Abstract
Disruption of the GABAergic system has been implicated in multiple developmental disorders, including epilepsy, autism spectrum disorder and schizophrenia. The human gene encoding uPAR (PLAUR) has been shown recently to be associated with the risk of autism. The uPAR-/- mouse exhibits a regionally selective reduction in GABAergic interneurons in frontal and parietal regions of the cerebral cortex as well as in the CA1 and dentate gyrus subfields of the hippocampus. Behaviorally, these mice exhibit increased sensitivity to pharmacologically-induced seizures, heightened anxiety, and atypical social behavior. Here, we explore potential alterations in GABAergic circuitry that may occur in the context of altered interneuron development. Analysis of gene expression for 13 GABAA receptor subunits using quantitative real-time PCR indicates seven subunit mRNAs (α1, α2, α3, β2, β3, γ2S and γ2L) of interest. Semi-quantitative in situ hybridization analysis focusing on these subunit mRNAs reveals a complex pattern of potential gene regulatory adaptations. The levels of α2 subunit mRNAs increase in frontal cortex, CA1 and CA3, while those of α3 decrease in frontal cortex and CA1. In contrast, α1 subunit mRNAs are unaltered in any region examined. β2 subunit mRNAs are increased in frontal cortex whereas β3 subunit mRNAs are decreased in parietal cortex. Finally, γ2S subunit mRNAs are increased in parietal cortex while γ2L subunit mRNAs are increased in the dentate gyrus, potentially altering the γ2S:γ2L ratio in these two regions. For all subunits, no changes were observed in forebrain regions where GABAergic interneuron numbers are normal. We propose that disrupted differentiation of GABAergic neurons specifically in frontal and parietal cortices leads to regionally-selective alterations in local circuitry and subsequent adaptive changes in receptor subunit composition. Future electrophysiological studies will be useful in determining how alterations in network activity in the cortex and hippocampus relate to the observed behavioral phenotype.
Keywords: neocortex, hippocampus, interneurons, neurodevelopmental disorders
The formation of appropriate inhibitory circuits during telencephalic development underlies the fine-tuning of sensory maps - through the regulation of the timing of the critical period - and the expression of normal behavior (Fagiolini et al., 2004, Hensch and Stryker, 2004). This requires a functional interaction during development between presynaptic elements, comprised of GABAergic interneurons, and postsynaptic elements, comprised of GABAA receptor subunits. Subtle disruptions in the differentiation of GABAergic interneurons, receptor subunit combinations, and the maturation of inhibitory innervation can result in significant functional alterations, such as occurs in epilepsy, schizophrenia and autism spectrum disorders (ASD; Levitt et al., 2004, Guidotti et al., 2005, Lewis et al., 2005, Eagleson et al., 2010). Recently, the plasminogen activator, urokinase receptor (PLAUR) gene was identified as an autism risk gene in a large genetic association study (Campbell et al., 2008) and we have previously described GABAergic interneuron deficits in the cerebral cortex of adult mice with a genetic deletion of the mouse homolog, the urokinase-type plasminogen activator receptor (uPAR) gene (Powell et al., 2003, Eagleson et al., 2005). From a mechanistic standpoint, upon binding of urokinase plasminogen activator, uPAR interacts with other proteins, including integrins and certain G-protein coupled receptors, to modulate cell migration, adhesion, proliferation and differentiation (reviewed in Binder et al., 2007). There are significant reductions in GABAergic interneurons in the adult uPAR-/- mouse, which are restricted to specific regions of neocortex (frontal and parietal). These reductions can be observed as early as postnatal day (P) 21 (Eagleson et al., 2005). The parvalbumin subpopulation is affected preferentially, with the other interneuron subclasses remaining intact. Of the other telencephalic regions examined (all amygdala subnuclei and striatum), only the adult hippocampus shows a loss of GABAergic interneurons, although the somatostatin, rather than the parvalbumin, subpopulation contributes to this loss. Behavioral analyses demonstrate that adult uPAR-/- mice exhibit alterations in anxiety and social behavior, as well as a high susceptibility to pharmacologically-induced convulsions (Powell et al., 2003, Levitt, 2005). Specifically, the performance of uPAR-/- mice in three behavioral paradigms is consistent with increased anxiety; thus, these mice spend significantly less time on the light side in a light-dark avoidance task, very little time in the open arms of an elevated plus maze and decreased time in the center of an open arena. In a modified version of the resident intruder task, uPAR-/- mice spend half the time interacting with a conspecific (assessed as time spent sniffing and in body contact) as do their wild type counterparts. Finally, when challenged with a single threshold dose of PTZ, all uPAR-/- mice tested exhibit motor convulsions, with the majority displaying full tonic extension. In contrast, only half of the wild type mice tested exhibit signs of motor seizure, with none progressing to tonic extension.
In the telencephalon, as in the rest of the central nervous system, fast inhibitory neurotransmission is mediated principally by GABAA receptors, which are chloride-selective ion channels composed of five subunits. The specific subunit composition of each receptor is the main determinant of its physiological and pharmacological properties. Nineteen subunits encoded by distinct genes have been identified in mammals, although those including α1β2/3γ2, α2β3γ2, or α3β3γ2 are the most prevalent in the brain (reviewed in Mohler, 2006). In addition, the diversity of GABAA receptors is increased by alternative splicing of some subunits, including the γ2 subunit (Whiting et al., 1990). From a clinical standpoint, GABAA receptors are the targets of multiple classes of therapeutic agents, such as benzodiazepines, that mediate their anxiolytic, muscle relaxant, sedative-hypnotic, amnesic, and anticonvulsant properties. Changes in GABAA receptor subunit expression have been observed under a variety of experimental and pathological conditions, including chronic ethanol exposure, temporal lobe epilepsy and schizophrenia (Akbarian et al., 1995, Brooks-Kayal et al., 1998, Bouilleret et al., 2000, Sanna et al., 2003, Nishimura et al., 2005, Evans et al., 2006, Hashimoto et al., 2007). While there are now a large number of animal models in which genes responsible for interneuron development have been studied (Roy et al., 2002, Carretta et al., 2004, Cobos et al., 2005, Carmona et al., 2006, Yabut et al., 2007, Andrews et al., 2008, Muller Smith et al., 2008, Wallis et al., 2008, Barber et al., 2009, Batista-Brito et al., 2009, Bond et al., 2009, Canty et al., 2009, Ibi et al., 2009, Mao et al., 2009, Price et al., 2009, Sakata et al., 2009), the impact of altered interneuron development on the long-term expression of GABAA receptor subunits remains to be elucidated. Here, we assess the possibility of GABAA receptor subunit adaptations in the adult male uPAR-/- mouse telencephalon, in the context of altered interneuron development through genetic manipulation.
Experimental Procedures
Animals
C57Bl/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). The uPAR-/- mouse line was obtained originally from P. Carmeliet (Center for Transgene Technology and Gene Therapy, Flanders Interuniversity Institute for Biotechnology, Leuven, Belgium). The line arrived in our colony on a mixed 129/Sv:C57Bl/6 background and was subsequently back-crossed on the C57Bl/6 strain for >10 generations. Animals were provided free access to food and water and housed in a 12 hour light:dark cycle. All research procedures using mice were approved by the Institutional Animal Care and Use Committee at Vanderbilt University and conformed to NIH guidelines. All wild type and null mice used in this study were generated using a heterozygous by heterozygous breeding strategy. Only male mice were analyzed as alterations in GABAA receptor subunit expression have been noted at different stages of the estrous cycle (Diaz-Veliz et al., 2000, Jorge et al., 2002, Maguire et al., 2005). Genotyping was performed by polymerase chain reaction (PCR) as described previously (Eagleson et al., 2005), using mouse tail genomic DNA as a template.
Real-time PCR assays
As an initial evaluation of potential alterations in the repertoire of GABAA receptor subunits in discrete areas of the uPAR-/- telencephalon, the relative levels of 13 GABAA receptor subunit mRNAs (α1,α2, α3, α4, α5, β1, β2, β3, γ1, γ2S, γ2L, γ3, δ) were analyzed using real-time PCR. Adult (>P90) male wild type (n=3) and uPAR-/- (n=3) mice were anesthetized with isoflurane vapor, decapitated, and the brains removed. Following a midsagittal cut, the diencephalon was removed. Five subregions of the telencephalon were dissected and immediately frozen on dry ice. Specifically, the regions comprised: 1) the entire striatum, 2) the entire hippocampus including all CA subfields and the dentate gyrus, 3) frontal cortex (predominantly frontal association and primary and secondary motor regions), 4) parietal cortex (predominantly primary and secondary somatosensory regions), and 5) occipital cortex (predominantly primary and secondary visual regions). Each animal was considered an independent sample. Total RNA was isolated using the Trizol method according to the manufacturer's protocol (Invitrogen, Carlsbad, CA). Following the initial extraction, RNA aliquots were further purified using RNeasy Mini spin columns (Qiagen, Valencia, CA). The concentration and purity of each sample was determined by spectrophotometry (NanoDrop, Wilmington, DE).
