Abstract
This study examined the effect of environmental enrichment on sucrose seeking in rats made abstinent from sucrose for 1 month, as measured by response for a tone + light cue previously associated with 10% sucrose self-administration. Rats were either enriched throughout the study (experiment 1) or only after sucrose self-administration training (experiment 2). Enrichment consisted of either housing the rats in pairs or grouping four rats (ENR4) in a large environment, both with novel objects. Controls (CON) were singly housed without novel objects. In experiment 1, ENR4 rats responded less to the sucrose-paired cue versus CON rats, but this difference was not statistically significant. In contrast, the decrease in response of ENR4 rats versus CON rats in experiment 2 was dramatic and significant. These findings, along with findings from other laboratories, support a hypothesis that the enrichment may provide individuals with a greater ability to discriminate the availability of reward. This may impart a decreased vulnerability to relapse behavior. Therefore, these results are relevant to both eating disorder and drug addiction – disorders characterized by relapse.
Keywords: cocaine, craving, eating disorders, enrichment, rat, relapse, sucrose
Introduction
Early education programs, such as Head Start (Shonkoff and Phillips, 2000), were developed for children of low-income families to help in providing an enriched learning experience, normally lacking in their socioeconomic circumstance (Gottlieb and Blair, 2004). To explore the impact of enrichment on behavior and neurobiological indices related to learning and motivation (Nithianantharajah and Hannan, 2006), a simple animal model was developed comparing rats living in isolated or normal (relative term) conditions to those in comparatively ‘enriched’ conditions (Rosenzweig, 2003). In this model, enrichment typically involves more space, greater social contact, and the opportunity to explore novel objects and engage in exercise. Environmental enrichment has repeatedly been shown to increase problem-solving abilities in rats, demonstrated as enhanced performance in the radial arm (Hellemans et al., 2004) and water mazes (Daniel et al., 1999; Pham et al., 1999) and increased efficiency in solving Hebb–Williams problems (Will et al., 1977; Murtha et al., 1990).
Other impacts of lower socio-economic status include drug use (Winstanley et al., 2008) and obesity (Drewnowski et al., 2007). Interestingly, environmental enrichment in rats tends to result in decreased self-administration of amphetamine (Bardo et al., 2001; Green et al., 2002). It is, however, not clear whether there is a positive effect of enrichment on reducing behaviors related to obesity. For example, environmental enrichment decreased sucrose intake in one study (Brenes and Fornaguera, 2008) but increased sucrose self-administration in another, if only temporarily (Bardo et al., 2001). It is possible that enriched rats are more adept at learning and relearning the significance of stimuli paired with reward or punishment. For example, enriched rats showed enhanced place preferences (Bowling and Bardo, 1994; Bardo et al., 1995; Smith et al., 2005) and aversion (Smith et al., 2003), accelerated extinction of conditioned fear (Pietropaolo et al., 2006), and accelerated extinction of lever pressing previously associated with either amphetamine or sucrose (Stairs et al., 2006). Therefore, enriched rats might be at an advantage, from a decreased vulnerability perspective, at avoiding conditioned addiction behaviors such as cue-induced ‘relapses’ characteristic of both eating (Marlatt, 1990) and drug addiction (Gawin, 1991; Childress et al., 1993).
Cue-induced relapse has been modeled using rats (e.g. Meil and See, 1996; Shalev et al., 2002 for review). In these studies, rats first lever press to self-administer a reinforcer that is directly paired with a stimulus (e.g. tone + light). After several training sessions, the reinforcer is removed and the rats respond in the absence of reinforcer and reinforcer-paired cues (extinction). After responding has decreased to low levels, responses were then allowed to produce the reinforcer-paired stimulus (responding for cue). Vigorous responding then ‘reinstates’ to near or above training levels; this is taken as a measure of cue-induced reward seeking and, in some instances, craving (Lu et al., 2004).
Using this procedure, we carried out two experiments to examine the effects of environmental enrichment on sucrose-seeking behavior in rats. In experiment 1, rats were enriched starting on postnatal (PN) day 38 and then for the duration of the experiment. The effects of enrichment on both sucrose self-administration and reinstatement of sucrose seeking after 29 days forced abstinence, were examined. Experiment 1 was designed to be a model of early life interventions on sucrose-seeking behaviors. In experiment 2, enrichment was used as an intervention for adult rats that already had sucrose self-administration experience. That is, rats were enriched for 29 days after 10 days of sucrose self-administration. Reinstatement of sucrose seeking was measured on both day 1 (preenrichment) and day 30 (postenrichment) of forced abstinence. In both the experiments, control (CON) rats were housed in standard conditions (singly housed; no enrichment ‘toys’). It was hypothesized that enrichment would attenuate responding for the sucrose-paired cue in both the experiments.
Methods
Subjects
Subjects were 62 male Long–Evans rats bred in the Western Washington University, Psychology Department, Washington, USA, vivarium. Rats were weighed each Monday, Wednesday, and Friday, immediately before the beginning of self-administration training and thereafter. At the onset of self-administration training, rats in experiment 1 were 9 weeks old, whereas the rats in experiment 2 were approximately 13 weeks old. Whenever possible, the rats were assigned to treatment groups in equal proportions from litters. Rats were maintained on nutritionally complete Mazuri Rodent Pellets (Purina Mills, St Louis, Missouri, USA), and water was provided freely except as noted in General procedures. Pellets and water were also freely available in the operant boxes, except as noted below (General procedures). All rats remained housed in the vivarium except during daily (08.30 h) training or testing sessions when they were brought to the operant boxes. The rats were maintained on a reversed 12 : 12 h light–dark cycle with lights off at 07.00 h. Housing conditions are described below (General procedures). All procedures performed on the rats followed the NIH guidelines for animal care, and were approved by the Western Washington University Animal Care and Use Committee, Washington, USA.
Apparatus
The operant boxes, controlled by a Med Associates (Georgia, Vermont, USA) system, had two levers, but only one lever (an active, retractable lever) activated the infusion pump. Presses on the other lever (an inactive, stationary lever) were also recorded. The 10% sucrose solution was delivered into a liquid drop receptacle for oral consumption (Med Associates). The boxes had four infrared emitters and detectors (Med Associates) aligned in a tic-tac-toe pattern (front beams each 10.5 cm from wall; side beams each 6 cm from the wall) across the operant box, each 4.5 cm above the stainless steel bar floor. The beams were set to count the number of complete breaks. The locomotor activity system was integrated into the Med Associates data collection system.
General procedures
Both experiments included a 10-day training phase and a 29-day forced-abstinence phase, with testing of sucrose cue reactivity on day 30 of forced abstinence in experiment 1 and on days 1 and 30 of forced abstinence in experiment 2. Rats were deprived of water in their home cages 17 h before the first self-administration training session. Water was provided in the home cages after 48 h of deprivation. Water was provided in the operant chambers when the rats learned to respond reliably for sucrose (≥ 20 sucrose deliveries/day). During the training phase (10 days), rats were placed in the operant chambers and allowed to lever press for sucrose. During the forced-abstinence phase (1 or 30 days), rats remained in the vivarium (Enrichment conditions).
