effect on the incubation of sucrose craving and free access to sucrose had only a limited effect–attenuating extinction responding. These results illustrate the strength of the incubation of craving and further suggest long-term changes in brain motivational circuitry following sucrose self-administration.
Keywords: Cocaine, Craving, Cue-induced, Eating disorder, Extinction, Incubation, Rat, Reinstatement, Relapse, Sucrose, Time-dependent
1. Introduction
Craving, a poorly understood subjective state that precedes and accompanies drug seeking [1] has been the target of a growing body of pre-clinical literature [2] that describes craving using animal models. In one animal model of cocaine craving (the reinstatement model [3,4]), lever presses for cocaine are presented with conditioned stimuli (CSs) such as a brief presentation of a tone and a stimulus light [5]. Craving is then assessed as lever pressing (“seeking behavior”) first in several daily sessions wherein rats press in the absence of cocaine and the CSs (“extinction”) and then in a subsequent session where responses result in delivery of the CSs alone (“responding for the reward-paired cue”). The latter condition has been suggested to model cue-induced craving [6], a phenomenon thought to contribute to cocaine craving and recidivism [7].
Using a modified version of the reinstatement procedure [2,8], we examined whether cocaine seeking increases over time away from cocaine (in general, abstinence applies to humans, “forced” abstinence to rats). In this procedure, rats self-administer cocaine during 10 daily sessions in which each cocaine infusion is paired with a discrete tone+light cue. Presses on the lever previously associated with cocaine during extinction (in the absence of the cue) and responding for the cue are determined on the same test day. We found that responding during these tests progressively increases from 1 to 60 days following self-administration training—a behavioral phenomenon that we hypothesize as a manifestation of an underlying processes of “incubation of craving” [9].
An important question is whether the incubation of craving generalizes to other drug and non-drug rewards. Indeed, incubation of craving has been observed following heroin [10], methamphetamine [11], and has been repeatedly demonstrated following sucrose self-administration [10]. We believe it is likely that incubation of craving for drug and non-drug rewards share a basic behavioral, and perhaps not yet identified molecular mechanism. Others have argued that study of the circuitry of “natural rewards” likely bears great significance on gaining an understanding of drug addiction [12].
Therefore, examining the incubation of craving using sucrose is informative to the question of how craving changes over time, and could reveal treatment strategies for chronic relapsing disorder such as eating disorders and drug addiction. Part of such an examination is to reveal conditions that might exacerbate or attenuate the incubation of craving. To this end, we parametrically evaluated the incubation of craving effect in two ways: Training duration was shortened, or rats were pre-loaded with sucrose just prior to testing.
2. Methods
2.1. Subjects
Subjects were 73 male Long-Evans rats (350–450 g) bred in the Western Washington University Psychology department vivarium. Rats were weighed each Monday, Wednesday, and Friday for the duration of the experiment. Rats were maintained on Mazuri Rodent Pellets and water was provided ad libitum except as noted in General procedures. Pellets and water were also available ad libitum in the self-administration boxes except as noted in General procedures. All rats remained singly housed in the vivarium except during daily training or testing sessions when they were brought to the self-administration boxes. Rats were maintained on a reversed 12:12 h light–dark cycle with lights off at 7 AM. All procedures performed on the rats followed the NIH guidelines for animal care, and were approved by the Western Washington University Animal Care and Use Committee.
2.2. Apparatus
The self-administration boxes, controlled by a Med Associates (Georgia, VT) system, had two levers located 11 cm above the floor, but only one lever (an active, retractable lever) activated the infusion pump. Presses on the other lever (an inactive, stationary lever) were also recorded. The 10% sucrose solution was delivered into liquid drop receptacles for oral consumption (Med Associates).