Primers (Oligos Etc) and probes (TaqMan probes, Applied Biosystem) were designed using Primer Express software (Applied Biosystem). Ribosomal RNA (18 S) probe and primers were purchased from Applied Biosystem. The sequence of primers and probes were: α1 primers, 5′-CCCCGGCTTGGCAACTA-3′ and 5′-TGGTTTTGTCTCAGGCTTGAC-3′, α1 probe, 5′-TGCTAAAAGTGCGACCATAGAACCGAAAGA-3′; α2 primers, 5′-GTATTACTGAAGTCTTCACTAACATT-3′ and 5′-CGAAAGAAAACATCTATTGTATACTCCATATC-3′, α2 probe, 5′-ACGTGACCAGTTTTGGCCCTGTCTCAGA-3′; α3 primers, 5′-CGCGACGGCCATGG-3′ and 5′-CAAATTCAATCAGTGCAGAAAATACAA-3′, α3 probe, 5′-CTGGTTCATGGCCGTCTGTTATGCC-3′; α4 primers, 5′-TGAAATCTTGAGGCTGAATAATATG-3′ and 5′-AGACAGATTTCTTTCCATTCCTGAAG-3′, α4 probe, 5′-TGGTCACCAAAGTTTGGACCCCTGAT-3′; α5 primers, 5′-GACTCTTGGATGGCTATGACA-3′ and 5′-CACCTGCGTGATTCGCTCT-3′, α5 probe, 5′-CAGACTGCGGCCTGGGCTGG-3′; β1 primers, 5′-CCGGCAAGGGGCGCA-3′ and 5′-TCAGTCAAGTCGGGGATCTTCACT-3′, β1 probe, 5′-CCGCAGGCGCGCCTCGCAGCTCA-3′; β2 primers, 5′-ACCCCAGGAGCACAATGC-3′ and 5′-AGGCAGCCCAGCTTTTCG-3′, β2 probe, 5′-TGCCTATGATGCCTCCAGCATCCAGTA-3′; β3 primers, 5′-AGAGCATGCCCAAGGAAGG-3′ and 5′-AGGTGGGTCTTCTTGTGCG-3′, β3 probe, 5′-CTTCTGTCTCCCATGTACCGCCCATG-3′; γ1 primers, 5′-CCCAGGTCTCCATGCTGG-3′ and 5′-TTCCCCTTGTGGCAAAGAAATG-3′, γ1 probe, 5′-CACTCTCATTCCCATGAACA-3′; γ2S primers, 5′-AAACCCTGCCCCTACCATTG-3′ and 5′-GGCATTGTTCATTTGAATGGT-3′, γ2S probe, 5′-TTCGTCCCAGATCAG-3′; γ2L primers, 5′-TTTCCTTCAAGGCCCCTACC-3′ and 5′-TGTGTGGCATTGTTCATTTG-3′, γ2L probe, 5′-TGATATTCGTCCCAGATCAGCAACCA-3′; γ3 primers, 5′-TCGTTATTACATCCAGATTCCACAAGA-3′ and 5′-GTCCAGTAGAGAGTAATTAGAGGGTG-3′, γ3 probe, 5′-CTGATCGAATAAGCCTTCAA-3′; δ primers, 5′-AGGGCAGAGATGGATGTGAG-3′ and 5′-TGACCCCAGCAGCTGAGAG-3′, δ probe, 5′-CCATCGTCCTTTTCTC-3′.
Quantitative one-step real-time PCR assays were performed in an ABI prism Applied Biosystems 7900HT Sequence Detection System using QuantiTect™ Probe RT-PCR kit (Qiagen). Standard curves for relative quantification were generated with 1 to 500 ng of total RNA from wild type mouse cortex. Total RNA (50 ng) from wild type and uPAR-/- mice was tested. Reactions were performed in triplicate in a total volume of 50 μl containing QuantiTect Probe RT-PCR master mixture, 250 μM receptor probe, 900 nM receptor primers, 1 μM 18 S rRNA probe, and 250 μM 18 S rRNA primers. Two aliquots of 20 μl/reaction were loaded in a 384-well plate and incubated as follows: 48 °C for 30 min, 95 °C for 10 min followed by 50 cycles of 95 °C for 15 s, and 60 °C for 1 min. The relative amount of each GABAA receptor subunit RNA was normalized to the relative amount of 18 S rRNA (internal control).
In situ hybridization
Based on the real time PCR data, select GABAA receptor subunits (α1, α2, α3, β2, β3, γ2L and γ2S) were analyzed using in situ hybridization. This method permits semi-quantitative analysis of transcript expression in more circumscribed anatomical subregions for the telencephalon. The tissue processing, probe synthesis and hybridization protocols were as described previously (Middleton et al., 2002, Campbell and Levitt, 2003). Briefly, adult (>P90) male wild type (n=6) and uPAR-/- (n=6) mice were anesthetized with isoflurane vapor and decapitated. Brains were removed and flash frozen using cold 2-methylbutane and stored at -80°C. Twenty-five μm sections were cut through the entire telencephalon with a cryostat. Three to four serial sections were collected on Superfrost Plus glass slides (VWR) and stored at -80°C until the day of hybridization. For each region analyzed (frontal, parietal and occipital cortices, striatum, and the CA1, CA3 and dentate gyrus subregions of the hippocampus), two slides from each mouse, processed in two independent hybridization runs, were used to examine the expression of each transcript. An additional two slides were used for sense controls. In each hybridization run, sections from all 12 brains were represented, with two slides from each animal (one antisense and one sense control) processed in parallel. Slides were hybridized with 3-5ng of [35S]-labeled sense or antisense probe at 55°C overnight, followed by a series of buffer washes. The probes for α2, α3, γ2L and γ2Shave been used previously in Northern blot and RNase protection assays (Steiger et al., 2002, Steiger et al., 2003). Plasmid clones for α1 (IMAGE 5360723), β2 (IMAGE 6847737) and β3 (IMAGE 4503196) were obtained from Open Biosystems (Huntsville, AL). After air drying, the slides were exposed to BioMax MR film (Eastman Kodak, Rochester, NY) for 1 day (α1, β2), 3-5 days (α3, β3,γ2L and γ2S) or 10-12 days (α2).
Image Analysis
For all analyses, the material was coded so that the individuals performing the analyses were blind to genotype. In each run, [14C]-labeled standards were included to cross calibrate signal intensity. The films containing the autoradiographic images were scanned at high resolution (2400dpi) and the average signal intensity across each region of interest quantified using Image J, version 1.33. For the frontal, parietal and occipital cortices, the region of interest spanned all cortical layers. For the hippocampus, a line was drawn through the pyramidal (CA1 and CA3) or granule (dentate gyrus) cell layers, while the entire striatum was outlined. Sections were analyzed between the following Bregma levels using stereotaxic coordinates: striatum (+1.4 and +0.1mm), hippocampus (-1.7 and -2.5mm), frontal cortex (+2.5 and +1.7mm), parietal cortex (+0.2 and -1.5mm), and occipital cortex (-2.5 and -3.4mm). For the cortical regions, it was not possible to define precisely the boundaries between cytoarchitectonic regions. Therefore, we placed the region of interest in the same position laterally such that the region of interest in frontal cortex comprised primarily secondary motor cortex, in parietal cortex comprised primarily primary somatosensory cortex and in occipital cortex comprised primarily primary visual cortex and lateral secondary visual cortex (Figure S1). Signal for each of the GABAA receptor subunits examined was virtually absent in the white matter. Thus, quantification was performed by subtracting the signal in the white matter from the signal in the area of interest. For each brain region in each animal, a single value was obtained by averaging the signal across six to eight sections.
Statistical Analyses
Data are expressed as means ± SEM. For each receptor subunit, a two-tailed t-test was used to determine significance, with the alpha level set at 0.05 for significance. All analyses were implemented in Statview, Version 5.0.1 (SAS Institute, Inc., Cary, NC).
Figures were prepared digitally in SigmaPlot 7.0 (SPSS Incorporated, Chicago, IL) and Adobe Photoshop 6.01 (Adobe Systems Incorporated, San Jose, CA).
Results
Regional-selective alterations in GABAA receptor subunit mRNA in uPAR-/- mice
An alteration in GABAA receptor number and/or subunit composition has been implicated in multiple behavioral states. The repertoire of behaviors displayed by adult uPAR-/- mice, namely increased anxiety, atypical social behavior and a higher susceptibility to pharmacologically-induced convulsions, prompted us to explore whether there are changes in GABAA receptor subunits in these mice. We first compared the expression level of 13 GABAA receptor subunit mRNAs (α1,α2, α3, α4, α5, β1, β2, β3, γ1, γ2S, γ2L, γ3, δ) in select regions of the telencephalon in wild type and uPAR-/- adult male mice using quantitative real-time PCR. We focused on these subunits because they are the most prevalent in the telencephalon; the remaining subunits are more prominent in more caudal brain structures, such as the hypothalamus and cerebellum, and in the periphery. Significant changes in expression were observed in select regions for two subunits. There was increased expression of the γ2S subunit in parietal cortex (wild type: 1.491 ± 0.119, uPAR-/-:2.851 ± 0.371, p = 0.0252) and of the β2 subunit in frontal cortex (wild type: 1.044 ± 0.017, uPAR-/-: 1.393 ± 0.099, p = 0.0254) and the hippocampus (wild type: 0.771 ± 0.030, uPAR-/-: 0.901 ± 0.035, p = 0.0469). We also noted potential alterations in the α2 (frontal, wild type: 0.140 ± 0.010, uPAR-/-: 0.236 ± 0.110; hippocampus, wild type: 0.246 ± 0.019, uPAR-/-: 0.470 ± 0.237), α3 (frontal, wild type: 1.226 ± 0.210, uPAR-/-: 0.841 ± 0.122) and β3 (frontal, wild type: 0.784 ± 0.392, uPAR-/-: 0.482 ± 0.042) subunits, but an increased degree of variability in either the null or wild type samples precluded this from reaching significance.