On the test day, rats were returned to the operant boxes and allowed to lever press on the previously active lever for 6 h (testing phase: extinction responding) in the absence of a discrete tone + light cue previously associated with sucrose self-administration (See et al., 1999). The rats were then tested during a 1-h session wherein cue presentations were contingent upon active lever presses (Grimm et al., 2000) (testing phase: responding for cue). Lever presses during testing were never reinforced with sucrose.
Training phase
Rats were trained to self-administer sucrose (0.4 ml for experiment 1 and 0.2 ml for experiment 2) delivered into the liquid drop receptacle. For experiment 1, training was conducted during six 1-h components that were separated by 5 min for 10 days under a continuous reinforcement schedule (each lever press was reinforced) with a 40-s timeout after each earned reinforcer. Lever presses were counted during timeouts. In experiment 2, training was conducted in 10 daily 4-h sessions under the same reinforcement contingency, but the sessions were not divided into 1-h components. A maximum of 15 sucrose deliveries were available in each hour of training for experiment 1, but there was no cutoff for experiment 2. At the end of each session, the houselight was turned off and the active lever retracted. If the number of deliveries reached 15 in one of the 1-h sessions (experiment 1), the houselight was turned off and the active lever retracted for the remainder of the hour. We chose a 4-h session with no cutoff for experiment 2 as part of our continuing parametric evaluation of the relationship between training conditions and strength of the ‘incubation of craving’ effect (operationally defined as a length of abstinence-dependent increase in reward seeking; Grimm et al., 2005). The lower volume of sucrose was to ensure that rats given unlimited access would not empty their sucrose syringes. As several of these training variables differed between studies, we did not intend to compare the results of experiments 1 and 2 quantitatively.
In both experiments, the training sessions began with the insertion of the active lever and the illumination of a red houselight that remained on for the entire session. A 5-s tone (2900 Hz, 20 dB above background) and light (7.5 W white light above the active lever) discrete compound cue accompanied each reinforcer delivery.
Enrichment conditions
Two enrichment conditions were present in experiment 1. The first, ENR2, paired rats in double-wide versions of wire bottom CON cages (width 40.6 cm by height 20.3 cm by depth 25cm vs. 20.3 by 20.3 by 25 cm). For reference, these double-wide cages are used in our vivarium to group house rats that have been weaned at PN day 21 (n = 4 per cage). The second enrichment condition, ENR4, had four rats placed in a large wire mesh environment that included a running wheel. ENR4 rats were placed in a moderately large environment (45.7 × 58.4 × 35.6 cm) for the first 20 days (PN days 38–58), and then were transferred to a large cage (83.8 × 88.9 × 45.7 cm) for the duration of the experiment (both cages from Quality Cage Company, Portland, Oregon, USA). The smaller cage was used with the young rats as the mesh was finer. The large cage was used exclusively in experiment 2 for the ENR4 condition. In experiment 2, ENR4 rats began enrichment in the afternoon of the final day of sucrose self-administration and remained enriched for the duration of the study. Rats in experiment 2 were housed singly 2 weeks prior to the beginning of self-administration training.
For the enriched rats (both ENR2 and ENR4), PVC pipe (20.3 cm length, 10.2 cm diameter) was provided at all times and a novel ‘toy’ was exchanged with a used toy every Monday, Wednesday, and Friday. Toys were purchased at a local pet store and included items designed for rodents but also toys typically used for cats and dogs.
Testing phase: extinction responding
On the test day, rats were given six 1-h extinction sessions that were separated by 5 min to reach an extinction criterion of less than 15 responses/1 h on the previously active lever. Three rats (experiment 1 only – one from each treatment condition) were given an additional 1-h extinction session to reach the 15 responses/1 h criterion, as they failed to meet it in six sessions. Each 1-h session began with the introduction of the active lever and illumination of the houselight. The tone + light discrete cue was not present during these sessions. At the end of each session, the houselight was turned off and the active lever retracted. Rats in experiment 1 were tested only on day 30 of forced abstinence, whereas rats in experiment 2 were tested on both days 1 and 30.
Testing phase: responding for cue
The test for cue-induced sucrose seeking consisted of a 1-h session wherein responses on the previously active lever led to the presentation of the tone + light cue on a continuous reinforcement schedule with a 40-s timeout. There was no maximum number of cue deliveries possible. This session started 5 min after the last 1-h extinction session. As with extinction responding, rats in experiment 1 were tested only on day 30 of forced abstinence, whereas rats in experiment 2 were tested on both days 1 and 30.
Testing phase: locomotor activity
Locomotor activity was recorded during all phases of the test procedure.
Cocaine challenge
Differences in locomotor sensitivity to cocaine after forced abstinence could indicate changes in sensitivity of the mesolimbic dopamine system. Therefore, we included a cocaine ‘challenge’ in the present set of experiments. Three days after the day 30 tests, rats were reintroduced to the operant chambers with the active lever retracted and the houselight turned off. Photobeam breaks recording commenced immediately after placing a rat in the chamber. After half an hour, rats were injected with saline (1 ml/kg, intraperitoneally) and immediately returned to the chambers. After half an hour, rats were injected with cocaine (10 mg/kg, intraperitoneally) and immediately returned to the chambers. Activity was measured for one more half an hour session.
Statistical analyses
Body weights
For both experiments, the 20 recorded weight measures were analyzed for change over time and differences between groups using two-way repeated-measures analysis of variance (RM ANOVA) with the factors of time (1–20) and enrichment (CON, ENR2, or ENR4 for experiment 1 and CON or ENR4 for experiment 2).
Training phase
Daily sucrose presentations (infusions), active lever responses, and inactive lever responses were analyzed with separate two-way RM ANOVAs using time (days 1–10 of training) and the between-group factor of enrichment. For experiment 1, this analysis was carried out to examine whether enrichment affected sucrose self-administration behavior, whereas for experiment 2, analysis was carried out to verify that rats subsequently placed into enrichment received the same training as their CON comparison rats.
Testing phase
Data from the full 6-h extinction sessions (extinction responding) and tests for cue-induced sucrose seeking (responding for cue) were analyzed separately for total responses, sucrose-paired cue deliveries, and responses on the inactive lever. Analyses were made using ANOVA in experiment 1 with the enrichment factor and with RM ANOVA in experiment 2 incorporating both days 1 and 30 behaviors as the factor of time versus the between-group factor of enrichment. To verify that rats reliably responded for the sucrose-paired cue, active lever responses in the final hour of extinction responding were compared with active responses in the responding for cue session using RM ANOVA (time by enrichment) for the single test in experiment 1 and for the day 1 or 30 tests in experiment 2.