2.3. General procedures
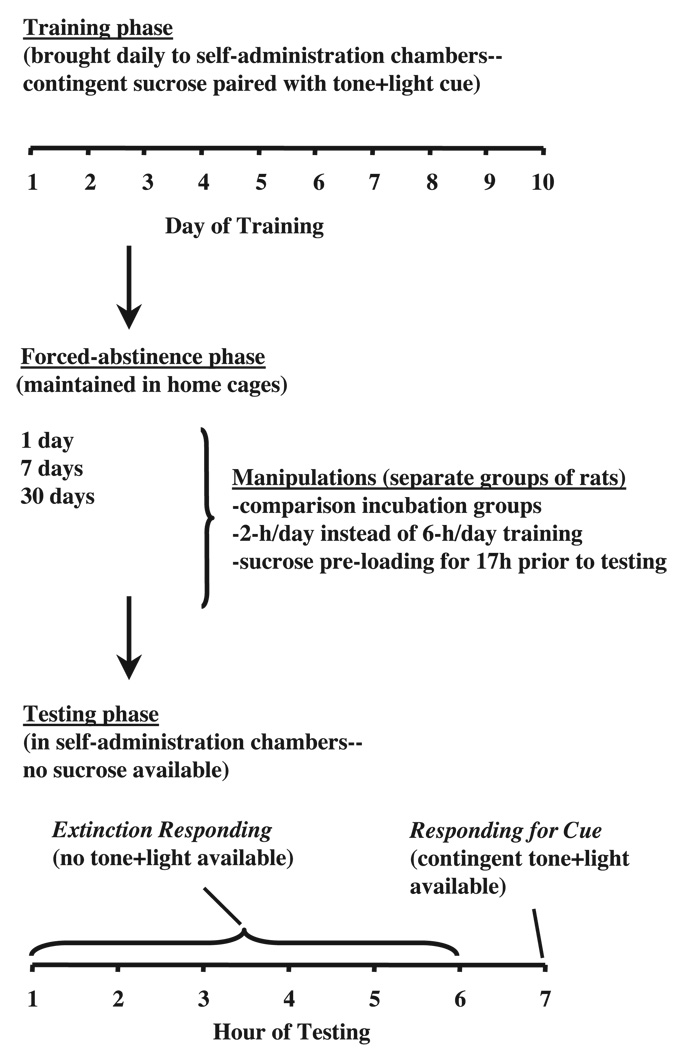
The experiment included three phases, depicted in Fig. 1. Rats were deprived of water in their home cages 17 h prior to the first training session. Water was not available in the self-administration boxes at this time. Water was returned to the self-administration chambers when rats learned to reliably respond for sucrose, or after 72 h of deprivation for rats that were slow to learn to press for sucrose. Water was returned to home cages after 48 h of deprivation. During the Training phase (10 days), rats were placed in the self-administration chambers and allowed to lever press for sucrose. During the Forced abstinence phase (1, 7, or 30 days), rats remained in their vivarium home cages. On the test day (Testing phase) rats were returned to the self-administration boxes. As described in the Introduction, responding in the testing phase (reinstatement conditions) is taken as an index of craving. Lever presses during testing were never reinforced with sucrose. Rats were first allowed to lever press on the previously active lever for 6 h (Testing phase: Extinction responding) in the absence of the discrete tone+light cue. Rats were then tested for cue-induced sucrose seeking during a 1-h session wherein lever presses led to cue presentations (Testing phase: Responding for cue).
Fig.1.
General procedure.
2.3.1. Training phase
Rats were trained to self-administer sucrose (0.4 ml) delivered into a liquid drop receptacle. Training was conducted during 6 (or 2—see reduced access manipulation), 1-h sessions that were separated by 5 min for 10 days under a continuous reinforcement schedule (each lever press is reinforced) with a 40-s timeout after each earned reward. Each session began with the insertion of the active lever and the illumination of a red houselight that remained on for the entire session. A 5-s tone (2900 Hz, 20 dB above background)-light (7.5 W white light above the active lever) discrete compound cue accompanied each reward delivery. At the end of each session, the house light was turned off and the active lever retracted. The number of rewards earned was limited to 15 per hour. If the maximum was earned in a session, the houselight was turned off and the active lever retracted for the remainder of the hour. Although well-trained rats typically achieved the maximum number of rewards in the first 15 minutes of each hour of training, rats were not in extinction conditions, defined as access to the lever and discrete cues predicting sucrose delivery, during these extended off periods. The 15 rewards maximum per hour limitation was imposed for two reasons. First, as some of our ongoing and previous studies compare rats self-administering sucrose vs. cocaine, we have found that 15 rewards per hour provide some level of similar intake. Second, as rats find sucrose to be highly palatable, they will self-administer at rates high enough to empty syringes during training sessions if given unlimited access. Rats were returned to home cages at the end of the final daily hour of training.