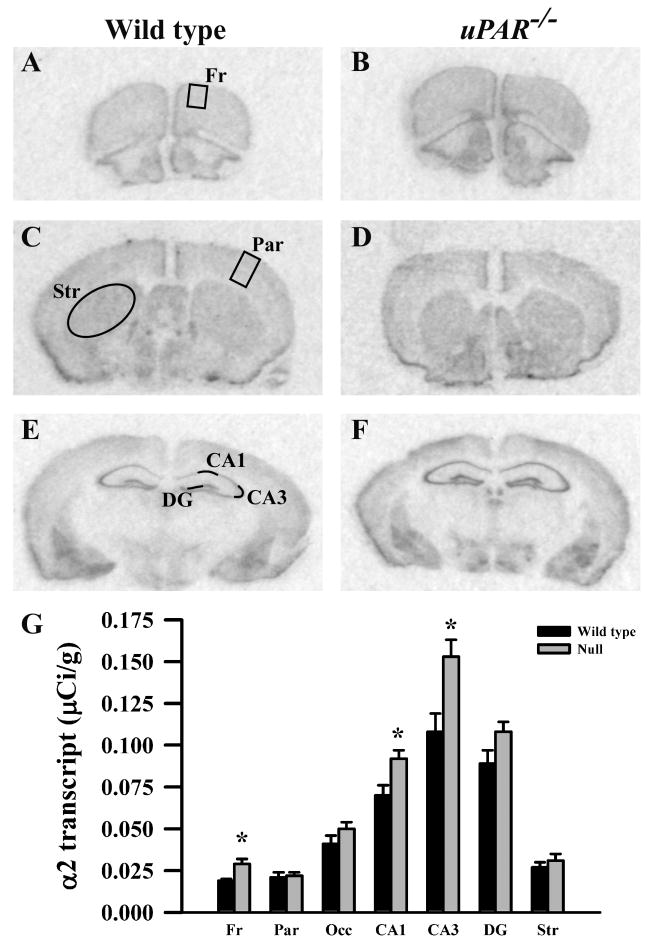
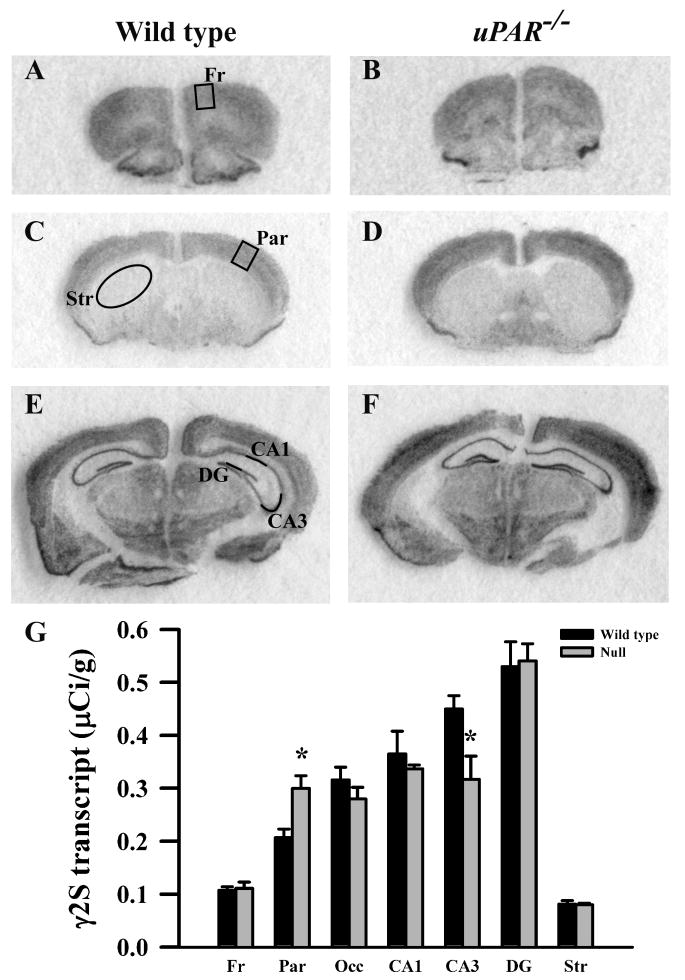
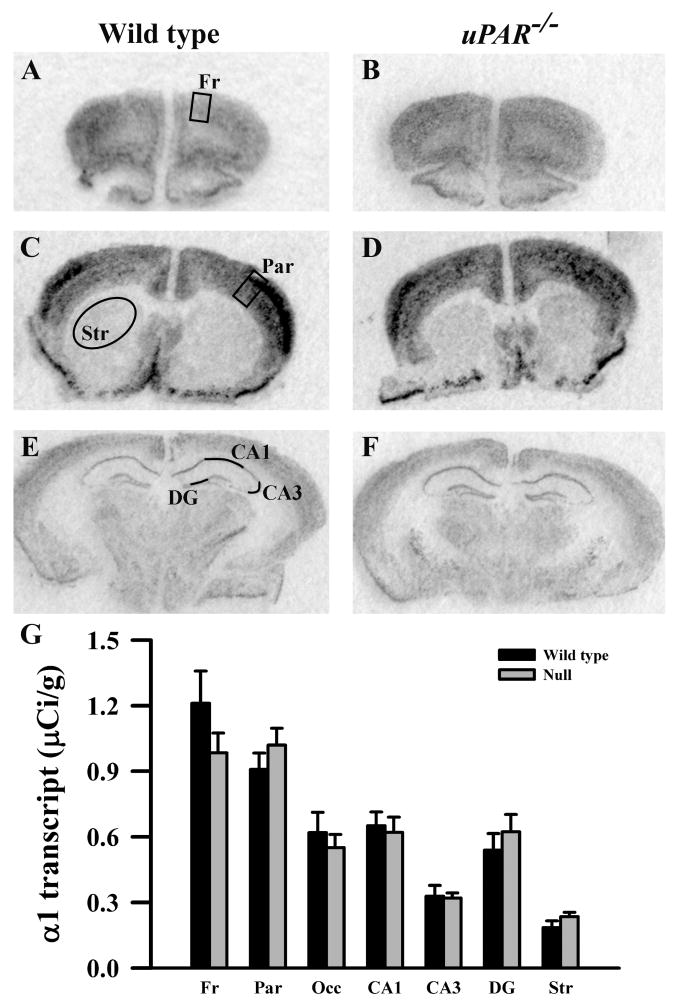
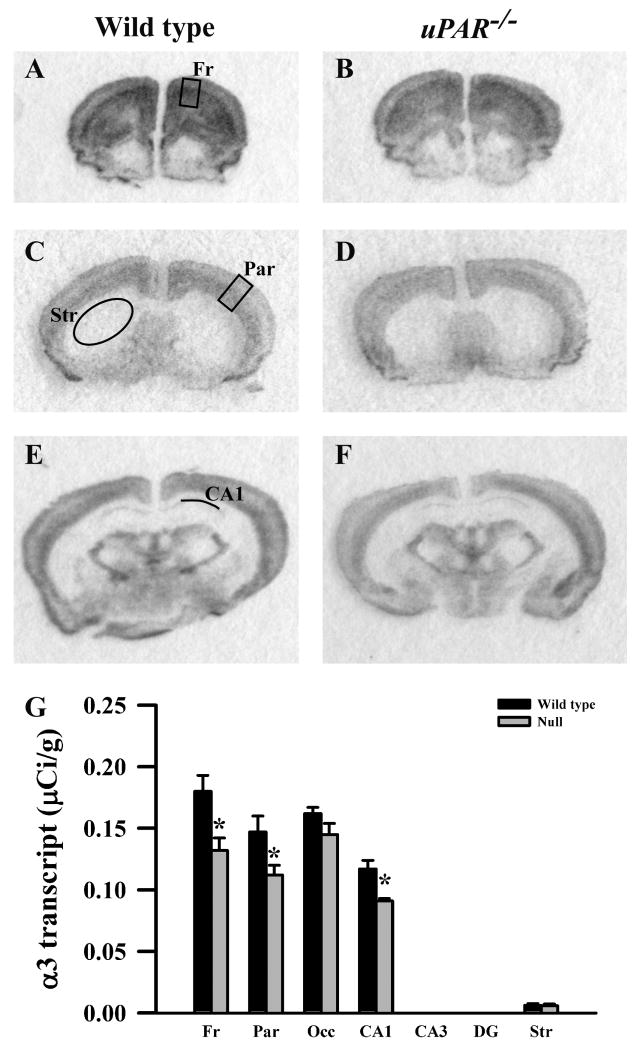
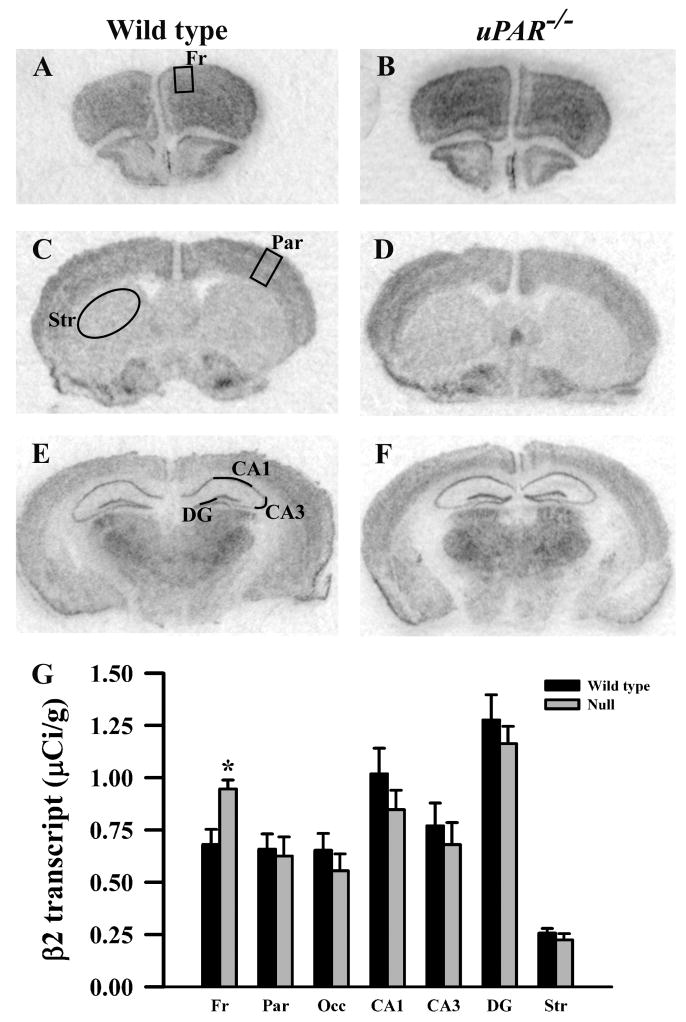
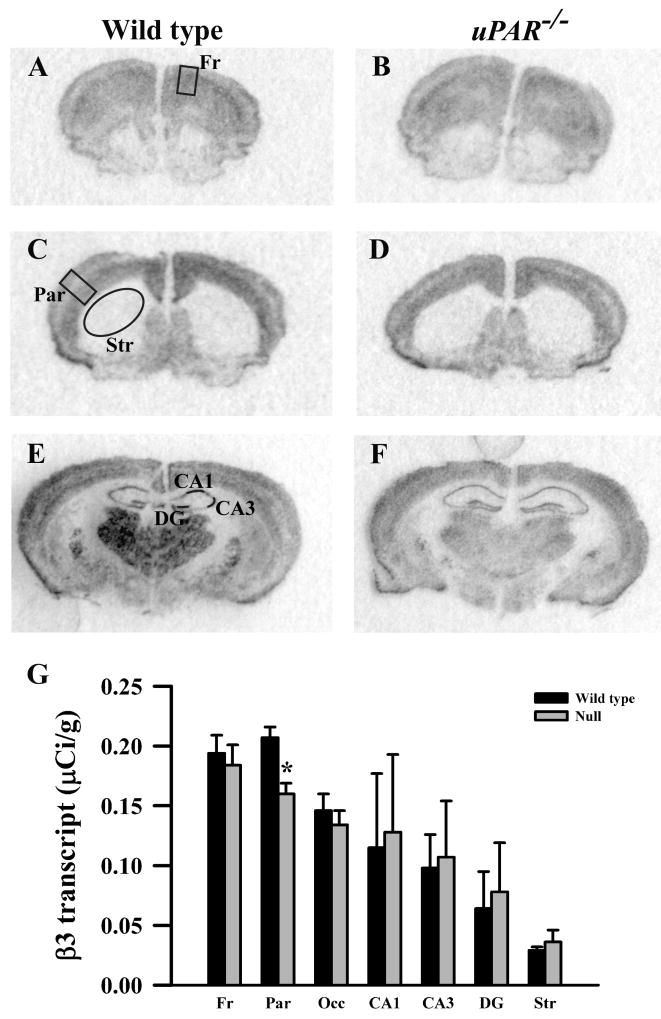
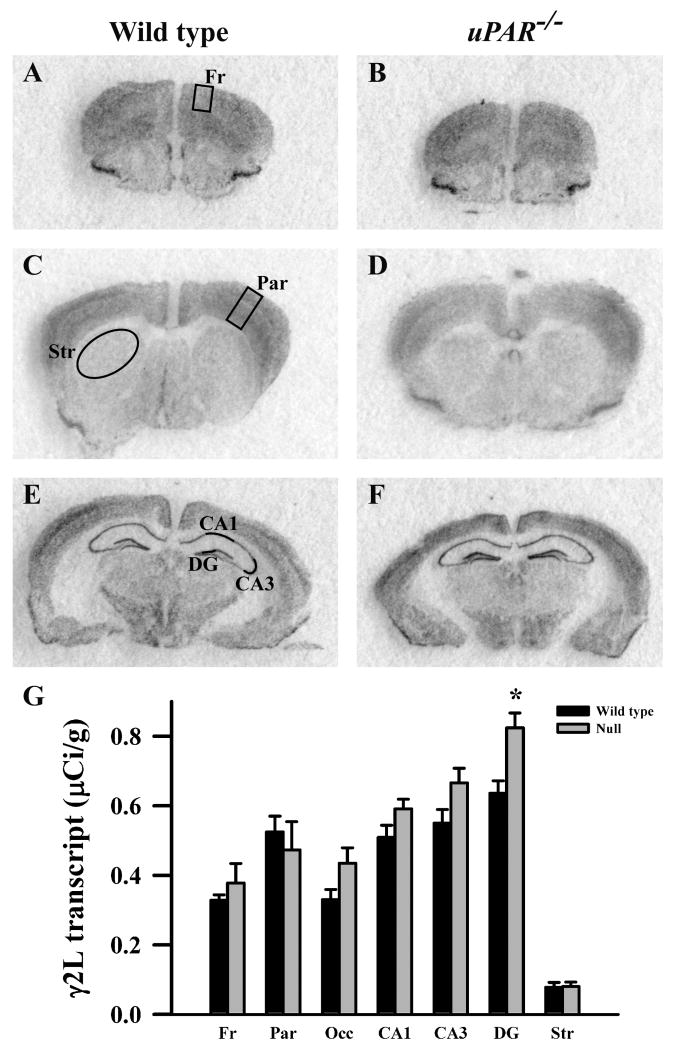
Based on the real-time PCR data, we focused on the expression levels of five GABAA receptor subunits (α2, α3, β2, β3 and γ2S) in our next level of analysis, semi-quantitative in situ hybridization. In addition, for reasons noted below, we included analysis of the α1 and γ2L subunits. In adult wild type mice, each subunit mRNA displayed a distinct pattern of probe hybridization (Figures 1-7) that is similar to that reported for adult C57Bl/6 mice (Heldt and Ressler, 2007). Hybridizations conducted with corresponding sense probes produced no specific labeling patterns. For each subunit, the anatomical patterns of expression were similar in the uPAR-/- brain compared to wild type. However, there were highly significant, quantitative differences in subunit expression. There was a significant increase in the levels of α2 subunit mRNAs in frontal cortex (p = 0.005) and in the CA1 (p = 0.013) and CA3 (p = 0.009) subregions of the hippocampus (Figure 1G). During normal development, as well as in some pathological conditions, an alteration in α2 subunit expression is accompanied by a reciprocal alteration in expression of the α1 subunit (Fritschy et al., 1994, Volk et al., 2002, Hashimoto et al., 2007, Hashimoto et al., 2009). However, there was no significant change in α1 subunit expression in any of the regions examined (Figure 2G). In contrast, there were significantly reduced levels of α3 subunit mRNAs in frontal cortex (p = 0.007), in parietal cortex (p = 0.039), and CA1 (p = 0.006) (Figure 3G). There was a significant increase in the levels of β2 subunit mRNAs in frontal cortex (p = 0.006) (Figure 4G), and significantly reduced levels of β3 subunit mRNAs in parietal cortex (p = 0.002) (Figure 5G). Messenger RNAs specific to the γ2S subunit were significantly increased in parietal cortex (p = 0.005) and decreased in CA3 (p = 0.016) (Figure 6G). Alterations in the ratio of γ2L subunit to γ2S subunit are thought to impact the function of the GABAA receptor (Moss et al., 1992). We