Cocaine challenge
For both experiments, the three half hour locomotor measures were analyzed using two-way RM ANOVA using the factors of time (habituation, saline challenge, cocaine challenge) and enrichment.
All statistical comparisons were made using SPSS version 15.0 (SPSS Inc., Chicago, Illinois, USA). Post-hoc comparisons were made using the Least Significant Difference method. Group data are presented as mean ± SEM in the text and figures.
Results
F or t values for nonsignificant effects are not indicated except in the case of an apparent trend or to clarify a comparison. Some data were lost in experiment 1 on the cocaine challenge day because of technical problems with the computer and photobeam system. The lowest group ‘n’ in these comparisons was 10.
Experiment 1
Body weight
Body weights increased over time [F(19,817) = 597.4, P < 0.001]. No significant effect of enrichment was observed. Body weight (for CON, ENR2, ENR4, respectively) averaged 311.5 ± 7.1, 301.2 ± 6.9, and 313.6 ± 8.6 g on the first measure and 444.2 ± 6.8, 451.4 ± 4.2, and 458.8 ± 15.1 g on the 20th measure.
Training phase
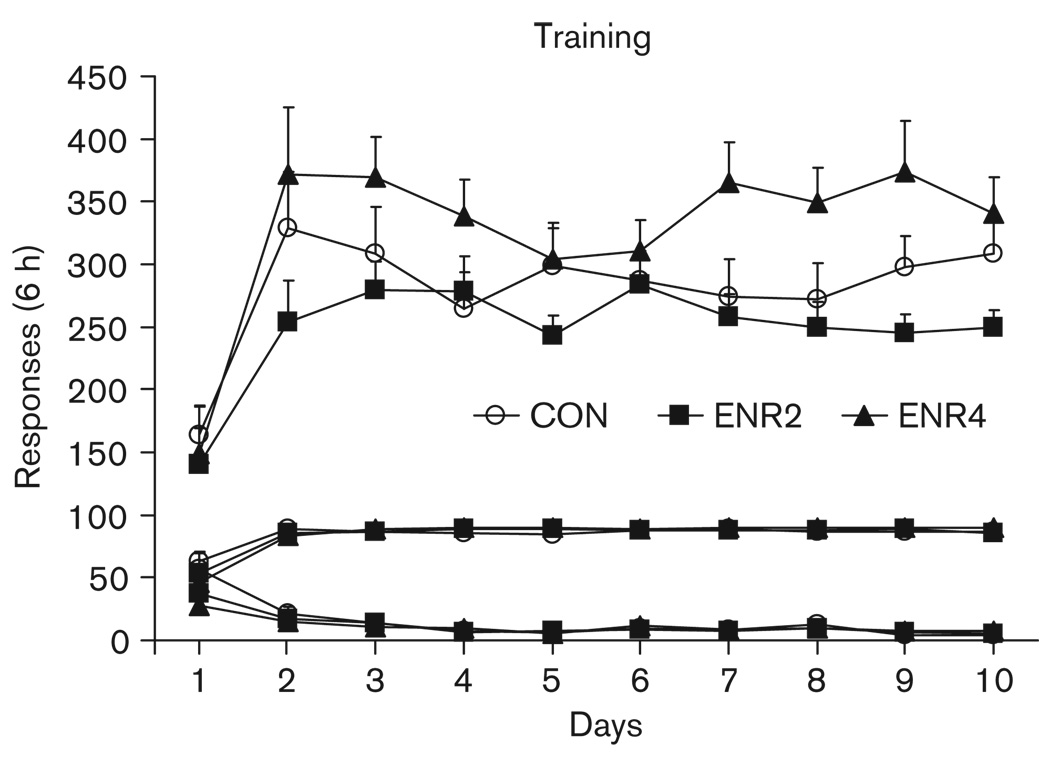
Training data are shown in Fig. 1. Active lever responding increased over 10 days of training, time [F(9,387) = 13.2, P < 0.001], and was higher in the ENR4 versus the ENR2 group, enrichment [F(2,43) = 4.0, P < 0.05] (significant between-group post hoc, P < 0.01). CON group responding did not differ significantly from either enriched group. On the basis of these statistical analyses and Fig. 1, we conclude that the ENR4 rats responded more overall, whereas CON and ENR2 groups responded at fairly similar levels. Sucrose intake also increased over training, time [F(9,387) = 35.8, P < 0.001]. No significant group differences for sucrose intake were observed. Inactive lever pressing decreased over training, time [F(9,387) = 27.8, P < 0.001], and there was a significant time by enrichment interaction [F(18,387) = 2.0, P < 0.01]. The interaction likely stemmed from slightly higher inactive lever responding in the CON group on day 1 of training, although there were no significant post-hoc group differences at that time point.
Fig. 1.
Experiment 1: sucrose self-administration training. Symbols represent means + SEMs, n = 12–18 per group. Active lever responses are top, sucrose deliveries are middle, and inactive lever responses are bottom sets of lines. CON, controls; ENR2, housing the rats in pairs; ENR4, grouping four rats.
