Abstract
Central sensitization is a fundamental mechanism contributing to acute and chronic pain conditions. Our previous studies have documented a glutamatergic-, purinergic- and glial-dependent central sensitization that can be induced in rat medullary dorsal horn (MDH) nociceptive neurons by mustard oil (MO) application to the tooth pulp. The present study demonstrated that carbenoxolone, a potent gap junction and hemichannel blocker, completely blocked all parameters of MO-induced central sensitization tested in functionally identified MDH nociceptive neurons. These results represent the first evidence suggesting that gap junctions and hemichannels may have a critical role in mediating central sensitization in dorsal horn nociceptive neurons and may account for the spread as well as development of central sensitization.
Keywords: carbenoxolone, mustard oil, P2X7 receptor, medullary dorsal horn, nociceptive-specific neuron, astroglia, central sensitization, tooth pulp inflammation
Introduction
Central sensitization is a fundamental mechanism underlying the increased excitability of nociceptive pathways following peripheral tissue injury and inflammation and is reflected in increases in nociceptive neuronal spontaneous activity, mechanoreceptive field (RF) size, and responses to noxious mechanical stimuli and in decreases in mechanical activation threshold [1–2]. The brainstem trigeminal subnucleus caudalis has many morphological and functional similarities with the spinal dorsal horn and indeed is nowadays often termed the medullary dorsal horn (MDH), and previous findings have documented that central sensitization can be induced in MDH nociceptive neurons by application to the tooth pulp of the inflammatory irritant and small-fibre excitant mustard oil (MO) [2–3]. In this acute dental inflammation model, we also documented that glutamatergic and purinergic mechanisms and non-neuronal cells (astroglia and microglia) in MDH are involved in this central sensitization [3–5].
Accumulating evidence indicates that gap junctions and hemichannels mediate cell-to-cell communication and propagation of postsynaptic events in neural networks in the central nervous system (CNS) [6–7]. A characteristic example is the transients and oscillatory waves of cytoplasmic Ca2+ in astroglia elicited by neuronal inputs, glutamate and ATP that may propagate to adjoining and/or distant astroglia through gap junctions and hemichannels [8]. These reactive astroglia then release gliotransmitters such as glutamate and ATP which in turn modulate synaptic transmission [7, 9]. The intercellular Ca2+ wave transmission may involve the cytoplasmic diffusion of Ca2+-mobilizing second messengers through the gap junction channels between astroglia, and extracellular diffusion of ATP released through connexin (Cx43) and pannexin (Panx-1) hemichannels and/or vesicular exocytosis; ATP then acts on purinergic receptors of adjacent and/or distant glia and neurons [10–14].
These findings led us to use electrophysiological techniques to test for a possible role of gap junctions and hemichannels in MDH central sensitization. Carbenoxolone (CBX) is a widely used potent blocker of the major astroglia-to-astroglia and astroglia-to-neuron gap junction and hemichannel protein Cx43 and plasmalemmal channel protein Panx-1 [10, 12–16]. In view of these features and our recent findings indicating the critical involvement of astroglia and purinergic receptor mechanisms in MDH central sensitization (see above), our aim was to test whether continuous intrathecal (i.t.) superfusion of CBX over the medulla can affect the MDH central sensitization induced by MO application to the tooth pulp.
Methods
Detailed descriptions of most of the methods have been reported [3–5], so only a brief outline follows.
Animal preparation
Male adult rats were anesthetized by intraperitoneal α-chloralose (50 mg/kg)/urethane (1 g/kg). The right maxillary first molar pulp was exposed, and covered with a saline-soaked cotton pellet, and the caudal medulla surgically exposed. The rat then received a continuous intravenous (i.v.) infusion of 70% urethane solution (0.2 g/ml) and 30 % pancuronium solution (2 mg/ml) at a rate of 0.3–0.4 ml/h, and was artificially ventilated throughout the whole experimental period. Heart rate, percentage expired CO2, and rectal temperature were continuously monitored. All surgeries and procedures were approved by the University of Toronto Animal Care Committee in accordance with the regulations of the Ontario Animal Research Act (Canada).
Recording and stimulation procedures
The activity of single neurons was recorded by a tungsten microelectrode (5–15 MΩ) in histologically verified sites in MDH (Lateral: 1.5–2.0 mm; Posterior: 1.5–2.0 mm referred to obex). Neuronal responses to stimulation of the orofacial region were amplified and displayed on oscilloscopes and also led to an analog-to-digital converter connected to a computer. Data were analyzed off-line with Spike 2 software (Cambridge Electronic Design, UK).