therefore included analysis of the expression levels of γ2L subunit mRNAs and found this subunit transcript was increased significantly in the dentate gyrus (p = 0.004) (Figure 7G). The regional specificity of changes in gene expression was noteworthy, as it paralleled the identical telencephalic regions that exhibit interneuron disruption. Thus, for all subunits examined, no significant changes were observed in striatum and occipital cortex (Figure 1G – 7G), in which the numbers of GABAergic interneurons are unaltered in the uPAR-/- mice.
Figure 1.
Film autoradiographs from coronal sections depict expression of the GABAA receptor α2 subunit in adult wild type (A,C,E) and uPAR-/- (B, D, F) mice. The region of interest analyzed in frontal (A) and parietal (C) cortex, striatum (C), and the different subfields of the hippocampus (E) are indicated. Quantitative analysis (E) reveals a significant increase in α2 message in frontal cortex, and in the CA1 and CA3 subfields of the hippocampus. Asterisks, significantly different than wild type (p< 0.05). Fr, frontal cortex; Par, parietal cortex; Occ, occipital cortex; DG, dentate gyrus; Str, striatum.
Figure 7.
Film autoradiographs from coronal sections depict expression of the GABAA receptor γ2S subunit in adult wild type (A,C,E) and uPAR-/- (B, D, F) mice. The region of interest analyzed in frontal (A) and parietal (C) cortex, striatum (C), and the different subfields of the hippocampus (E) are indicated. Quantitative analysis (E) reveals a significant increase in γ2S message in parietal cortex, and a decrease in the CA3 subfield of the hippocampus. Asterisks, significantly different than wild type (p< 0.05). Fr, frontal cortex; Par, parietal cortex; Occ, occipital cortex; DG, dentate gyrus; Str, striatum.
Figure 2.
Film autoradiographs from coronal sections depict expression of the GABAA receptor α1 subunit in adult wild type (A,C,E) and uPAR-/- (B, D, F) mice. The region of interest analyzed in frontal (A) and parietal (C) cortex, striatum (C), and the different subfields of the hippocampus (E) are indicated. Quantitative analysis (E) reveals no significant difference in α1 message in any region examined. Fr, frontal cortex; Par, parietal cortex; Occ, occipital cortex; DG, dentate gyrus; Str, striatum.