Testing phase: extinction responding
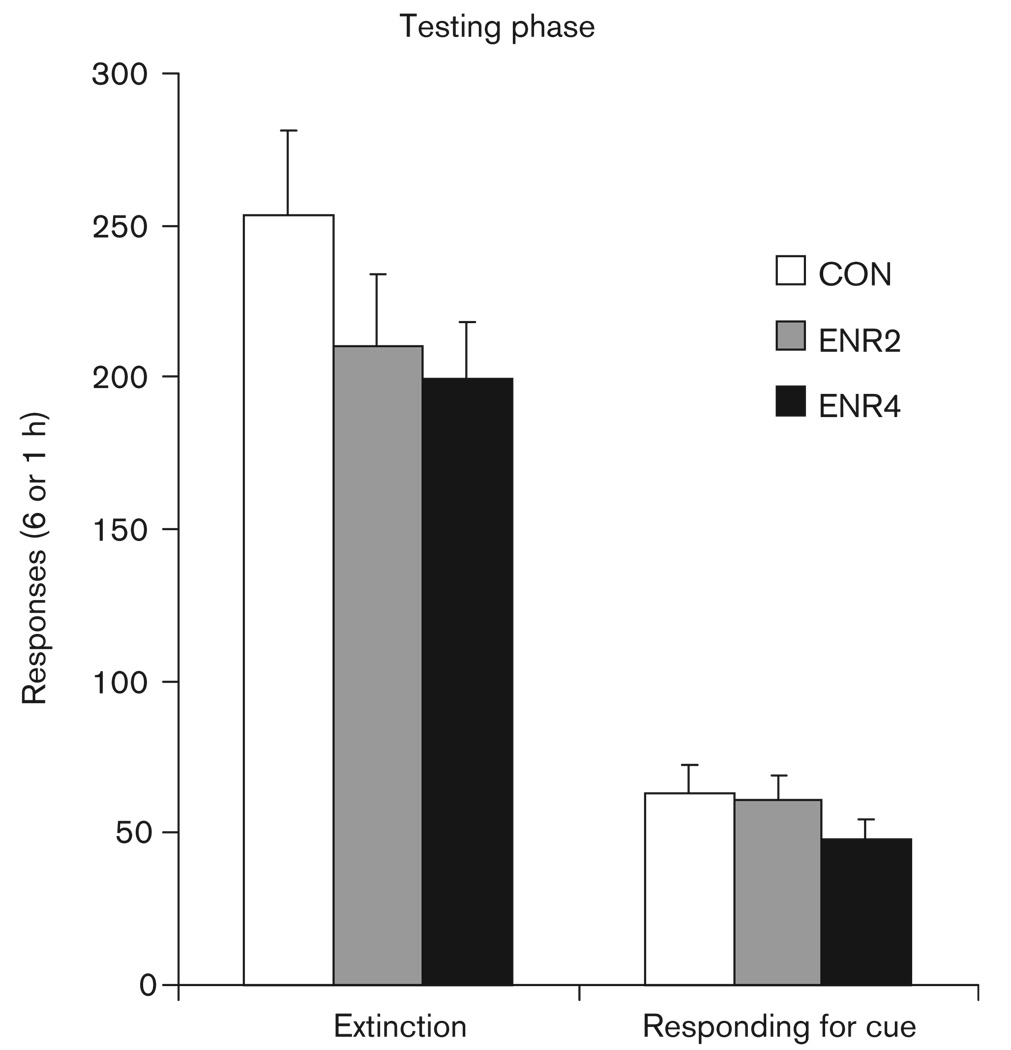
Testing phase data for experiment 1 are shown in Fig. 2. The 6-h active lever responding was generally lower in the ENR4 group (e.g. 199.8 ± 18.7 by ENR4 vs. 253.0 ± 28.6 responses by CON, Fig. 2), but there was no significant effect of enrichment. No significant effect of enrichment on inactive lever responses was observed: these were 14.3 ± 3.5, 10.0 ± 2.7, and 13.9 ± 3.9 responses for CON, ENR2, and ENR4, respectively. There was also no significant effect of enrichment on locomotor activity: photobeam counts were 4640.7 ± 529.4, 4170.6 ± 225.5, and 4920.5 ± 237.8 beam breaks for CON, ENR2, and ENR4, respectively.
Fig. 2.
Experiment 1: testing phase. Bars represent means + SEMs, n = 12–18 per group. Extinction sessions totaled 6 h; the responding for cue session lasted 1 h. CON, controls; ENR2, housing the rats in pairs; ENR4, grouping four rats.
Testing phase: responding for cue
As with 6-h extinction responding, responding for the sucrose-paired cue was generally lower in the ENR4 group (e.g. 47.9 ± 6.4 vs. 62.9 ± 9.3, ENR4 vs. CON, for active lever responding; Fig. 2), but this was not statistically significant (overall ANOVA enrichment effect: P = 0.15). No significant effects of enrichment on cue deliveries, inactive lever responses, or locomotor activity were observed: for CON, ENR2, and ENR4, cue deliveries were 15.4 ± 1.6, 15.7 ± 1.6, and 13.0 ± 1.5; active lever responses were 1.1 ± 0.4, 0.7 ± 0.3, and 1.8 ± 0.4; photobeam break counts were 846.7 ± 114.8, 645.3 ± 81.8, and 770.3 ± 116.5.
The RM ANOVA comparing the final hour of extinction responding (h6) and responding for cue active lever responding indicated that all groups reinstated responding for the sucrose-paired cue, time [F(1,43) = 142.1, P < 0.001], and also that there was no significant effect of enrichment or time by enrichment interaction. As there was an apparent trend when visually inspecting the responding for cue active lever data (Fig. 2), we statistically compared the CON versus ENR4 groups separate from ENR2. This revealed a nonsignificant trend [CON vs. ENR4, t(26) = 1.5, P = 0.08]. We conclude from these subjective and objective analyses that there is some effect of the ENR4 manipulation on cue reactivity for rats raised in an enriched environment; however, it is not robust.
Cocaine challenge
For all groups, locomotor activity decreased over habituation, remained low after saline, and increased after cocaine [time, F(2,58) = 44.8, P < 0.001, all three time points significantly different from each other, P < 0.05]. No significant effect of enrichment was observed. Photobeam break means (CON, ENR2, ENR4) were 661.0 ± 30.3, 678.4 ± 55.1, and 593.3 ± 76.2 for habituation, 412.9 ± 44.3, 420.0 ± 60.3, and 438.3 ± 62.7 for the saline challenge, and 862.6 ± 63.9, 864.8 ± 63.4, and 785.2 ± 86.4 for the cocaine challenge.
Experiment 2
Body weight
Body weights increased over time [F(19,266) = 39.2, P < 0.001]. No significant effect of enrichment was observed. Body weight (CON, ENR4) averaged 431.6 ± 13.1 and 437.3 ± 9.4 g on the first measure and 504.0 ± 11.0 and 501.0 ± 9.2 g on the 20th measure.
Training phase
Active lever responding increased over the 10 days of training, time [F(9,126) = 2.6, P < 0.01], and did not differ significantly between groups. Sucrose intake also increased over training, time [F(9,126) = 5.2, P < 0.001]. No significant group differences for sucrose intake were observed. Inactive lever pressing decreased over training, time [F(9,126) = 11.7, P < 0.001], and did not differ significantly between groups. Average active responses/sucrose deliveries/inactive lever responses over the final 3 days of training were (CON, ENR4) 136.9 ± 9.1/82.4 ± 6.4/3.1 ± 0.6 and 136.0 ± 3.6/85.0 ± 4.4/2.9 ± 0.4.
