Abstract
The hypertriglyceridemia of infection was traditionally thought to represent the mobilization of substrate to fuel the body's response to the infectious challenge. However, we have previously shown that triglyceride-rich lipoproteins can protect against endotoxin-induced lethality. The current studies examine the mechanism by which this protection occurs. Rats infused with a lethal dose of endotoxin preincubated with chylomicrons had a reduced mortality compared with rats infused with endotoxin alone (15 vs. 76%, P < 0.001). Preincubation with chylomicrons increased the rate of clearance of endotoxin from plasma and doubled the amount of endotoxin cleared by the liver (30 +/- 1 vs. 14 +/- 2% of the total infused radiolabel, P < 0.001). In addition, autoradiographic studies showed that chylomicrons directed more of the endotoxin to hepatocytes and away from hepatic macrophages. Rats infused with endotoxin plus chylomicrons also showed reduced peak serum levels of tumor necrosis factor as compared with controls (14.2 +/- 3.3 vs. 44.9 +/- 9.5 ng/ml, mean +/- SEM, P = 0.014). In separate experiments, chylomicrons (1,000 mg triglyceride/kg) or saline were infused 10 min before the infusion of endotoxin. Chylomicron pretreatment resulted in a reduced mortality compared with rats infused with endotoxin alone (22 vs. 78%, P < 0.005). Therefore, chylomicrons can protect against endotoxin-induced lethality with and without preincubation with endotoxin. The mechanism by which chylomicrons protect against endotoxin appears to involve the shunting of endotoxin to hepatocytes and away from macrophages, thereby decreasing macrophage activation and the secretion of cytokines.
Full text
PDF

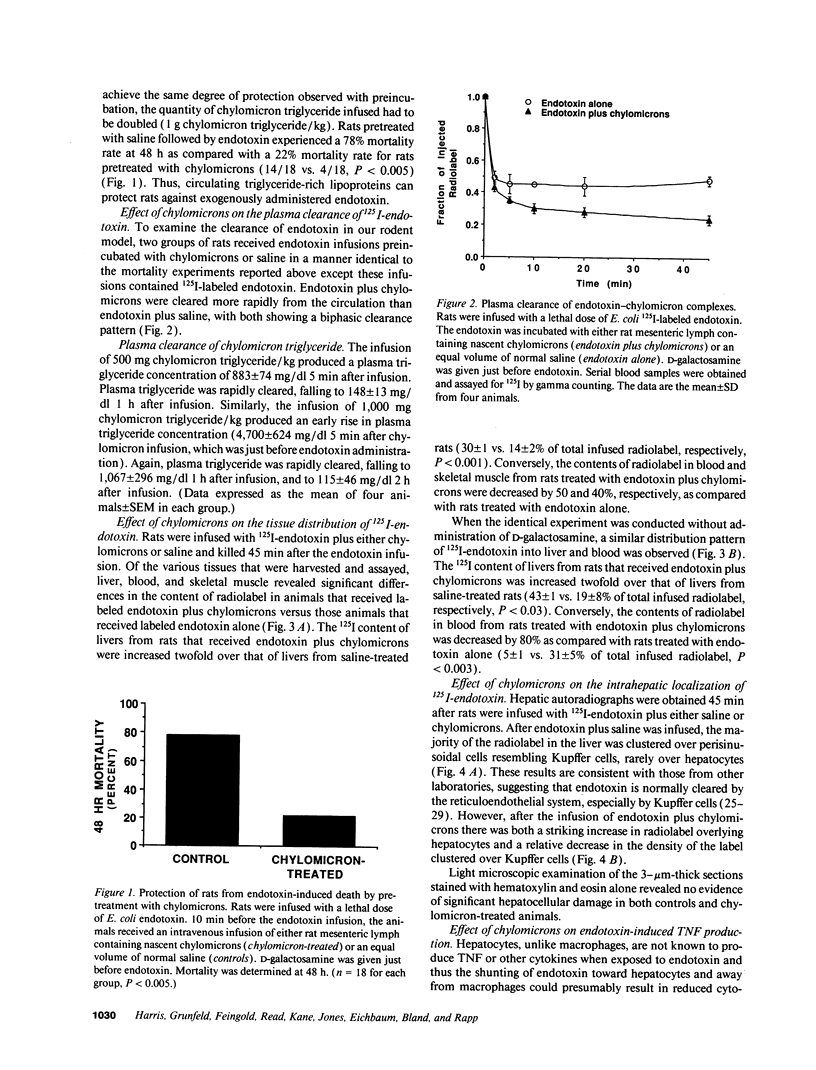