Mechanical (brush, pressure and pinch) and noxious thermal (radiant heat, 51–53 °C) stimuli were applied to classify nociceptive-specific (NS) neurons in the deep laminae of MDH [3–5]. Each NS neuron’s spontaneous activity was determined over an initial 1-min recording period, its cutaneous orofacial RF was determined with non-serrated forceps, and its activation threshold to mechanical stimuli applied to its RF was assessed by force-monitoring forceps or an electronic von Frey monofilament (Stoelting Co., Wood Dale. Illinois, USA). Its responses to graded pressure or pinch were recorded (25 g, 50 g, 75 g, 100 g and 200 g applied in ascending order, each for 5 s at an interval of >45 s), and the number of spikes evoked by each of these graded stimuli were summed.
Experimental paradigm
Continuous i.t. superfusion over the exposed ipsilateral medulla of either CBX (50 µM; freshly dissolved in phosphate-buffered saline (PBS), at pH 7.4; Sigma-Aldrich, Canada) or PBS (as vehicle control) started soon after surgery at a rate of 0.6 ml/h. At least 1 hour after surgery, recording of NS neuronal activity in MDH began. Two assessments of recorded neuronal properties were carried out in an identified neuron at an interval of 10 min to determine its baseline properties. Thereafter, the saline-soaked cotton pellet covering the exposed pulp was quickly replaced by a segment of a dental absorbent point (Lux & Zwingenberger, Canada) soaked with MO (0.2 µl, allyl isothiocynate, 95%; Aldrich Chemical, U.S.A.), and the exposed pulp promptly sealed with CAVIT (ESPE, Germany). Three minutes after MO application, neuronal properties were repeatedly assessed at 10-min intervals over the next 50 min.
Statistical analyses
Statistical analyses were based on normalized data (in percentage). Differences between baseline values and values at different time points after MO application in each of the 2 groups of neurons in which CBX or PBS was superfused over the medulla were treated by 1-way repeated measures (RM) ANOVA or ANOVA on ranks, followed by Dunnett’s test. Differences between the PBS and CBX groups were treated by 2-way ANOVA followed by Dunnett’s test. For evaluating the effect on neuronal properties of long-time superfusion of drugs, differences in baseline values of RF size, threshold and responses between PBS and CBX groups were treated by t-test. The level of significance was set at P<0.05. All values are presented as mean ± S.E.M.
Results
The recording sites of all 12 NS neurons tested were histologically verified and located in the deep MDH laminae (Fig. 1d). Only 1 NS neuron in the PBS group (n=6) had baseline spontaneous activity (0.07Hz), and this neuron responded abruptly to MO application to the pulp, followed by a short-lasting period of tonic activity (<4 Hz); the other 5 neurons had no responses evoked by MO application and no ensuing spontaneous activity. No spontaneous activity appeared in any neurons in the CBX group (n=6) but 1 of these neurons in this group was excited immediately and transiently (<13 s) after MO application; however, no spontaneous activity appeared thereafter.
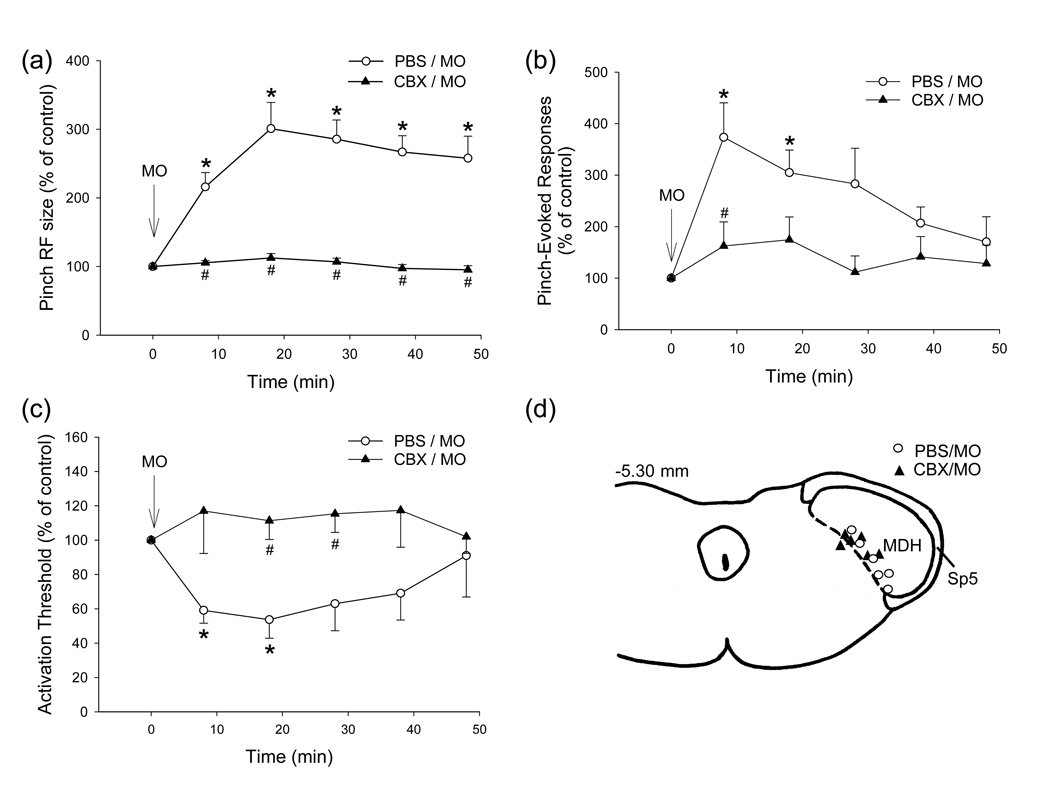
Fig. 1.