Testing phase: extinction responding
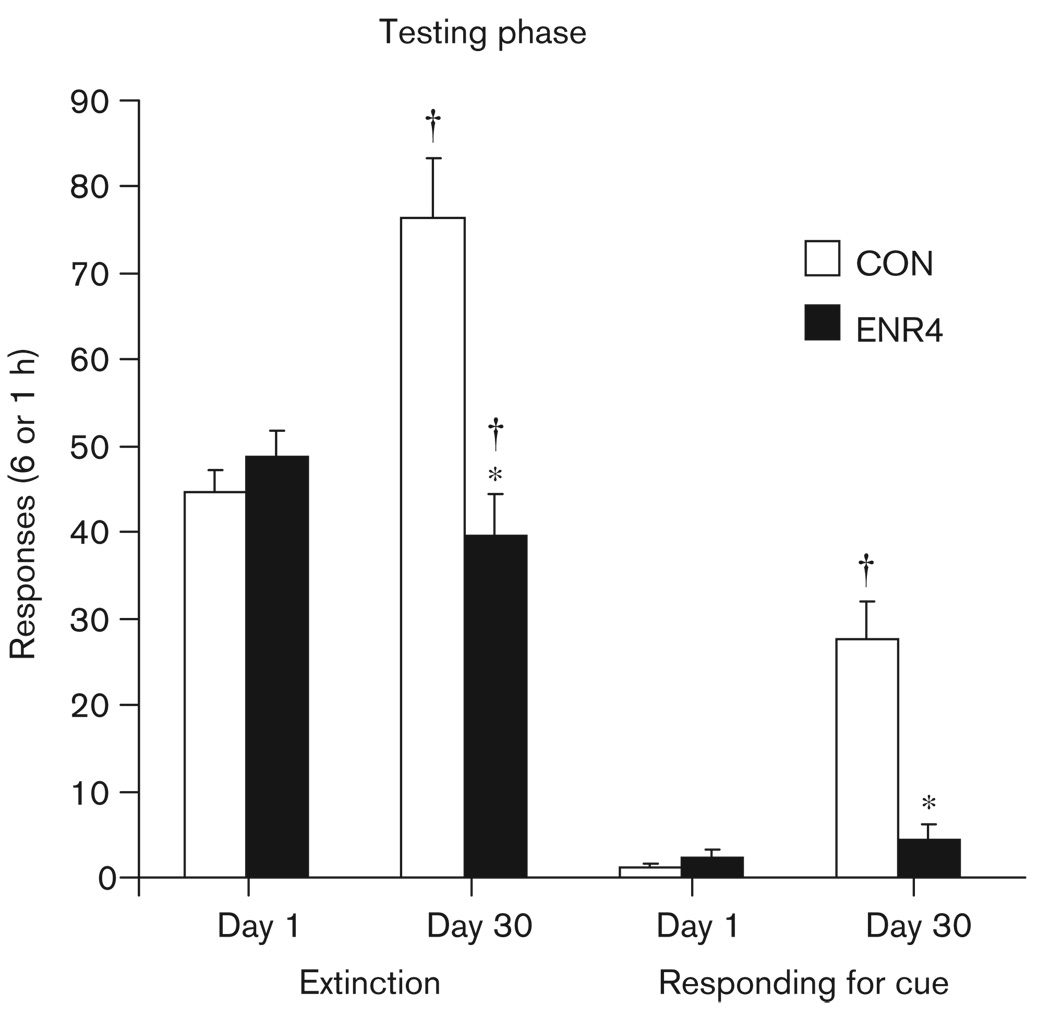
Testing phase data for experiment 2 are shown in Fig. 3. Significant effect of time [F(1,14) = 4.8, P < 0.05], enrichment [F(1,14) = 10.0, P < 0.01], and time by enrichment interaction were observed [F(1,14) = 21.3, P < 0.001]. Post-hoc analyses revealed no significant difference between groups for responding on day 1, but a significant difference for responding on day 30. The CON group responded more. In addition, there was a significant increase in responding by the CON group comparing day 1 with day 30 (day 30 higher), whereas the ENR4 group actually responded less on day 30 versus day 1. Inactive lever responding was greater on day 30 versus day 1, time [F(1,14) = 14.4, P < 0.01]. No significant effects of enrichment on inactive lever responding were observed. Inactive presses means were (CON days 1 and 30; ENR4 days 1 and 30) 6.3 ± 1.7 and 17.6 ± 4.0; 4.4 ± 1.5 and 17.3 ± 5.5. Locomotor activity was similar. It was greater on day 30 versus day 1 [F(1,14) = 5.5, P < 0.05], but was not affected by enrichment. Photobeam break means were (CON days 1 and 30; ENR4 days 1 and 30) 3447.9 ± 286.9 and 4752.1 ± 480.9; 3888.5 ± 289.4 and 4299.3 ± 463.4.
Fig. 3.
Experiment 2: testing phase. Vertical bars represent means + SEMs, *Significant difference from control (CON) group at that time point and in that phase of testing. †Significant difference from same testing phase and group on day 1, P value of less than 0.05, n = 8 per group. Extinction sessions totaled 6 h; the responding for cue session lasted 1 h. ENR2, housing the rats in pairs; ENR4, grouping four rats.
Testing phase: responding for cue
The responding for cue results were similar to the extinction responding results (Fig. 3). For active lever responding, there were significant effects of time [F(1,14) = 40.5, P < 0.001], enrichment [F(1,14) = 47.4, P < 0.01], and a significant time by enrichment interaction [F(1,14) = 29.3, P < 0.001]. Post-hoc analyses revealed no significant difference between groups for responding on day 1, but a significant difference for responding on day 30. The CON group responded more. In addition, there was a significant increase in responding for the CON group comparing day 1 with day 30, but no significant difference in responding for the ENR4 group, comparing day 1 with day 30. The results for the sucrose-paired cue presentations were similar to those of the active lever responding. Significant effects of time [F(1,14) = 24.1, P < 0.001], enrichment [F(1,14) = 15.7, P < 0.01], and a significant time by enrichment interaction [F(1,14) = 38.5, P < 0.001] were observed. Post-hoc analyses revealed no significant difference between groups for responding on day 1, but a significant difference for responding on day 30. The CON group responded more. In addition, there was a significant increase in responding for the CON group comparing day 1 with day 30, but no significant difference in responding for the ENR4 group comparing day 1 with day 30. Cue delivery means were (CON days 1 and 30; ENR4 days 1 and 30) 1.1 ± 0.3 and 9.8 ± 1.6; 2.0 ± 0.5 and 1.0 ± 0.5. Neither the significant effects of time or enrichment on inactive lever responding during the responding for cue sessions, nor the significant effects on locomotor activity were observed. However, there was a trend for locomotor activity to be higher on day 30 versus day 1, regardless of enrichment condition, time [F(1,14) = 4.1, P = 0.06]. Inactive lever press means were (CON days 1 and 30; ENR4 days 1 and 30) 0.4 ± 0.2 and 1.4 ± 0.8; 0.3 ± 0.2 and 0.9 ± 0.4. Photobeam break means were (CON days 1 and 30; ENR4 days 1 and 30) 342.8 ± 61.3 and 589.4 ± 60.6; 532.7 ± 103.4 and 692.5 ± 116.1.
The RM ANOVA comparing h6 and responding for cue active lever responding on day 1 indicated that neither group reinstated responding for the sucrose-paired cue. The factors of time and enrichment were both nonsignificant. In contrast, analysis of active lever responding on day 30 revealed reinstatement of responding for the sucrose-paired cue only in the CON group. This was indicated by significant effects of time [F(1,14) = 19.1, P < 0.01], enrichment [F(1,14) = 20.5, P < 0.001], and a significant time by enrichment interaction [F(1,14) = 21.9, P < 0.001]. Post-hoc analyses indicated h6 of extinction only for the CON group. Active lever responding in the final hour of extinction did not significantly differ between groups.