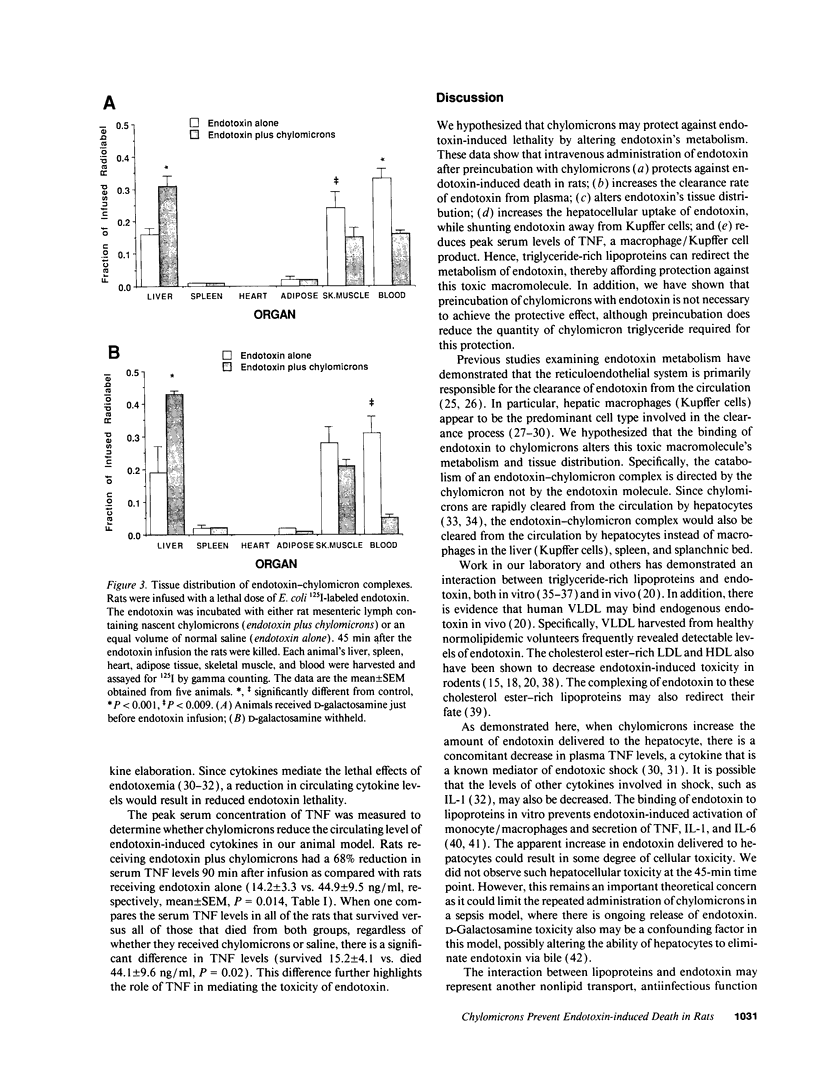
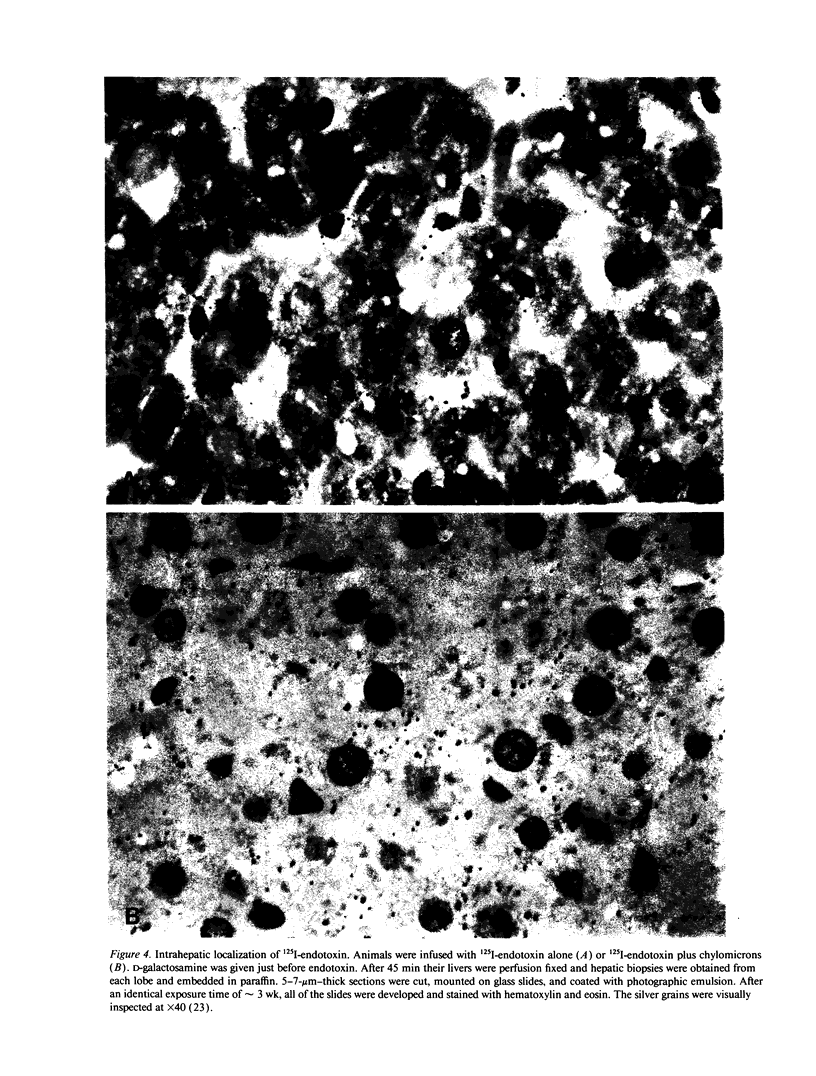


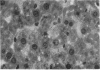
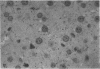
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beisel W. R. Metabolic response to infection. Annu Rev Med. 1975;26:9–20. doi: 10.1146/annurev.me.26.020175.000301. [DOI] [PubMed] [Google Scholar]
- Beutler B. A., Cerami A. Recombinant interleukin 1 suppresses lipoprotein lipase activity in 3T3-L1 cells. J Immunol. 1985 Dec;135(6):3969–3971. [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Black D. D., Tso P., Weidman S., Sabesin S. M. Intestinal lipoproteins in the rat with D-(+)-galactosamine hepatitis. J Lipid Res. 1983 Aug;24(8):977–992. [PubMed] [Google Scholar]
- Castell J. V., Gómez-Lechón M. J., David M., Andus T., Geiger T., Trullenque R., Fabra R., Heinrich P. C. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989 Jan 2;242(2):237–239. doi: 10.1016/0014-5793(89)80476-4. [DOI] [PubMed] [Google Scholar]
- Cavaillon J. M., Fitting C., Haeffner-Cavaillon N., Kirsch S. J., Warren H. S. Cytokine response by monocytes and macrophages to free and lipoprotein-bound lipopolysaccharide. Infect Immun. 1990 Jul;58(7):2375–2382. doi: 10.1128/iai.58.7.2375-2382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari F. V., Curtiss L. K., Jensen F. C. Physiologic concentrations of normal human plasma lipoproteins inhibit the immortalization of peripheral B lymphocytes by the Epstein-Barr virus. J Clin Invest. 1981 Aug;68(2):329–336. doi: 10.1172/JCI110260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington G. J., Wilson D. R., Lachman L. B. Monocyte-conditioned medium, interleukin-1, and tumor necrosis factor stimulate the acute phase response in human hepatoma cells in vitro. J Cell Biol. 1986 Sep;103(3):787–793. doi: 10.1083/jcb.103.