Figure 3.
Film autoradiographs from coronal sections depict expression of the GABAA receptor α3 subunit in adult wild type (A,C,E) and uPAR-/- (B, D, F) mice. The region of interest analyzed in frontal (A) and parietal (C) cortex, striatum (C), and the CA1 subfield of the hippocampus (E) are indicated. Quantitative analysis (E) reveals a significant decrease in α3 message in frontal and parietal cortices, and in the CA1 subfield of the hippocampus. No signal was detected in the CA3 and dentate gyrus subfields in either genotype. Asterisks, significantly different than wild type (p< 0.05). Fr, frontal cortex; Par, parietal cortex; Occ, occipital cortex; DG, dentate gyrus; Str, striatum.
Figure 4.
Film autoradiographs from coronal sections depict expression of the GABAA receptor β2 subunit in adult wild type (A,C,E) and uPAR-/- (B, D, F) mice. The region of interest analyzed in frontal (A) and parietal (C) cortex, striatum (C), and the different subfields of the hippocampus (E) are indicated. Quantitative analysis (E) reveals a significant increase in β2 message in frontal cortex. Asterisks, significantly different than wild type (p< 0.05). Fr, frontal cortex; Par, parietal cortex; Occ, occipital cortex; DG, dentate gyrus; Str, striatum.
Figure 5.
Film autoradiographs from coronal sections depict expression of the GABAA receptor β3 subunit in adult wild type (A,C,E) and uPAR-/- (B, D, F) mice. The region of interest analyzed in frontal (A) and parietal (C) cortex, striatum (C), and the different subfields of the hippocampus (E) are indicated. Quantitative analysis (E) reveals a significant decrease in β3 message in parietal cortex. Asterisks, significantly different than wild type (p< 0.05). Fr, frontal cortex; Par, parietal cortex; Occ, occipital cortex; DG, dentate gyrus; Str, striatum.
Figure 6.
Film autoradiographs from coronal sections depict expression of the GABAA receptor γ2L subunit in adult wild type (A,C,E) and uPAR-/- (B, D, F) mice. The region of interest analyzed in frontal (A) and parietal (C) cortex, striatum (C), and the different subfields of the hippocampus (E) are indicated. Quantitative analysis (E) reveals a significant increase in γ2L message in the dentate gyrus subfield of the hippocampus. Asterisks, significantly different than wild type (p< 0.05). Fr, frontal cortex; Par, parietal cortex; Occ, occipital cortex; DG, dentate gyrus; Str, striatum.
Discussion
The present study reveals a selective interaction between GABAergic interneuron development and adaptive changes in GABAA receptor subunit expression. The relationship appears to be complex, as the data reveal distinct patterns of altered subunit expression that vary regionally. Thus, it is likely that unique features of input and local circuitry may contribute to the differential adaptive changes in GABAA receptor composition.
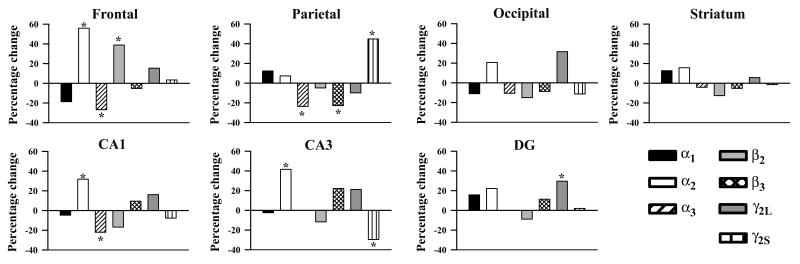
While a large number of studies have defined multiple mechanisms that control different aspects of interneuron development (Roy et al., 2002, Carretta et al., 2004, Cobos et al., 2005, Carmona et al., 2006, Yabut et al., 2007, Andrews et al., 2008, Muller Smith et al., 2008, Wallis et al., 2008, Barber et al., 2009, Batista-Brito et al., 2009, Canty et al., 2009, Mao et al., 2009, Sakata et al., 2009), none have extended these findings to investigate directly potential adaptive changes to the receptors that ultimately are responsible for mediating physiological activity. In the present study, we identified a complex pattern of region-specific alterations in GABAA receptor subunit gene expression in the adult uPAR-/- telencephalon (Figure 8), which may contribute to the functional alterations exhibited by this genetically altered mouse. The magnitude of these changes ranged from 25-55%, similar to the range that has been reported in mouse models of Rett syndrome (30%), Fragile X syndrome (35-50%) and lissencephaly (50%) (Samaco et al., 2005, D'Hulst et al., 2006, Valdes-Sanchez et al., 2007), although these studies did not examine potential variability in receptor changes across different brain regions. Altered mRNA levels may reflect changes in the number of GABAA receptors at the cell surface and/or in the subunit composition of GABAA receptors. We hypothesize that these changes in mRNA levels reflect alterations in network activity in the cortex and hippocampus. Although the precise molecular mechanisms are currently unknown, previous data suggest that uPAR-mediated signaling influences the differentiation and maturation of GABAergic neurons arising from the medial ganglionic eminence, leading to subtype specific deficits in select regions of the cortex and hippocampus during the period of cortical circuit formation. The subsequent disruption of inhibitory control in more rostral regions of the cortex and the hippocampus likely impacts the development of local circuits in affected regions leading to a regionally-selective adaptive response in GABAA receptor subunit expression.
Figure 8.
Percentage change in the expression of the α1, α2, α3, β2, β3, γ2S, and γ2L subunits of the GAGBAA receptor in uPAR-/- mice compared to wild type in seven regions of the telencephalon (same data as in Figures 1-7). Note that the pattern of change varies across different regions of the uPAR-/-telencephalon. Asterisks, significant difference between wild type and null.
Alterations in GABAA receptor subunit composition have been observed following a variety of environmental challenges in model systems and, when examined, usually accompany changes in receptor pharmacology and physiology. Chronic ethanol or benzodiazepine exposure in rodents leads to region-specific alterations in the expression levels of discrete receptor subunits [for example, (Tseng et al., 1994, Devaud et al., 1995, Holt et al., 1996, Pesold et al., 1997, Tyndale et al., 1997, Chen et al., 1999, Tietz et al., 1999, Raol et al., 2005)]. Furthermore, following ethanol exposure in vitro, hippocampal neurons demonstrate an altered sensitivity to several benzodiazepine ligands, which is associated with changes in the expression levels of α and γ subunit mRNAs (Sanna et al., 2003). During development, prenatal malnutrition, stress and maternal behavior modulate GABAA receptor subunit expression, as does prolonged social isolation in adult mice (Steiger et al., 2002, Caldji et al., 2003, Steiger et al., 2003, Pinna et al., 2006, Jacobson-Pick et al., 2008). In patients with temporal lobe epilepsy and in animal models of this disorder, there is an increase in several key GABAA receptor subunits in the dentate granule cell layer, which are thought to reflect axon sprouting and the formation of new circuits in response to increased excitability (Brooks-Kayal et al., 1998, Nishimura et al., 2005). A reduction in α3-containing receptors in the cortex and an increase in the α2 subunit in the CA regions also have been reported in this disorder (Loup et al., 2006). The subunit changes observed in temporal lobe epilepsy are reminiscent of, although not identical to, those observed in the uPAR-/- telencephalon, in which there are altered levels of excitability. Given that the α2 subunit is localized primarily at the axon initial segment, the potential increase in α2 containing receptors in the uPAR-/- frontal cortex, CA1 and CA3 may reflect an attempt to dampen the output of pyramidal cells in these regions. In addition, the γ2 subunit is important for targeting the GABAA receptor to the synapse (Essrich et al., 1998, Schweizer et al., 2003). Thus, the increase in γ2 subunit gene expression in the parietal cortex and dentate gyrus may, in effect, increase the number of receptors at the synapse in these regions.
Comparison of qPCR and in situ analyses
Potential alterations in seven GABAA receptor subunits were assessed by both qPCR and in situ hybridization. With one exception, all changes observed by qPCR were recapitulated by the in situ analyses. In addition, significant changes were revealed by in situ hybridization that had not been appreciated by qPCR. This is particularly evident in the hippocampus, and most likely reflects the increased anatomical precision achieved with the in situ analysis, where each major subregion (CA1, CA3 and dentate gyrus) was considered independently, compared to the qPCR, where the hippocampus was considered a single entity. Similarly, the ability to detect changes in α3 and β3 subunit mRNA expression in parietal cortex by in situ hybridization that are not apparent by qPCR is likely a consequence of the ability to analyze a more circumscribed region of cortex in the former (primary somatosensory only) compared to the latter (primary and secondary somatosensory). Further analyses will need to be done using both methods in new cohorts of mice to reconcile the apparent increase in α3 subunit expression observed by qPCR in frontal cortex with the decrease in the same subunit seen by in situ hybridization.