Cocaine challenge
The results of the cocaine challenge for experiment 2 were very similar to those for experiment 1. For all groups, locomotor activity decreased over habituation, remained low after saline, and increased after cocaine, time [F(2,28) = 11.4, P < 0.001], all three time points differed from each other (P < 0.05). Photobeam break means were (CON; ENR4) 617.9 ± 69.8; 542.6 ± 58.4 for habituation, 377.4 ± 27.4; 329.0 ± 71.3 for the saline challenge, and 565.1 ± 70.9; 517.3 ± 58.7 for the cocaine challenge. No significant effect of enrichment was observed.
Discussion
Experiment 1: enrichment before sucrose self-administration
Enrichment did not have a consistent effect on sucrose intake, although ENR4 rats maintained the highest response rates for sucrose (Fig. 1). ENR4 rats seemed to respond less in the extinction and responding for cue components on the test day compared with CON rats (Fig. 2), but there was no significant effect of enrichment. Between all three groups, locomotor activity was similar both before and in response to cocaine.
Experiment 1 was similar to many enrichment studies where enrichment was initiated early postweaning and through testing. In contrast to one earlier report (Bardo et al., 2001), we did not find an increase in sucrose intake by the enriched animals. Even then, this report (Bardo et al., 2001) described only a transient increase in sucrose intake by enriched rats, and another report (Brenes and Fornaguera, 2008) reported that enriched rats actually drank less sucrose solution than isolated rats. Many possible differences for the lack of effect are found in this study, one most likely being the maximum of 15 sucrose deliveries/hour. This imposed a ceiling on intake for our rats.
What was most salient in this experiment was the observation that ENR4 rats responded generally less in extinction and for the sucrose-paired cue than CON rats. Although the robustness of the enrichment effect on cue reactivity was not great in experiment 1, we felt there was a definite suggestion that the ENR4 had more potential for reducing sucrose cue reactivity. Therefore, experiment 2 was designed to use the ENR4 procedure exclusively.
Experiment 2: enrichment after sucrose self-administration
In contrast to experiment 1, enrichment in experiment 2 had a dramatic and statistically significant effect on both extinction responding and responding for cue behaviors (Fig. 3). As observed in experiment 1, locomotor behavior both before and after cocaine was similar between conditions.
We were surprised to find that responding for cue behavior was nonexistent on the day 1 test for all rats tested (Fig. 3). We have found small but significant increases in responding for cue versus the final hour of extinction on day 1 in all our relapse studies to date. The main difference in experiment 2 was that we used a 4 h/day ‘unlimited’ self-administration period. As noted in Methods, this was done as part of our continuing evaluation of factors that contribute to cue reactivity and especially to the incubation of craving. This procedure contrasts with the majority of our earlier studies (other than Grimm et al., 2007) having either 2 or 6 h/day periods that are broken into 1-h components with a maximum of 15 sucrose deliveries per hour. It is not clear from this study whether the volume of the sucrose delivery, overall time in the chamber, or presence/absence of a maximum intake has the most effect on cue reactivity.
The minimal cue reactivity on day 1 did provide a clear baseline for examining the effects of enrichment on the incubation of sucrose craving. That is, it was clear that only CON rats responded more for the sucrose-paired cue on day 30 versus day 1 (Fig. 3). These findings complement the findings of Stairs et al. (2006), who reported faster extinction of previously amphetamine or sucrose-reinforced responding in enriched animals over several daily tests. This study extends these findings to responding for a discrete reward-paired cue, and additionally shows how an enrichment ‘intervention’ can block the incubation of sucrose craving (Grimm et al., 2005).
General discussion
These positive results support the hypothesis that the ENR4 rats have a better understanding of their environment because of enhanced learning ability. This hypothesis fits with the long emerging picture of enriched rats having enhanced ability to learn not only about their environment (Will et al., 1977; Murtha et al., 1990; Daniel et al., 1999; Pham et al., 1999), but also of the value of reinforcers. For example, enriched rats show reduced impulsivity in nose poking for sucrose (Wood et al., 2006), and show less anticipatory behavior in response to a cue-signaling sucrose availability (Van der Harst et al., 2003), enhanced conditioned place avoidance for spiradoline (κ agonist) (Smith et al., 2003), enhanced conditioned place preference for some µ opiate agonists (Smith et al., 2005) and amphetamine (Bowling and Bardo, 1994; Bardo et al., 1995), and accelerated extinction of lever pressing previously reinforced with amphetamine or sucrose (Stairs et al., 2006). Even more, enriched rats respond less for a novel light stimulus (Cain et al., 2006). Overall, enriched animals seem to better discriminate whether a stimulus is rewarding or not.
In general, the effect of enriching early PN or in adulthood was similar, albeit more robust in adulthood. Other than this dramatic effect of enrichment on responding for cue in experiment 2, the most salient feature of the experiment was the fact that it was an ‘intervention’ approach. That is, experiment 2 was less like typical enrichment studies in that rats were housed in normal conditions until adulthood and then placed into enriched conditions temporarily. A handful of studies have used variants of this approach and have observed positive effects of temporary enrichment in various learning paradigms (Rosenzweig et al., 1962; Will et al., 1977; Alexander et al., 1981; Murtha et al., 1990; Hellemans et al., 2004; Elliott and Grunberg, 2005; for a review of early work on this topic, see Rosenzweig, 2003). The enrichment-mediated decrease in cue reactivity in experiment 2 was especially striking in that it might be assumed that the longer enrichment in experiment 1 would have produced a greater effect. One potential characteristic of experiment 2 that might have led to overall greater ‘enrichment’ was the relative novelty of the procedure for the rats. The enriched rats in experiment 1 had been enriched for a longer period of time, and may have habituated to the novelty of being enriched.