2.3.2. Forced-abstinence phase
At the end of the training phase, rats (n=8–10 per group) were assigned to one of the forced-abstinence periods (1, 7, or 30 days). Rats lived in the vivarium for the duration of forced abstinence. For the sucrose pre-loading manipulation, rats received bottles of sucrose (300 ml) exchanged with their drinking water for the full 17 h immediately prior to testing. The 17 h sucrose pre-loading period was chosen as 17 h covers the time from just after the end of a training session one day (3:30 PM) and the start time of testing the next day (8:30 AM). A 17-h period is therefore the maximum time available for a free-access manipulation for rats tested on Day 1 of abstinence. Sucrose consumption was recorded as ml/h.
2.3.3. Testing phase: extinction responding
On the test day, all rats were given 6, 1-h extinction sessions that were separated by 5 min until they reached an extinction criterion of less than 15-responses/1 h on the previously active lever. Approximately 20% of the rats were given an additional 1-h extinction session to reach the 15-responses/h criterion if they failed to meet it in six sessions. The tone+light discrete cue was not present during these sessions. Each 1-h session began with the introduction of the active lever and illumination of the houselight. At the end of each session, the house light was turned off and the active lever was retracted.
2.3.4. Testing phase: responding for cue
The test for cue-induced sucrose seeking consisted of a 1-h session wherein responses on the previously active lever led to the presentation of the tone+light cue on a continuous reinforcement schedule with a 40-s timeout. This session started 5 min after the last 1-h extinction session.
2.4. Manipulations
The main comparison groups consisted of separate groups of rats trained to self-administer sucrose and subsequently tested for reinstatement at either days 1, 7, or 30 of forced abstinence. Amount of sucrose intake during training was manipulated by having separate groups of rats self-administer sucrose for 2 h/day instead of 6. Sucrose pre-loading was manipulated for some groups by allowing 17-h free access to sucrose immediately prior to testing on days 1, 7, or 30. This free access manipulation was only done with rats that had self-administered sucrose for 6 h/day during the Training phase.
2.5. Statistical analyses
2.5.1. Training phase
Daily sucrose presentations (infusions) were analyzed with repeated measures ANOVA (RM ANOVA). Data from all rats that self-administered sucrose 6 h/day were analyzed using the additional between group factors of DAY (1, 7, or 30) and MANIPULATION (comparison groups, 2 h/day groups, or sucrose 17-h free access groups) to verify that rats tested at different time points and with different manipulations received equivalent training. Similarly, daily infusions data from rats that self-administered sucrose 2 h/day were analyzed with RM ANOVA with the additional between group factor of DAY (1, 7, 30).
2.5.2. Testing phase
Data from the extinction sessions (Extinction responding) and tests for cue-induced sucrose seeking (Responding for cue) were analyzed separately for total non-reinforced responses on the previously active lever and responses on the inactive lever. These data were analyzed using ANOVA with the between-groups factor of DAY (1, 7, 30) and MANIPULATION (comparison groups, 2 h/day groups, or sucrose 17-h free access groups). Additional ANOVAs were run comparing active lever responding in the final hour of extinction to examine whether response rates in the different groups were comparable prior to responding for the sucrose-paired cue.