(a–c) Time course of MO-induced central sensitization in NS neurons in MDH during continuous i.t. superfusion of PBS (n=6) or CBX (n=6) over medulla. (a) Neuronal pinch/pressure RF. (b) Responses to mechanical stimuli. (c) Mechanical activation threshold. Mean ± SEM values in percentage are shown. *P<0.05 compared with the baseline within the group (RM ANOVA with post-hoc Dunnett’s test); #P<0.05 compared with PBS group at the different time-points tested (two-way ANOVA with post-hoc Dunnett’s test). (d) Histologically confirmed NS neuronal recording sites in MDH. Sp5, trigeminal spinal tract.
MO-induced central sensitization in the control (PBS) group
The baseline values of NS neuronal properties following 170±12 min period of continuous superfusion of PBS are shown in Table 1. Central sensitization became apparent 5–10 min after MO application to the pulp (Fig. 1): MO application significantly increased RF size, decreased mechanical activation threshold and increased pressure- or pinch-evoked responses in all 6 neurons (P<0.02–0.001, 1-way RM ANOVA; Table 1 and Fig. 1a–c). The RF size at all post-MO time-points, and the responses to the graded mechanical stimuli and the activation threshold at 8, and 18 min after MO application were significantly different from the baseline values (P<0.05, Dunnett's test).
Table 1.
Changes in pinch RF size and response properties of NS neurons in MDH induced by MO application to the rat molar tooth pulp
| Treatment | Pinch RF size (cm2) |
Activation threshold (g) |
Responses to graded stimuli (sum of spikes) |
|---|---|---|---|
| PBS/MO experiments (n=6) | |||
| Baseline value | 1.85 ± 0.54 | 97.0 ± 35.5 | 60.7 ± 7.8 |
| Value 18' after MO | 4.89 ± 0.93* | 49.5 ± 16.5* | 129.5 ± 30.8* |
| Value 48' after MO | 4.19 ± 0.81* | 83.8 ± 30.4 | 87.3 ± 45.8 |
| CBX/MO experiments (n=6) | |||
| Baseline value | 0.89 ± 0.07 | 90.7 ± 18.4 | 127.3 ± 34.5 |
| Value 18' after MO | 0.97 ± 0.09 | 91.8 ± 14.8 | 155.2 ± 24.3 |
| Value 48' after MO | 0.84 ± 0.05 | 88.7 ± 19.2 | 126.5 ± 28.2 |
| Statistical comparisons | |||
| PBS/MO vs CBX/MOa | F (1,60)=146.18;P <0.001 | F (1,60)=19.52;P <0.001 | F (1,60)=8.34;P <0.005 |
All values are shown as mean ± S.E.M.
P<0.05 for comparison between baseline value and after MO/pulp values of each indicated time point in each group (RM ANOVA or RM ANOVA on ranks).
2-way ANOVA results for comparison of all time points between PBS/MO and CBX/MO groups.