Alterations in the γ2L:γ2S subunit mRNA ratio
The γ2 subunit is the predominant γ isoform expressed throughout the brain. The two alternatively spliced variants differ only by the addition of 8 amino acids, which include a protein kinase C (PKC) phosphorylation consensus sequence, in the γ2L form (Whiting et al., 1990). In addition, both of the splice variants can be phosphorylated by PKC on serine 327 (Krishek et al., 1994). Thus, because the two splice variants possess a differential capacity to be phosphorylated by PKC and PKC-induced phosphorylation negatively modulates GABAA receptor function, it has been suggested that an alteration in the γ2L:γ2S subunit ratio leads to a change in GABAA receptor function (Moss et al., 1992). Consistent with this, mice in which the γ2L subunit is deleted exhibit an increased level of anxiety and increased sensitivity to benzodiazepines (Quinlan et al., 2000). Region-specific increases in mRNA levels of the γ2S, but not the γ2L, subunit have been reported in animals following ethanol treatment (Devaud et al., 1995), while the γ2L:γ2S mRNA ratio is altered in cultured cerebellar neurons after pentobarbital treatment (Tyndale et al., 1997). In the uPAR-/- mouse telencephalon, there seems to be a differential adaptation in the gene expression of γ2S and γ2L subunits, such that there is an increase in mRNA levels of the γ2S subunit in parietal cortex and in the γ2L subunit in the dentate gyrus, with no compensatory change in the other variant.
GABAA receptor expression and neurodevelopmental disorders
Genetic and postmortem studies have indicated altered GABAergic interneuron development and receptor expression in several neurodevelopmental disorders. Perhaps the most compelling evidence, coming from multiple studies, is for schizophrenia, with most attention focused on the prefrontal cortex. Here, as in the uPAR-/- frontal cortex, there is a reduction in GAD67 expression, with the parvalbumin-expressing population particularly affected (Volk et al., 2000, Reynolds and Beasley, 2001, Reynolds et al., 2002, Hashimoto et al., 2003). In addition, there is an increase in α2 subunit expression, detected at both the message and protein level, as well as a reduction in the levels α1, γ2 and δ subunit mRNA, all of which are thought to occur in response to decreased GABA levels (Volk et al., 2002, Hashimoto et al., 2007). Furthermore, a marked decrease in γ2S transcripts, with no significant change in γ2L transcripts, has also been reported (Huntsman et al., 1998). Together, these changes are thought to reflect alterations in the underlying GABAergic circuitry in this brain region and to contribute to the cognitive deficits, in particular those in working memory, in patients with schizophrenia (Lewis and Hashimoto, 2007, Hashimoto et al., 2008).
We identified a functional common polymorphism in the human homologue of the uPAR gene, PLAUR, which increases the risk for ASD (Campbell et al., 2008). Several authors have postulated that alterations in the GABAergic system may contribute to the etiology of this disorder and the behavioral phenotypes expressed (Rubenstein and Merzenich, 2003, Levitt et al., 2004, Geschwind and Levitt, 2007, Eagleson et al., 2010). Consistent with this, alleleic variants and chromosomal inversion in genes encoding GABAA receptor subunits, including the β3 subunit, have been associated with ASD (McCauley et al., 2004, Ma et al., 2005, Ashley-Koch et al., 2006, Vincent et al., 2006, Hogart et al., 2007). Furthermore, a reduced expression of the β3 subunit in the frontal cortex, as well as a decreased density of GABAA receptors in anterior cingulate cortex, has been reported in patients with ASD (Samaco et al., 2005, Oblak et al., 2009). The current study contributes additional evidence for a GABAergic contribution to ASD, providing an experimental link between an autism susceptibility gene and alterations in GABAA receptor expression. Alterations in the expression of the GABAA receptor have also been noted in rarer disorders, including Tuberous Sclerosis, Prader-Willi, Angelman and Rett syndromes (White et al., 2001, Dan and Boyd, 2003, Lucignani et al., 2004, Samaco et al., 2005, Valencia et al., 2006). Indeed, there is a cluster of three GABAA receptor subunits (α5, β3 and γ3) in the chromosomal regions 15q11-q13 that are deleted in most patients with Prader-Willi or Angelman syndromes, and it has been hypothesized that altered neurobehavioral function seen in patients with Prader-Willi could arise directly from an altered GABAA receptor composition and expression (Lucignani et al., 2004). Similar to patients with ASD, reduced expression of the β3 subunit of the GABAA receptor has been reported in subjects with Angelman and Rett syndromes (Samaco et al., 2005). Finally, alterations in GABAA receptor subunit expression have been observed in mouse models of Rett syndrome, Fragile X syndrome and lissencephaly (Samaco et al., 2005, D'Hulst et al., 2006, Valdes-Sanchez et al., 2007).
The present study demonstrates that in the telencephalon of uPAR-/- mutant mice, the gene expression levels of six GABAA receptor subunits are altered in a region-specific manner. The regional and subunit specificity that we report here will help focus examination of potential parallel changes in clinical samples. Moreover, the documentation of these changes lays the foundation for determining whether an alteration in the subunit composition of GABAA receptors may underlie, at least in part, the abnormal behavioral repertoire of uPAR-/- mutant mice. The role that adaptive gene regulation plays in GABAA receptor subtype expression, and how this may contribute to genomic control over interneuron development, will be pursued in the future.
Supplementary Material
Photomicrographs of cresyl violet-stained coronal sections through the forebrain of an adult wild type mouse illustrating the cytoarchitecture of the regions of interest analyzed. The areas boxed in A, C and E are shown at higher magnification in B, D and F, respectively. Fr, frontal cortex; Par, parietal cortex; Occ, occipital cortex; DG, dentate gyrus; Str, striatum. Scale bar = 1mm (A, C, E, G and H) and 300μm (B, D, F).
Acknowledgments
We thank Paula Woods, Donte Smith and Frank Liu for assistance in maintaining the mouse colony and genotyping, Kathryn Spencer, Neeti Sasi and Duncan Leitch for their technical assistance with the in situ hybridization experiments, and Dr Gregg Stanwood for helpful comments on the manuscript. This study was supported by NIMH grant 67842 (PL), NICHD P30 grant 15052, NIAA grant RO1 AA11697-09 and NIMH grant 049469 (DHF).
Abbreviations
- ASD
autism spectrum disorder
- CA
cornu ammonis
- P
postnatal day
- PCR
polymerase chain reaction
- PLAUR
plasminogen activator, urokinase receptor
- uPAR
urokinase –type plasminogen activator receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbarian S, Huntsman MM, Kim JJ, Tafazzoli A, Potkin SG, Bunney WE, Jr, Jones EG. GABAA receptor subunit gene expression in human prefrontal cortex: comparison of schizophrenics and controls. Cereb Cortex. 1995;5:550–560. doi: 10.1093/cercor/5.6.550. [DOI] [PubMed] [Google Scholar]
- Andrews W, Barber M, Hernadez-Miranda LR, Xian J, Rakic S, Sundaresan V, Rabbitts TH, Pannell R, Rabbitts P, Thompson H, Erskine L, Murakami F, Parnavelas JG. The role of Slit-Robo signaling in the generation, migration and morphological differentiation of cortical interneurons. Dev Biol. 2008;313:648–658. doi: 10.1016/j.ydbio.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Ashley-Koch AE, Mei H, Jaworski J, Ma DQ, Ritchie MD, Menold MM, Delong GR, Abramson RK, Wright HH, Hussman JP, Cuccaro ML, Gilbert JR, Martin ER, Pericak-Vance MA. An analysis paradigm for investigating multi-locus effects in complex disease: examination of three GABA receptor subunit genes on 15q11-q13 as risk factors for autistic disorder. Ann Hum Genet. 2006;70:281–292. doi: 10.1111/j.1469-1809.2006.00253.x. [DOI] [PubMed] [Google Scholar]
- Barber M, Di Meglio T, Andrews WD, Hernandez-Miranda LR, Murakami F, Chedotal A, Parnavelas JG. The role of Robo3 in the development of cortical interneurons. Cereb Cortex. 2009;19 1:i22–31. doi: 10.1093/cercor/bhp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Rossignol E, Hjerling-Leffler J, Denaxa M, Wegner M, Lefebvre V, Pachnis V, Fishell G. The cell-intrinsic requirement of Sox6 for cortical interneuron development. Neuron. 2009;63:466–481. doi: 10.1016/j.neuron.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BR, Mihaly J, Prager GW. uPAR-uPA-PAI-1 interactions and signaling: a vascular biologist's view. Thromb Haemost. 2007;97:336–342. [PubMed] [Google Scholar]
- Bond AM, Vangompel MJ, Sametsky EA, Clark MF, Savage JC, Disterhoft JF, Kohtz JD. Balanced gene regulation by an embryonic brain ncRNA is critical for adult hippocampal GABA circuitry. Nat Neurosci. 2009;12:1020–1027. doi: 10.1038/nn.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouilleret V, Loup F, Kiener T, Marescaux C, Fritschy JM. Early loss of interneurons and delayed subunit-specific changes in GABA(A)-receptor expression in a mouse model of mesial temporal lobe epilepsy. Hippocampus. 2000;10:305–324. doi: 10.1002/1098-1063(2000)10:3<305::AID-HIPO11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care alter GABA(A) receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology. 2003;28:1950–1959. doi: 10.1038/sj.npp.1300237. [DOI] [PubMed] [Google Scholar]