Post-hoc analyses for the main effect of DAY were done with PLSD tests. The aim for between-group comparisons of active lever responding was to see whether a manipulation resulted in a change in the amount of responding in either extinction or responding for cue conditions compared to the comparison group at that same forced-abstinence time point. Therefore, post-hoc comparisons for overall MANIPULATION main effects are not reported. Instead, planned comparisons are reported for groups at a single time point that significantly differed from the comparison group. To identify whether an incubation of sucrose craving occurred in the comparison group, planned comparisons were made between these groups alone comparing day 1 responding with days 7 and 30. Planned comparisons were made using independent t-tests and significant differences are reported for p<0.006. This conservative probability value was chosen to keep the family-wise error rate for the 8 comparisons of either the extinction or responding for cue active lever data at p<0.05. Planned comparisons were only made on the extinction and responding for cue active lever data.
Sucrose consumption was compared within the sucrose pre-loading manipulation using ANOVA with the between-groups factor of DAY (1, 7, 30). This manipulation required further analyses described in the results section. Significant differences are reported for p’s<0.05.
3. Results
3.1. Training phase
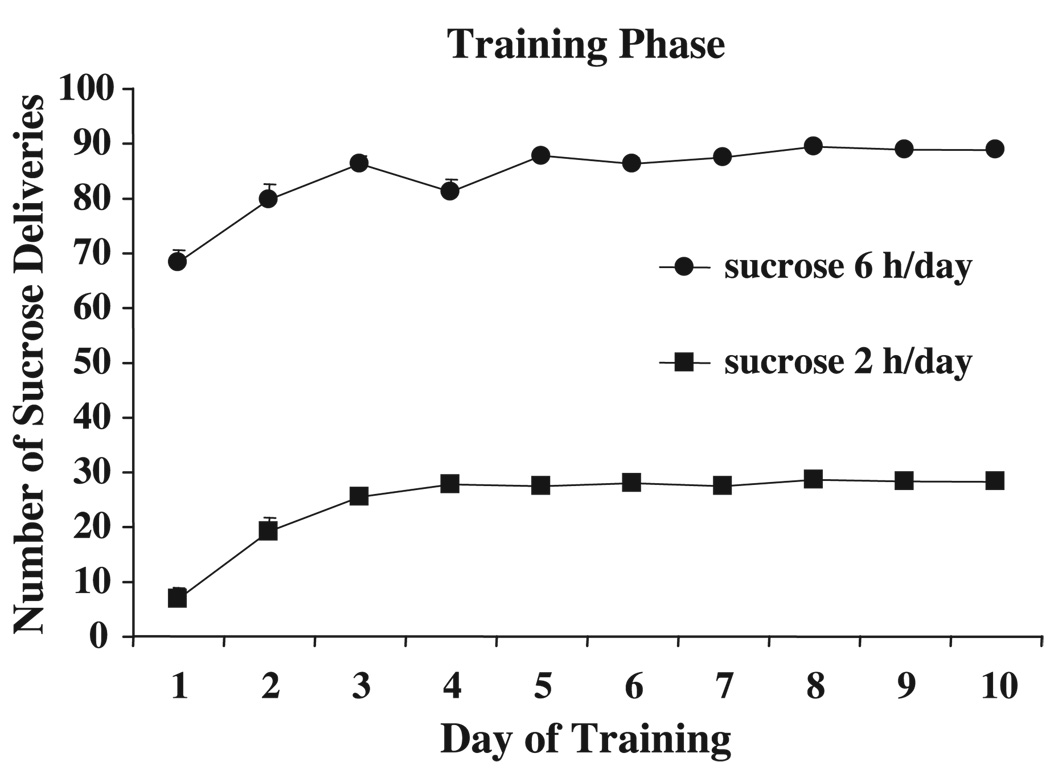
Training infusion data are depicted in Fig. 2 for all rats. Rats demonstrated reliable sucrose self-administration behavior, and no significant differences were observed for the between-group variable DAY or MANIPULATION (p’s>.05). There were significant effects of TIME (day of training), indicating increased intake over the 10 days of training, F(9,387)=18.5 for sucrose 6 h and F(9,189)=34.2 for sucrose 2 h, both p’s<0.05.