MO-induced central sensitization in the CBX group
The baseline values of NS neuronal properties following 152±13 min period of continuous i.t. superfusion of CBX are shown in Table 1. This pretreatment did not itself produce any significant changes in the baseline values of pinch RF size, activation threshold, and pinch or pressure-evoked responses (P>0.05 ; t-test; n=6). However, the CBX superfusion completely blocked the MO-induced central sensitization since MO application to the pulp did not produce any significant changes in RF size, mechanical activation threshold and pinch or pressure-evoked responses (P>0.05; 1-way RM ANOVA; Table 1, Fig. 1a–c). In addition, these 3 features in the CBX group were all significantly less than those in the PBS group (all P<0.005–0.001, 2-way ANOVA; Table 1). Post-hoc analysis indicated that there were significant differences in values at different post-MO time-points between these 2 groups (Dunnett’s test; see Fig. 1a–c).
Discussion
Our finding that application of the inflammatory irritant MO to the rat molar pulp can induce MDH central sensitization, as reflected in increases in RF size and responses of nociceptive neurons to mechanical noxious stimuli and the decrease in mechanical activation threshold, is consistent with our previous findings [3–5]. However, this study has also provided the first documentation that i.t. superfusion of CBX completely blocks these parameters of central sensitization, suggesting that gap junctions and/or hemichannels play an important role in mediating central sensitization in the MDH.
CBX has been shown to block both Cx43 gap junctions and hemichannels and P2X7 receptor-associated Panx-1 hemichannels [12, 13, 16]. While more than 6 connexins have been identified in CNS neurons and glia, Cx43 is the most abundant gap junction protein in astroglia [14, 15]. It preferentially exists in a closed state under resting conditions, but its open probability can be increased under appropriate conditions [13, 17]. However recent studies, especially those in Cx43 knock-out animals, indicate that CBX can also block other channels including P2X7 receptor-associated Panx-1 channels in spinal cord astroglia [16]. Panx-1 has been shown to form nonselective, large conductance plasmalemmal channels permeable to ATP in immune cells, neurons and glia [10, 12, 18], and has recently been suggested as an anionic transporter [19]. In cultured cortical astroglia, Panx-1 can be activated by strong depolarization or by P2X7 receptor stimulation, and studies on cultured astroglia from wild and Cx43-null mice suggest that Panx-1, rather than Cx43, is responsible for ATP release from astroglia and is readily blocked by CBX [10, 12, 16]. Additionally, recent in vitro studies have demonstrated that CBX can also modulate astroglial volume-regulated anion channels [20,21], modulate synaptic transmission and neuronal membrane properties [22, 23], and increase reactive oxygen species formation in neurons [24]. Despite the many varied potential effects of CBX, our previous studies indicating an important role of astroglia in MDH sensitization suggest that at least part of the observed CBX-mediated suppression of central sensitization documented in the present study is most likely due to its blocking Cx43 and/or Panx-1 channels in astroglia, although the possibility cannot be ruled out of a contribution from Cx43 or Panx-1 in other cell types (e.g., microglia, neurons) or other actions of CBX.
There is evidence that neuronal-glial communications via gap junctions and paracrine signaling are involved in the capsaicin-evoked peripheral sensitization in the trigeminal ganglion, the site of trigeminal primary afferent cell bodies [25]. It is very unlikely that the CBX effects observed in this study resulted from an action on the trigeminal ganglion since it is located far rostral (over 3 mm) to the MDH recording site and upstream to the superfused medullary area. It is also noteworthy that all MDH nociceptive neurons manifested central sensitization that started 5–10 min following MO application to the pulp and lasted over 40 min, yet most neurons had no immediate responses evoked by the MO application. These spatio-temporal features of MDH central sensitization could have resulted from the intercellular Ca2+ wave-mediated asynchronous excitation of neurons and non-neuronal cells involving mainly astroglial gap junctions [7, 9]; a propagated astroglial Ca2+ wave can travel several hundred µm in tens of seconds [11]. Such events could explain how a MO-evoked excitatory input to only a small subset of MDH nociceptive neurons could result in a more generalized effect reflecting central sensitization in all the other nearby neurons tested in the MDH.
In conclusion, this study has provided the first demonstration in an in vivo preparation that i.t. application to the CNS of a potent gap junction and hemichannel blocker (CBX) can completely block central sensitization in functionally identified nociceptive neurons in central nociceptive pathways. These findings point to novel mechanisms that may be crucially involved in the production and spread of central sensitization that are considered critical in the development of pain and its referral.