In contrast to the enrichment-mediated effects, we observed that enrichment had no effect on either basal or cocaine-induced locomotor activity. Both these findings are counter to what has been described previously. For example, several reports indicated reduced locomotor activity by enriched rats in a novel environment (Bowling et al., 1993; Hellemans et al., 2004; Neugebauer et al., 2004; Zhu et al., 2004; Del Arco et al., 2007; Segovia et al., 2008), but a greater response to amphetamine or the dopamine reuptake blocker GBR 12935 versus this reduced baseline activity compared with nonenriched animals (Bowling et al., 1993; Bardo et al., 1995; Zhu et al., 2004). One factor that differs between our study and these earlier studies is how we measured locomotor activity. We measured activity in the operant chambers in which rats were exposed to repeatedly as opposed to earlier studies in which locomotor activity was assessed in a novel environment. It could be that any difference in locomotion related to enrichment was masked in our study by repeated habituation to the testing environment. Another, perhaps more likely, scenario was that our enrichment procedure selectively favored a reduction in cue reactivity, as opposed to psychomotor activation. Our enrichment procedure was relatively moderate compared with that used in earlier studies. That is, our most ‘successful’ enrichment protocol required cohousing of four animals with handling and toy changes three times a week whereas, for example, Bardo and colleagues cohoused nine animals with daily handling and toy changes (Stairs et al., 2006). It could be that following the more ‘enriched’ enrichment procedure of Bardo and others would have resulted in locomotor changes in our animals. The fact that we did observe motivational changes in our enriched animals does point to the effectiveness of a less-intense enrichment protocol. This novel finding fits with a conception of enrichment-mediated motivational changes occurring along a ‘gradient’. That is, the enrichment intensity of our procedure might be seen to fall somewhere between exposure to brief novelty before or during an operant procedure (Klebaur et al., 2001) and the relatively greater social and environmental stimulation provided by Stairs et al. (2006).
We also did not observe any effect of enrichment on body weight. This was in contrast to two studies indicating lower body weights from enrichment (Hellemans et al., 2004; Zaias et al., 2008) but consistent with others indicating no effect of enrichment on body weight (Huck and Price, 1975; Carughi et al., 1989). We did notice that in both experiments the ENR4 rats had noticeably leaner body morphology. The ENR4 rats likely had a higher ratio of muscle to fat, although this was not examined systematically. Examination of body fat composition is a consideration for future studies. A likely contribution to this particular effect of enrichment was the types of behaviors the enriched animals engaged in. That is, the behavior of the ENR4 animals was more ‘wild’. Specifically, these animals were actually difficult to gather for testing and weighing, whereas the CON rats, and to some extent the ENR2 rats, were mostly docile. The more ‘extroverted’ behavior of the ENR4 rats leads us to speculate that although exercise may have been a factor in their dampened cue reactivity, the running wheel was likely not the cause of it. We observed minimal running activity by our rats despite the fact that we observed them in the dark part of their light cycle. The ENR4 rats were, however, very active in other ways (climbing, wrestling, chasing each other – more social behaviors). Alternately, as stated above with regard to locomotor activity, the lack of enrichment-induced body weight change could also have been because of the ‘less enriched’ enrichment procedure we used.
Finally, although this study was not designed to clearly establish neurobiological substrates of the enrichment effects on responding for rewarding stimuli, there are published studies that indicate potential roles for mesolimbic and mesocortical dopamine and glutamate (Bowling et al., 1993; Neugebauer et al., 2004; Zhu et al., 2004; Del Arco et al., 2007; Rahman and Bardo, 2008). We expected that if mesolimbic dopamine were heavily impacted by enrichment, we would have observed basal locomotor differences and/or cocaine-induced locomotor differences. Neither effect was observed. Methodological issues were likely critical, as discussed previously (e.g. locomotor measures were not in a novel environment). It is, however, clear that other transmitters as targets and/or other behavioral probes may be necessary to elucidate the neurobiological substrates of the enrichment effects observed in this study.
Concluding remarks
Most data from studies examining the effects of enrichment on appetitive or aversive learning supports a working hypothesis that enrichment does not change perception of incentive value of stimuli just by decreasing brain reward threshold. Rather, enrichment additionally imparts greater ability to make a distinction between reward availability and nonavailability. This ability, supported by the present data, results in a decreased vulnerability to relapse by enriched animals. This led to a dramatically attenuated incubation of craving for sucrose in this study. This is the first example of a behavioral, versus pharmacological (Grimm et al., 2007; Uejima et al., 2007), intervention that selectively attenuates reward seeking after protracted abstinence from self-administration. Therefore, these results have implications for disorders characterized by relapse, such as obesity and drug addiction. As enrichment leads to a marked decrease in reactivity to sucrose-paired cues, these results support the inclusion of enrichment as a therapeutic tool in the treatment of obesity.
Acknowledgements
The authors dedicate this study to the late Dr Lanny ‘Bip’ Sokol. This study was supported by NIDA/NIH Grant R15 DA016285-01-S1 and Western Washington University.
References
- Alexander BK, Beyerstein BL, Hadaway PF, Coambs RB. Effect of early and later colony housing on oral ingestion of morphine in rats. Pharmacol Biochem Behav. 1981;15:571–576. doi: 10.1016/0091-3057(81)90211-2. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bowling SL, Rowlett JK, Manderscheid P, Buxton ST, Dwoskin LP. Environmental enrichment attenuates locomotor sensitization, but not in vitro dopamine release, induced by amphetamine. Pharmacol Biochem Behav. 1995;51:397–405. doi: 10.1016/0091-3057(94)00413-d. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Bowling SL, Bardo MT. Locomotor and rewarding effects of amphetamine in enriched, social, and isolate reared rats. Pharmacol Biochem Behav. 1994;48:459–464. doi: 10.1016/0091-3057(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology. 1993;32:885–893. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- Brenes JC, Fornaguera J. Effects of environmental enrichment and social isolation on sucrose consumption and preference: associations with depressive-like behavior and ventral striatum dopamine. Neurosci Lett. 2008;436:278–282. doi: 10.1016/j.neulet.2008.03.045. [DOI] [PubMed] [Google Scholar]