Fig. 2.
Mean daily sucrose intake. Data points indicate (mean±SEM) of sucrose in self-administration sessions totaling either 6 or 2 h per day (sucrose 6 h, n=49, sucrose 2 h, n=24).
3.2. Testing phase
Responding on the inactive lever was low during both extinction responding and responding for the sucrose-paired cue (an average of less than 2 responses per hour) across all groups, and there were no statistically significant main effects or interactions found in the ANOVAs. Therefore, the inactive lever data are not shown.
3.2.1. Testing phase: extinction responding
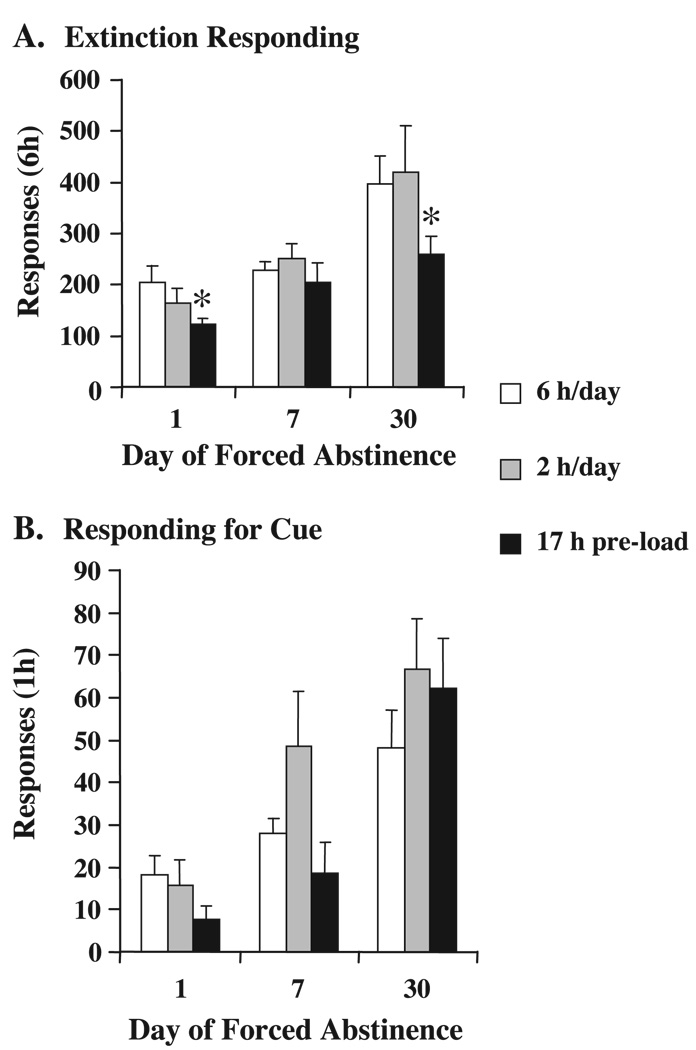
ANOVA of extinction responding revealed main effects of DAY, F(2,64)=15.9, and MANIPULATION, F(2,64)=3.8, both p<0.05. For the main effect of DAY, post hoc comparisons revealed that Day 30 responding was significantly greater than either Day 1 or Day 7 responding. Planned comparisons between the comparison groups alone revealed a significant increase between Days 1 and 30. Planned comparisons between manipulated groups at each time point and the comparison group at that time point revealed a significant attenuation of responding in the 17-h free access groups tested on Days 1 or 30 of forced abstinence (Fig. 3A). There were no significant main effects or interactions for active or inactive lever responding for the final hour of extinction (data not shown).
Fig. 3.