Acknowledgements
The authors acknowledge Ms S. Carter for her technical assistance. This study was supported by NIH Grant DE-04786 to B.J.S. and CIHR Grant MOP-82831 to J.O.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Salter MW. Cellular neuroplasticity mechanisms mediating pain persistence. J Orofac Pain. 2004;18:318–324. [PubMed] [Google Scholar]
- 2.Sessle BJ. Trigeminal central sensitization Reviews in Analgesia. 2005;8:85–102. [Google Scholar]
- 3.Chiang CY, Zhang S, Xie YF, Hu JW, Dostrovsky JO, Sessle BJ. Endogenous ATP involvement in mustard oil-induced central sensitization in trigeminal subnucleus caudalis (medullary dorsal horn) J Neurophysiol. 2005;94:1751–1760. doi: 10.1152/jn.00223.2005. [DOI] [PubMed] [Google Scholar]
- 4.Chiang CY, Wang J, Xie YF, Zhang S, Hu JW, Dostrovsky JO, Sessle BJ. Astroglial glutamate-glutamine shuttle is involved in central sensitization of nociceptive neurons in rat medullary dorsal horn. J Neurosci. 2007;27:9068–9076. doi: 10.1523/JNEUROSCI.2260-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang CY, Li Z, Dostrovsky JO, Sessle BJ. Glutamine uptake contributes to central sensitization in the medullary dorsal horn. NeuroReport. 2008;19:1151–1154. doi: 10.1097/WNR.0b013e3283086781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, McCarthy KD. What is the role of astrocyte calcium in neurophysiology? Neuron. 2008;59:932–946. doi: 10.1016/j.neuron.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scemes E, Giaume C. Astrocyte calcium waves: what they are and what they do. Glia. 2006;54:716–725. doi: 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dani JW, Chernjavsky A, Smith SJ. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron. 1992;8:429–440. doi: 10.1016/0896-6273(92)90271-e. [DOI] [PubMed] [Google Scholar]
- 9.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 10.Scemes E, Spray DC, Meda P. Connexins, Pannexins, innexins: novel roles of “hemi-channels”. Pflugers Arch. 2009;457:1207–1226. doi: 10.1007/s00424-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowser DN, Khakh BS. Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes. J Gen Physiol. 2007;129:485–491. doi: 10.1085/jgp.200709780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte "hemichannels". J Neurosci. 2009;29:7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orellana JA, Saez PJ, Shoji KF, Schalper KA, Palacios-Prado N, Velarde V, Giaume C, Bennett MV, Saez JC. Modulation of brain hemichannels and gap junction channels by pro-inflammatory agents and their possible roles in neurodegeneration. Antioxi Redox Signal. 2009;11:369–399. doi: 10.1089/ars.2008.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagy JI, Dudek FE, Rash JE. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res Brain Res Rev. 2004;47:191–215. doi: 10.1016/j.brainresrev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sáez JC, Contreras JE, Bukauskas FF, Retamal MA, Bennett MV. Gap junction hemichannels in astrocytes of the CNS. Acta Physiol Scand. 2003;179:9–22. doi: 10.1046/j.1365-201X.2003.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelegrin P, Surprenant A. Pannexin-1 mediate large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma W, Hui H, Pelegrin P, Surprenant A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther. 2009;328:409–418. doi: 10.1124/jpet.108.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benfenati V, Caprini M, Nicchia GP, Rossi A, Dovizio M, Cervetto C, Nobile M, Ferroni S. Carbenoxolone inhibits volume-regulated anion conductance in cultured rat cortical astroglia. Channels (Austin) 2009;3:323–336. doi: 10.4161/chan.3.5.9568. [DOI] [PubMed] [Google Scholar]
- 21.Ye ZC, Oberheim N, Kettenmann H, Ransom BR. Pharmacological "cross-inhibition" of connexin hemichannels and swelling activated anion channels. Glia. 2009;57:258–269. doi: 10.1002/glia.20754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chepkova AN, Sergeeva OA, Haas HL. Carbenoxolone impairs LTP and blocks NMDA receptors in murine hippocampus. Neuropharmacology. 2008;55:139–147. doi: 10.1016/j.neuropharm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Tovar KR, Maher BJ, Westbrook GL. Direct actions of carbenoxolone on synaptic transmission and neuronal membrane properties. J Neurophysiol. 2009;102:974–978. doi: 10.1152/jn.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zündorf G, Kahlert S, Reiser G. Gap-junction blocker carbenoxolone differentially enhances NMDA-induced cell death in hippocampal neurons and astrocytes in co-culture. J Neurochem. 2007;102:508–521. doi: 10.1111/j.1471-4159.2007.04509.x. [DOI] [PubMed] [Google Scholar]
- 25.Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, Durham PL. Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache. 2007;47:1008–1023. doi: 10.1111/j.1526-4610.2007.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]