- Cain ME, Green TA, Bardo MT. Environmental enrichment decreases responding for visual novelty. Behav Process. 2006;73:360–366. doi: 10.1016/j.beproc.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carughi A, Carpenter KJ, Diamond MC. Effect of environmental enrichment during nutritional rehabilitation on body growth, blood parameters and cerebral cortical development of rats. J Nutr. 1989;119:2005–2016. doi: 10.1093/jn/119.12.2005. [DOI] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Daniel JM, Roberts SL, Dohanich GP. Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiol Behav. 1999;66:11–20. doi: 10.1016/s0031-9384(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Canales JJ, Garrido P, de Blas M, Garcia-Verdugo JM, Mora F. Environmental enrichment reduces the function of D1 dopamine receptors in the prefrontal cortex of the rat. J Neural Transm. 2007;114:43–48. doi: 10.1007/s00702-006-0565-8. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Rehm CD, Solet D. Disparities in obesity rates: analysis by ZIP code area. Soc Sci Med. 2007;65:2458–2463. doi: 10.1016/j.socscimed.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott BM, Grunberg NE. Effects of social and physical enrichment on open field activity differ in male and female Sprague-Dawley rats. Behav Brain Res. 2005;165:187–196. doi: 10.1016/j.bbr.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Gottlieb G, Blair C. How early experience matters in intellectual development in the case of poverty. Prev Sci. 2004;5:245–252. doi: 10.1023/b:prev.0000045358.12782.6b. [DOI] [PubMed] [Google Scholar]
- Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology (Berl) 2002;162:373–378. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Kruzich PJ, See RE. Contingent access to stimuli associated with cocaine self-administration is required for reinstatement of drug-seeking behavior. Psychobiology. 2000;28:383–386. [Google Scholar]
- Grimm JW, Fyall AM, Osincup DP. Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiol Behav. 2005;84:73–79. doi: 10.1016/j.physbeh.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Manaois M, Osincup D, Wells B, Buse C. Naloxone attenuates incubated sucrose craving in rats. Psychopharmacology (Berl) 2007;194:537–544. doi: 10.1007/s00213-007-0868-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Benge LC, Olmstead MC. Adolescent enrichment partially reverses the social isolation syndrome. Brain Res Dev Brain Res. 2004;150:103–115. doi: 10.1016/j.devbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Huck UW, Price EO. Differential effects of environmental enrichment on the open-field behavior of wild and domestic Norway rats. J Comp Physiol Psychol. 1975;89:892–898. doi: 10.1037/h0077160. [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Phillips SB, Kelly TH, Bardo MT. Exposure to novel environmental stimuli decreases amphetamine self-administration in rats. Exp Clin Psychopharmacol. 2001;9:372–379. [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47 Suppl 1:214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Marlatt GA. Cue exposure and relapse prevention in the treatment of addictive behaviors. Addict Behav. 1990;15:395–399. doi: 10.1016/0306-4603(90)90048-3. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- Murtha S, Pappas BA, Raman S. Neonatal and adult forebrain norepinephrine depletion and the behavioral and cortical thickening effects of enriched/impoverished environment. Behav Brain Res. 1990;39:249–261. doi: 10.1016/0166-4328(90)90031-9. [DOI] [PubMed] [Google Scholar]
- Neugebauer NM, Cunningham ST, Zhu J, Bryant RI, Middleton LS, Dwoskin LP. Effects of environmental enrichment on behavior and dopamine transporter function in medial prefrontal cortex in adult rats prenatally treated with cocaine. Brain Res Dev Brain Res. 2004;153:213–223. doi: 10.1016/j.devbrainres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- Pham TM, Soderstrom S, Winblad B, Mohammed AH. Effects of environmental enrichment on cognitive function and hippocampal NGF in the non-handled rats. Behav Brain Res. 1999;103:63–70. doi: 10.1016/s0166-4328(99)00019-4. [DOI] [PubMed] [Google Scholar]
- Pietropaolo S, Feldon J, Alleva E, Cirulli F, Yee BK. The role of voluntary exercise in enriched rearing: a behavioral analysis. Behav Neurosci. 2006;120:787–803. doi: 10.1037/0735-7044.120.4.787. [DOI] [PubMed] [Google Scholar]
- Rahman S, Bardo MT. Environmental enrichment increases amphetamine-induced glutamate neurotransmission in the nucleus accumbens: a neurochemical study. Brain Res. 2008;1197:40–46. doi: 10.1016/j.brainres.2007.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig MR. Effects of differential experience on the brain and behavior. Dev Neuropsychol. 2003;24:523–540. doi: 10.1080/87565641.2003.9651909. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Krech D, Bennett EL, Diamond MC. Effects of environmental complexity and training on brain chemistry and anatomy: a replication and extension. J Comp Physiol Psychol. 1962;55:429–437. doi: 10.1037/h0041137. [DOI] [PubMed] [Google Scholar]
- See RE, Grimm JW, Kruzich PJ, Rustay N. The importance of a compound stimulus in conditioned drug-seeking behavior following one week of extinction from self-administered cocaine in rats. Drug Alcohol Depend. 1999;57:41–49. doi: 10.1016/s0376-8716(99)00043-5. [DOI] [PubMed] [Google Scholar]
- Segovia G, Del Arco A, de Blas M, Garrido P, Mora F. Effects of an enriched environment on the release of dopamine in the prefrontal cortex produced by stress and on working memory during aging in the awake rat. Behav Brain Res. 2008;187:304–311. doi: 10.1016/j.bbr.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shonkoff J, Phillips D. From Neurons to Neighborhoods: the science of early child development. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- Smith MA, Bryant PA, McClean JM. Social and environmental enrichment enhances sensitivity to the effects of kappa opioids: studies on antinociception, diuresis and conditioned place preference. Pharmacol Biochem Behav. 2003;76:93–101. doi: 10.1016/s0091-3057(03)00189-8. [DOI] [PubMed] [Google Scholar]
- Smith MA, Chisholm KA, Bryant PA, Greene JL, McClean JM, Stoops WW, Yancey DL. Social and environmental influences on opioid sensitivity in rats: importance of an opioid’s relative efficacy at the mu-receptor. Psychopharmacology (Berl) 2005;181:27–37. doi: 10.1007/s00213-005-2218-2. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Klein ED, Bardo MT. Effects of environmental enrichment on extinction and reinstatement of amphetamine self-administration and sucrose-maintained responding. Behav Pharmacol. 2006;17:597–604. doi: 10.1097/01.fbp.0000236271.72300.0e. [DOI] [PubMed] [Google Scholar]
- Uejima JL, Bossert JM, Poles GC, Lu L. Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of sucrose craving in rats. Behav Brain Res. 2007;181:292–296. doi: 10.1016/j.bbr.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Van der Harst JE, Baars AM, Spruijt BM. Standard housed rats are more sensitive to rewards than enriched housed rats as reflected by their anticipatory behaviour. Behav Brain Res. 2003;142:151–156. doi: 10.1016/s0166-4328(02)00403-5. [DOI] [PubMed] [Google Scholar]
- Will BE, Rosenzweig MR, Bennett EL, Hebert M, Morimoto H. Relatively brief environmental enrichment aids recovery of learning capacity and alters brain measures after postweaning brain lesions in rats. J Comp Physiol Psychol. 1977;91:33–50. doi: 10.1037/h0077306. [DOI] [PubMed] [Google Scholar]
- Winstanley EL, Steinwachs DM, Ensminger ME, Latkin CA, Stitzer ML, Olsen Y. The association of self-reported neighborhood disorganization and social capital with adolescent alcohol and drug use, dependence, and access to treatment. Drug Alcohol Depend. 2008;92:173–182. doi: 10.1016/j.drugalcdep.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DA, Siegel AK, Rebec GV. Environmental enrichment reduces impulsivity during appetitive conditioning. Physiol Behav. 2006;88:132–137. doi: 10.1016/j.physbeh.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Zaias J, Queeney TJ, Kelley JB, Zakharova ES, Izenwasser S. Social and physical environmental enrichment differentially affect growth and activity of preadolescent and adolescent male rats. J Am Assoc Lab Anim Sci. 2008;47:30–34. [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Green T, Bardo MT, Dwoskin LP. Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res. 2004;148:107–117. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]