(A) Extinction responding. Extinction responding on days 1, 7, or 30 of forced abstinence. Manipulations abbreviated in the legend are 6/h day (6 h/day comparison groups), 2 h/day (2 h/day training instead of 6 h/day), 17 h pre-load (17-h free access to sucrose just prior to testing). There was an overall main effect of DAY with day 30 responding greater than either day 1 or day 7. Planned comparisons between the comparison groups alone revealed a significant difference between days 1 and 30. * Indicates significantly different from the 6-h/day comparison group at that time point. (B) Responding for cue. Responding for the sucrose-paired cue on days 1,7, or 30 of forced abstinence. Manipulations abbreviated in the legend are 6 h/day (6 h/day comparison groups), 2 h/day (2 h/day training instead of 6 h/day), 17 h pre-load (17-h free access to sucrose just prior to testing). There was an overall main effect of DAY-post hoc analyses revealed that day 30 responding was greater than either day 1 or day 7, and that day 7 responding was greater than day 1. Planned comparisons between the comparison groups alone revealed a significant difference between days 1 and 30. * Indicates significantly different from the 6-h/day comparison group at that time point.
3.2.2 Testing phase: responding for cue
ANOVA of responding for the sucrose-paired cue revealed a main effect of DAY, F(2,64)=21.1, p<0.05. For the main effect of DAY, post-hoc comparisons revealed that day 30 responding was significantly greater than either day 1 or day 7 responding and that day 7 responding was greater than day 1. Planned comparisons between the comparison groups alone revealed a significant increase in responding between days 1 and 30. Planned comparisons between manipulated groups and the comparison group at each time point revealed no significant differences (Fig. 3B).
3.3. Sucrose intake during sucrose pre-loading manipulation
Intake of sucrose varied significantly across the time points [significant effect of DAY, F(2,22)=7.4, p<0.01]. As depicted in Table 1, rats consumed more sucrose (ml/h) in the free access period prior to day 7 testing compared to days 1 or 30. Water intake by a group of naive rats was subsequently monitored and it is clear that sucrose intake by rats in the free access manipulation was much greater than this water intake comparison group (Table 1).
Table 1.
Sucrose pre-loading manipulation: home cage sucrose consumption (ml/h) for 17 h immediately prior to testing
Intake is expressed as mean (SEM) of 17-h access.
Indicates statistically significant difference of days 1 or 30, p<0.05.
Water consumption measured over a 17-h period in experimentally naive rats (n=9).
4. Discussion
In general, the manipulations had minimal effects on the incubation of sucrose craving. Free access to sucrose did attenuate extinction responding on days 1 and 30. However, the same sucrose pre-loading was without effect on responding for the sucrose-paired cue after extinction. Reduced training had no effect on incubation of sucrose craving. We argue that these findings are indicative of the strength of the incubation of craving. In the following sections, the results of each manipulation are discussed in light of previous studies. The subsequent General remarks section proposes an integration of these findings pointing to the strength of the incubation of craving, its possible neural substrates, and implications of the findings for chronic relapsing disorders.
4.1. Reduced training (2 h/day vs. 6 h/day)
Following a reduced training regimen (2 h/day), incubation of sucrose craving was similar to the comparison groups (6 h/day, a training condition used in our previous studies). A lack of effect was observed despite two factors that discriminated the training situation for rats in the two conditions. As a result of having 2-h/day access, rats in the reduced training condition could only obtain a maximum of 30 deliveries of sucrose per day compared to 90 for the 6 h/day rats. As shown in Fig. 2, rats trained using either procedure achieved nearly maximum hourly deliveries early in training. The second factor was simply that rats trained 6 h/day had four more hours a day in the reward-predictive environment (self-administration boxes). A possible reason for the lack of effect of reduced training on the incubation of craving is that rats in both the 2-h and 6-h/day training conditions were overtrained. Kruzich and See [13] found that only two 3-h sessions where a tone+light stimulus was explicitly paired with reward, in that case with cocaine, resulted in subsequent responding for the tone+light alone.