- Campbell DB, Levitt P. Regionally restricted expression of the transcription factor c-myc intron 1 binding protein during brain development. J Comp Neurol. 2003;467:581–592. doi: 10.1002/cne.10958. [DOI] [PubMed] [Google Scholar]
- Campbell DB, Li C, Sutcliffe JS, Persico AM, Levitt P. Genetic evidence implicating multiple genes in the MET receptor tyrosine kinase pathway in autism spectrum disorder. Autism Res. 2008;1:159–168. doi: 10.1002/aur.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canty AJ, Dietze J, Harvey M, Enomoto H, Milbrandt J, Ibanez CF. Regionalized loss of parvalbumin interneurons in the cerebral cortex of mice with deficits in GFRalpha1 signaling. J Neurosci. 2009;29:10695–10705. doi: 10.1523/JNEUROSCI.2658-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona MA, Pozas E, Martinez A, Espinosa-Parrilla JF, Soriano E, Aguado F. Age-dependent spontaneous hyperexcitability and impairment of GABAergic function in the hippocampus of mice lacking trkB. Cereb Cortex. 2006;16:47–63. doi: 10.1093/cercor/bhi083. [DOI] [PubMed] [Google Scholar]
- Carretta D, Santarelli M, Sbriccoli A, Pinto F, Catini C, Minciacchi D. Spatial analysis reveals alterations of parvalbumin- and calbindin-positive local circuit neurons in the cerebral cortex of mutant mdx mice. Brain Res. 2004;1016:1–11. doi: 10.1016/j.brainres.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Chen S, Huang X, Zeng XJ, Sieghart W, Tietz EI. Benzodiazepine-mediated regulation of alpha1, alpha2, beta1-3 and gamma2 GABA(A) receptor subunit proteins in the rat brain hippocampus and cortex. Neuroscience. 1999;93:33–44. doi: 10.1016/s0306-4522(99)00118-9. [DOI] [PubMed] [Google Scholar]
- Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- D'Hulst C, De Geest N, Reeve SP, Van Dam D, De Deyn PP, Hassan BA, Kooy RF. Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res. 2006;1121:238–245. doi: 10.1016/j.brainres.2006.08.115. [DOI] [PubMed] [Google Scholar]
- Dan B, Boyd SG. Angelman syndrome reviewed from a neurophysiological perspective. The UBE3A-GABRB3 hypothesis. Neuropediatrics. 2003;34:169–176. doi: 10.1055/s-2003-42213. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Smith FD, Grayson DR, Morrow AL. Chronic ethanol consumption differentially alters the expression of gamma-aminobutyric acidA receptor subunit mRNAs in rat cerebral cortex: competitive, quantitative reverse transcriptase-polymerase chain reaction analysis. Mol Pharmacol. 1995;48:861–868. [PubMed] [Google Scholar]
- Diaz-Veliz G, Butron S, Benavides MS, Dussaubat N, Mora S. Gender, estrous cycle, ovariectomy, and ovarian hormones influence the effects of diazepam on avoidance conditioning in rats. Pharmacol Biochem Behav. 2000;66:887–892. doi: 10.1016/s0091-3057(00)00283-5. [DOI] [PubMed] [Google Scholar]
- Eagleson KL, Bonnin A, Levitt P. Region- and age-specific deficits in gamma-aminobutyric acidergic neuron development in the telencephalon of the uPAR(-/-) mouse. J Comp Neurol. 2005;489:449–466. doi: 10.1002/cne.20647. [DOI] [PubMed] [Google Scholar]
- Eagleson KL, Hammock EAD, Levitt P. Interneuron pathologies: Pathes to neurodevelopmental disorders. In: Pallas SL, editor. Developmental Plasticity of Inhibitory Circuitry. Springer-Verlag; New York: 2010. [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Evans MS, Cady CJ, Disney KE, Yang L, Laguardia JJ. Three brief epileptic seizures reduce inhibitory synaptic currents, GABA(A) currents, and GABA(A)-receptor subunits. Epilepsia. 2006;47:1655–1664. doi: 10.1111/j.1528-1167.2006.00634.x. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy JM, Low K, Mohler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. J Neurosci. 1994;14:5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Dong E, Grayson DR, Veldic M, Zhang X, Costa E. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology (Berl) 2005;180:191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Nguyen QL, Rotaru D, Keenan T, Arion D, Beneyto M, Gonzalez-Burgos G, Lewis DA. Protracted developmental trajectories of GABAA receptor alpha1 and alpha2 subunit expression in primate prefrontal cortex. Biol Psychiatry. 2009;65:1015–1023. doi: 10.1016/j.biopsych.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. Forebrain and midbrain distribution of major benzodiazepine-sensitive GABAA receptor subunits in the adult C57 mouse as assessed with in situ hybridization. Neuroscience. 2007;150:370–385. doi: 10.1016/j.neuroscience.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK, Stryker MP. Columnar architecture sculpted by GABA circuits in developing cat visual cortex. Science. 2004;303:1678–1681. doi: 10.1126/science.1091031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogart A, Nagarajan RP, Patzel KA, Yasui DH, Lasalle JM. 15q11-13 GABAA receptor genes are normally biallelically expressed in brain yet are subject to epigenetic dysregulation in autism-spectrum disorders. Hum Mol Genet. 2007;16:691–703. doi: 10.1093/hmg/ddm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt RA, Bateson AN, Martin IL. Chronic treatment with diazepam or abecarnil differently affects the expression of GABAA receptor subunit mRNAs in the rat cortex. Neuropharmacology. 1996;35:1457–1463. doi: 10.1016/s0028-3908(96)00064-0. [DOI] [PubMed] [Google Scholar]
- Huntsman MM, Tran BV, Potkin SG, Bunney WE, Jr, Jones EG. Altered ratios of alternatively spliced long and short gamma2 subunit mRNAs of the gamma-amino butyrate type A receptor in prefrontal cortex of schizophrenics. Proc Natl Acad Sci U S A. 1998;95:15066–15071. doi: 10.1073/pnas.95.25.15066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibi D, Nagai T, Koike H, Kitahara Y, Mizoguchi H, Niwa M, Jaaro-Peled H, Nitta A, Yoneda Y, Nabeshima T, Sawa A, Yamada K. Combined effect of neonatal immune activation and mutant DISC1 on phenotypic changes in adulthood. Behav Brain Res. 2009 doi: 10.1016/j.bbr.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson-Pick S, Elkobi A, Vander S, Rosenblum K, Richter-Levin G. Juvenile stress-induced alteration of maturation of the GABAA receptor alpha subunit in the rat. Int J Neuropsychopharmacol. 2008;11:891–903. doi: 10.1017/S1461145708008559. [DOI] [PubMed] [Google Scholar]
- Jorge JC, McIntyre KL, Henderson LP. The function and the expression of forebrain GABA(A) receptors change with hormonal state in the adult mouse. J Neurobiol. 2002;50:137–149. doi: 10.1002/neu.10021. [DOI] [PubMed] [Google Scholar]
- Krishek BJ, Xie X, Blackstone C, Huganir RL, Moss SJ, Smart TG. Regulation of GABAA receptor function by protein kinase C phosphorylation. Neuron. 1994;12:1081–1095. doi: 10.1016/0896-6273(94)90316-6. [DOI] [PubMed] [Google Scholar]
- Levitt P. Disruption of interneuron development. Epilepsia. 2005;46 7:22–28. doi: 10.1111/j.1528-1167.2005.00305.x. [DOI] [PubMed] [Google Scholar]
- Levitt P, Eagleson KL, Powell EM. Regulation of neocortical interneuron development and the implications for neurodevelopmental disorders. Trends Neurosci. 2004;27:400–406. doi: 10.1016/j.tins.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T. Deciphering the disease process of schizophrenia: the contribution of cortical gaba neurons. Int Rev Neurobiol. 2007;78:109–131. doi: 10.1016/S0074-7742(06)78004-7. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Loup F, Picard F, Andre VM, Kehrli P, Yonekawa Y, Wieser HG, Fritschy JM. Altered expression of alpha3-containing GABAA receptors in the neocortex of patients with focal epilepsy. Brain. 2006;129:3277–3289. doi: 10.1093/brain/awl287. [DOI] [PubMed] [Google Scholar]
- Lucignani G, Panzacchi A, Bosio L, Moresco RM, Ravasi L, Coppa I, Chiumello G, Frey K, Koeppe R, Fazio F. GABA A receptor abnormalities in Prader-Willi syndrome assessed with positron emission tomography and [11C]flumazenil. Neuroimage. 2004;22:22–28. doi: 10.1016/j.neuroimage.2003.10.050. [DOI] [PubMed] [Google Scholar]
- Ma DQ, Whitehead PL, Menold MM, Martin ER, Ashley-Koch AE, Mei H, Ritchie MD, Delong GR, Abramson RK, Wright HH, Cuccaro ML, Hussman JP, Gilbert JR, Pericak-Vance MA. Identification of significant association and gene-gene interaction of GABA receptor subunit genes in autism. Am J Hum Genet. 2005;77:377–388. doi: 10.1086/433195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Mao R, Page DT, Merzlyak I, Kim C, Tecott LH, Janak PH, Rubenstein JL, Sur M. Reduced conditioned fear response in mice that lack Dlx1 and show subtype-specific loss of interneurons. J Neurodev Disord. 2009;1:224–236. doi: 10.1007/s11689-009-9025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley JL, Olson LM, Delahanty R, Amin T, Nurmi EL, Organ EL, Jacobs MM, Folstein SE, Haines JL, Sutcliffe JS. A linkage disequilibrium map of the 1-Mb 15q12 GABA(A) receptor subunit cluster and association to autism. Am J Med Genet B Neuropsychiatr Genet. 2004;131:51–59. doi: 10.1002/ajmg.b.30038. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Mirnics K, Pierri JN, Lewis DA, Levitt P. Gene expression profiling reveals alterations of specific metabolic pathways in schizophrenia. J Neurosci. 2002;22:2718–2729. doi: 10.1523/JNEUROSCI.22-07-02718.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler H. GABA(A) receptor diversity and pharmacology. Cell Tissue Res. 2006;326:505–516. doi: 10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- Moss SJ, Doherty CA, Huganir RL. Identification of the cAMP-dependent protein kinase and protein kinase C phosphorylation sites within the major intracellular domains of the beta 1, gamma 2S, and gamma 2L subunits of the gamma-aminobutyric acid type A receptor. J Biol Chem. 1992;267:14470–14476. [PubMed] [Google Scholar]