4.2. Sucrose pre-loading
Free access to sucrose immediately prior to testing only moderately attenuated the incubation of sucrose craving, an effect that was limited to extinction responding. The same sucrose pre-loading had no effect on the time-dependent increase in responding for the sucrose-paired cue. We believe that these findings indicate that extended access (and intake, as indicated in Table 1) to sucrose was without effect on cue-induced sucrose seeking assessed as lever pressing for a sucrose-paired cue. These findings complement those describing how reward-paired cues can override satiety to produce eating in sated humans [14] and rats [15]. Specifically, our findings suggest that in a “sated” individual, reward-paired cues can override satiety signals even in the absence of the availability of the primary reward. While we do not have direct evidence that our rats were satiated for sucrose following extended free access, it is common in the behavioral literature to define satiation as having been exposed to extended free access to a reward [15]. A more rigorous definition of sucrose satiation for the present study would have required perhaps detailed observations of drinking behavior [16]. Table 1, however, illustrates that the rats in the present study maintained intake levels of sucrose during the free access periods well above rats drinking unsweetened water.
The present results also shed light on the reasons why day 1 responding is lower than subsequent days in incubation of craving studies. We have suggested [17] that cocaine responding on day 1 is especially low due to potential anhedonia in the animals, or perhaps memories of the aversive qualities of cocaine that decrease over the first few days of forced abstinence. The case with sucrose is complicated in that there are no studies indicating such an abstinence syndrome following sucrose self-administration. In addition, stress does not reinstate responding for sucrosepaired cues [18]. If the decreased responding on day 1 is simply a matter of time since previous exposure to the reward, we should have seen very low responding in the rats given extended access to sucrose just prior to day 30 testing, perhaps at levels similar to day 1. Instead, responding was just as high as that of day 30 rats. This suggests that the incubation of sucrose craving is for the sucrose-paired stimuli in the operant chambers. Unlimited access to sucrose did not “sate” cue-induced craving for sucrose.
Interestingly, sucrose intake was greatest in the access period prior to day 7 of forced abstinence. Intake was similar to day 1 levels in the 17-h period prior to day 30 (Table 1). While free access intake may be a crude measure of the rewarding effects of sucrose, these findings suggest that sucrose was more rewarding at day 7. If this is so, then these findings support dissociation between the primary rewarding effects of sucrose and conditioned reward. These findings are supported by neuroanatomical studies wherein bilateral reversible inactivation of the nucleus accumbens attenuates responding for cocaine itself, but not responding for a cocaine-paired cue while bilateral inactivation of the basolateral amygdala results in the direct opposite outcome [19].
4.3. Concluding remarks
We suggest that the incubation of craving is a somewhat inflexible increased motivational response to reward-paired cues, either to contact the lever in the presence of the reward-paired environment or to contact the lever for the presentation of reward-paired cues. It is not the craving for the sucrose per se that grows in strength, but the motivational reaction to reward-paired cues.
Perhaps the incubation of craving is a psychological process with adaptive value. It has been suggested that “incubation of fear” [20], where the response to a shock-predictive stimulus increases over time, may occur to allow the individual to be maximally reactive to more of the features of the conditioned stimulus (CS) later in time, perhaps by decreased discrimination among those most predictive of punishment [21]. The incubation of craving could be adaptive in a similar manner by allowing the individual to be receptive to the greatest amounts of reward-predictive stimuli in an environment that has not been visited for some time. The time-dependent increase in reactivity to a conditioned sucrose stimulus following removal of sucrose is similar in pattern to the time-dependent increase in saccharin consumption following removal of saccharin, referred to as the “saccharin deprivation/elation effect” [22]. However, in such studies actual consumption of saccharin increased following withdrawal. In the present study, sucrose consumption did increase just prior to day 7 of forced abstinence, but it was at day 1 levels when testing revealed increased responding for the sucrose-paired cue on day 30. Further study is required to establish whether the incubation of craving and the saccharin deprivation/elation effects are manifestations of the same phenomenon.