- Muller Smith K, Fagel DM, Stevens HE, Rabenstein RL, Maragnoli ME, Ohkubo Y, Picciotto MR, Schwartz ML, Vaccarino FM. Deficiency in inhibitory cortical interneurons associates with hyperactivity in fibroblast growth factor receptor 1 mutant mice. Biol Psychiatry. 2008;63:953–962. doi: 10.1016/j.biopsych.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Schwarzer C, Gasser E, Kato N, Vezzani A, Sperk G. Altered expression of GABA(A) and GABA(B) receptor subunit mRNAs in the hippocampus after kindling and electrically induced status epilepticus. Neuroscience. 2005;134:691–704. doi: 10.1016/j.neuroscience.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Oblak A, Gibbs TT, Blatt GJ. Decreased GABAA receptors and benzodiazepine binding sites in the anterior cingulate cortex in autism. Autism Res. 2009;2:205–219. doi: 10.1002/aur.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesold C, Caruncho HJ, Impagnatiello F, Berg MJ, Fritschy JM, Guidotti A, Costa E. Tolerance to diazepam and changes in GABA(A) receptor subunit expression in rat neocortical areas. Neuroscience. 1997;79:477–487. doi: 10.1016/s0306-4522(96)00609-4. [DOI] [PubMed] [Google Scholar]
- Pinna G, Agis-Balboa RC, Zhubi A, Matsumoto K, Grayson DR, Costa E, Guidotti A. Imidazenil and diazepam increase locomotor activity in mice exposed to protracted social isolation. Proc Natl Acad Sci U S A. 2006;103:4275–4280. doi: 10.1073/pnas.0600329103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell EM, Campbell DB, Stanwood GD, Davis C, Noebels JL, Levitt P. Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J Neurosci. 2003;23:622–631. doi: 10.1523/JNEUROSCI.23-02-00622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MG, Yoo JW, Burgess DL, Deng F, Hrachovy RA, Frost JD, Jr, Noebels JL. A triplet repeat expansion genetic mouse model of infantile spasms syndrome, Arx(GCG)10+7, with interneuronopathy, spasms in infancy, persistent seizures, and adult cognitive and behavioral impairment. J Neurosci. 2009;29:8752–8763. doi: 10.1523/JNEUROSCI.0915-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan JJ, Firestone LL, Homanics GE. Mice lacking the long splice variant of the gamma 2 subunit of the GABA(A) receptor are more sensitive to benzodiazepines. Pharmacol Biochem Behav. 2000;66:371–374. doi: 10.1016/s0091-3057(00)00225-2. [DOI] [PubMed] [Google Scholar]
- Raol YH, Zhang G, Budreck EC, Brooks-Kayal AR. Long-term effects of diazepam and phenobarbital treatment during development on GABA receptors, transporters and glutamic acid decarboxylase. Neuroscience. 2005;132:399–407. doi: 10.1016/j.neuroscience.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Beasley CL. GABAergic neuronal subtypes in the human frontal cortex--development and deficits in schizophrenia. J Chem Neuroanat. 2001;22:95–100. doi: 10.1016/s0891-0618(01)00113-2. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Beasley CL, Zhang ZJ. Understanding the neurotransmitter pathology of schizophrenia: selective deficits of subtypes of cortical GABAergic neurons. J Neural Transm. 2002;109:881–889. doi: 10.1007/s007020200072. [DOI] [PubMed] [Google Scholar]
- Roy K, Thiels E, Monaghan AP. Loss of the tailless gene affects forebrain development and emotional behavior. Physiol Behav. 2002;77:595–600. doi: 10.1016/s0031-9384(02)00902-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata K, Woo NH, Martinowich K, Greene JS, Schloesser RJ, Shen L, Lu B. Critical role of promoter IV-driven BDNF transcription in GABAergic transmission and synaptic plasticity in the prefrontal cortex. Proc Natl Acad Sci U S A. 2009;106:5942–5947. doi: 10.1073/pnas.0811431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Hogart A, LaSalle JM. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum Mol Genet. 2005;14:483–492. doi: 10.1093/hmg/ddi045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Busonero F, Talani G, Tranquilli S, Mameli M, Spiga S, Follesa P, Biggio G. Changes in GABA(A) receptor gene expression associated with selective alterations in receptor function and pharmacology after ethanol withdrawal. J Neurosci. 2003;23:11711–11724. doi: 10.1523/JNEUROSCI.23-37-11711.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer C, Balsiger S, Bluethmann H, Mansuy IM, Fritschy JM, Mohler H, Luscher B. The gamma 2 subunit of GABA(A) receptors is required for maintenance of receptors at mature synapses. Mol Cell Neurosci. 2003;24:442–450. doi: 10.1016/s1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- Steiger JL, Alexander MJ, Galler JR, Farb DH, Russek SJ. Effects of prenatal malnutrition on GABAA receptor alpha1, alpha3 and beta2 mRNA levels. Neuroreport. 2003;14:1731–1735. doi: 10.1097/00001756-200309150-00015. [DOI] [PubMed] [Google Scholar]
- Steiger JL, Galler JR, Farb DH, Russek SJ. Prenatal protein malnutrition reduces beta(2), beta(3) and gamma(2L) GABA(A) receptor subunit mRNAs in the adult septum. Eur J Pharmacol. 2002;446:201–202. doi: 10.1016/s0014-2999(02)01815-0. [DOI] [PubMed] [Google Scholar]
- Tietz EI, Huang X, Chen S, Ferencak WF., 3rd Temporal and regional regulation of alpha1, beta2 and beta3, but not alpha2, alpha4, alpha5, alpha6, beta1 or gamma2 GABA(A) receptor subunit messenger RNAs following one-week oral flurazepam administration. Neuroscience. 1999;91:327–341. doi: 10.1016/s0306-4522(98)00516-8. [DOI] [PubMed] [Google Scholar]
- Tseng YT, Wellman SE, Ho IK. In situ hybridization evidence of differential modulation by pentobarbital of GABAA receptor alpha 1- and beta 3-subunit mRNAs. J Neurochem. 1994;63:301–309. doi: 10.1046/j.1471-4159.1994.63010301.x. [DOI] [PubMed] [Google Scholar]
- Tyndale RF, Bhave SV, Hoffmann E, Hoffmann PL, Tabakoff B, Tobin AJ, Olsen RW. Pentobarbital decreases the gamma-aminobutyric acidA receptor subunit gamma-2 long/short mRNA ratio by a mechanism distinct from receptor occupation. J Pharmacol Exp Ther. 1997;283:350–357. [PubMed] [Google Scholar]
- Valdes-Sanchez L, Escamez T, Echevarria D, Ballesta JJ, Tabares-Seisdedos R, Reiner O, Martinez S, Geijo-Barrientos E. Postnatal alterations of the inhibitory synaptic responses recorded from cortical pyramidal neurons in the Lis1/sLis1 mutant mouse. Mol Cell Neurosci. 2007;35:220–229. doi: 10.1016/j.mcn.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Valencia I, Legido A, Yelin K, Khurana D, Kothare SV, Katsetos CD. Anomalous inhibitory circuits in cortical tubers of human tuberous sclerosis complex associated with refractory epilepsy: aberrant expression of parvalbumin and calbindin-D28k in dysplastic cortex. J Child Neurol. 2006;21:1058–1063. doi: 10.1177/7010.2006.00242. [DOI] [PubMed] [Google Scholar]
- Vincent JB, Horike SI, Choufani S, Paterson AD, Roberts W, Szatmari P, Weksberg R, Fernandez B, Scherer SW. An inversion inv(4)(p12-p15.3) in autistic siblings implicates the 4p GABA receptor gene cluster. J Med Genet. 2006;43:429–434. doi: 10.1136/jmg.2005.039693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- Wallis K, Sjogren M, van Hogerlinden M, Silberberg G, Fisahn A, Nordstrom K, Larsson L, Westerblad H, Morreale de Escobar G, Shupliakov O, Vennstrom B. Locomotor deficiencies and aberrant development of subtype-specific GABAergic interneurons caused by an unliganded thyroid hormone receptor alpha1. J Neurosci. 2008;28:1904–1915. doi: 10.1523/JNEUROSCI.5163-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Hua Y, Scheithauer B, Lynch DR, Henske EP, Crino PB. Selective alterations in glutamate and GABA receptor subunit mRNA expression in dysplastic neurons and giant cells of cortical tubers. Ann Neurol. 2001;49:67–78. doi: 10.1002/1531-8249(200101)49:1<67::aid-ana10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Whiting P, McKernan RM, Iversen LL. Another mechanism for creating diversity in gamma-aminobutyrate type A receptors: RNA splicing directs expression of two forms of gamma 2 phosphorylation site. Proc Natl Acad Sci U S A. 1990;87:9966–9970. doi: 10.1073/pnas.87.24.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabut O, Renfro A, Niu S, Swann JW, Marin O, D'Arcangelo G. Abnormal laminar position and dendrite development of interneurons in the reeler forebrain. Brain Res. 2007;1140:75–83. doi: 10.1016/j.brainres.2005.09.070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Photomicrographs of cresyl violet-stained coronal sections through the forebrain of an adult wild type mouse illustrating the cytoarchitecture of the regions of interest analyzed. The areas boxed in A, C and E are shown at higher magnification in B, D and F, respectively. Fr, frontal cortex; Par, parietal cortex; Occ, occipital cortex; DG, dentate gyrus; Str, striatum. Scale bar = 1mm (A, C, E, G and H) and 300μm (B, D, F).