In conclusion, our study demonstrates robust incubation of sucrose craving which was minimally altered by reducing the amount of sucrose exposure during training and by allowing rats free access to sucrose prior to testing. These data and those from previous studies on incubation of craving for cocaine, methamphetamine and heroin raise the possibility that the incubation of craving for sucrose, psychostimulants, and opiates share similar neurobiological substrates. For example, in rats a diet rich in sugar results in opioid dependence [23], and sugar intake results in cross-sensitization to amphetamine [24]. The incubation of craving may be an exaggerated expression of an adaptive behavior-exaggerated due to neuroplastic changes mediated by the effects of high-density reward on brain reward circuitry. Understanding the parametrics of the manifestation of incubation of craving and ultimately its neurobiology may have implications for the treatment for chronic relapsing disorders where craving may be a potential factor in relapse, such as eating disorders and drug addiction.
Acknowledgments
This study was supported by funds from Western Washington University. The authors wish to thank Yavin Shaham for thoughtful comments on early versions of the manuscript.
References
- 1.O’Brien CP, Childress AR, Mclellan TA, Ehrman R. Classical conditioning in drug dependent humans. Ann NY Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- 2.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Davis WM, Smith SG. Role of conditioned reinforcers in the initiation, maintenance and extinction of drug-seeking behavior. Pavlovian J Biol Sci. 1976;11:222–236. doi: 10.1007/BF03000316. [DOI] [PubMed] [Google Scholar]
- 4.de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- 5.See RE, Grimm JW, Kruzich PJ, Rustay N. The importance of a compound stimulus in conditioned drug-seeking behavior following one week of extinction from self-administered cocaine in rats. Drug Alcohol Depend. 1999;57:41–49. doi: 10.1016/s0376-8716(99)00043-5. [DOI] [PubMed] [Google Scholar]
- 6.Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- 7.Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- 8.Tran-Nguyen TL, Fuchs RA, Coffey GP, O’Dell LE, Baker DA, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and dopamine overflow in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- 9.Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shalev U, Morales M, Hope BT, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- 11.Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 12.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kruzich PJ, See RE. Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J Neurosci. 2001;21:RC155. doi: 10.1523/JNEUROSCI.21-14-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornell CE, Rodin J, Weingarten H. Stimulus-induced eating when satiated. Physiol Behav. 1989;45:695–704. doi: 10.1016/0031-9384(89)90281-3. [DOI] [PubMed] [Google Scholar]
- 15.Petrovich GD, Setlow B, Holland PC, Gallagher M. Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. J Neurosci. 2002;22:8748–8753. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao P, Harvey M, Mook DG, Zeigler HP. A “pre-satiety sequence” in rats drinking sucrose solutions. Physiol Behav. 1998;65:355–359. doi: 10.1016/s0031-9384(98)00177-2. [DOI] [PubMed] [Google Scholar]
- 17.Grimm JW, Shaham Y, Hope BT. Effect of cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement, and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav Pharmacol. 2002;13:379–388. doi: 10.1097/00008877-200209000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buczek Y, Le AD, Wang A, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology. 1999;144:183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- 19.Grimm JW, See RE. Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology. 2000;22:473–479. doi: 10.1016/S0893-133X(99)00157-8. [DOI] [PubMed] [Google Scholar]
- 20.Houston FP, Stevenson GD, McNaughton BL, Barnes CA. Effects of age on the generalization and incubation of memory in the F344 rat. Learn Mem. 1999;6:111–119. [PMC free article] [PubMed] [Google Scholar]
- 21.Gabriel M, Vogt J. Incubation of avoidance CRs in the rabbit produced by increase over time in stimulus generalization to apparatus. Behav Biol. 1972;7:113–125. doi: 10.1016/s0091-6773(72)80193-7. [DOI] [PubMed] [Google Scholar]
- 22.Zakharova E, Malyshkin A, Kashkin V, Neznanova O, Sukhotina I, Danysz W, et al. The NMDA receptor channel blocker memantine and opioid receptor antagonist naltrexone inhibit the saccharin deprivation effect in rats. Behav Pharmacol. 2004;15:273–278. doi: 10.1097/01.fbp.0000137213.85321.8e. [DOI] [PubMed] [Google Scholar]
- 23.Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, et al. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10:478–488. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- 24.Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience. 2003;122:17–20. doi: 10.1016/s0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]