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and its biologically related cytokines. Adv Immunol. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- Eichbaum E. B., Harris H. W., Kane J. P., Rapp J. H. Chylomicrons can inhibit endotoxin activity in vitro. J Surg Res. 1991 Nov;51(5):413–416. doi: 10.1016/0022-4804(91)90143-a. [DOI] [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Grunfeld C. Tumor necrosis factor-alpha stimulates hepatic lipogenesis in the rat in vivo. J Clin Invest. 1987 Jul;80(1):184–190. doi: 10.1172/JCI113046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold K. R., Serio M. K., Adi S., Moser A. H., Grunfeld C. Tumor necrosis factor stimulates hepatic lipid synthesis and secretion. Endocrinology. 1989 May;124(5):2336–2342. doi: 10.1210/endo-124-5-2336. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Soued M., Adi S., Staprans I., Neese R., Shigenaga J., Doerrler W., Moser A., Dinarello C. A., Grunfeld C. Effect of interleukin-1 on lipid metabolism in the rat. Similarities to and differences from tumor necrosis factor. Arterioscler Thromb. 1991 May-Jun;11(3):495–500. doi: 10.1161/01.atv.11.3.495. [DOI] [PubMed] [Google Scholar]
- Flegel W. A., Wölpl A., Männel D. N., Northoff H. Inhibition of endotoxin-induced activation of human monocytes by human lipoproteins. Infect Immun. 1989 Jul;57(7):2237–2245. doi: 10.1128/iai.57.7.2237-2245.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Freudenberg N., Galanos C. Time course of cellular distribution of endotoxin in liver, lungs and kidneys of rats. Br J Exp Pathol. 1982 Feb;63(1):56–65. [PMC free article] [PubMed] [Google Scholar]
- Grunfeld C., Adi S., Soued M., Moser A., Fiers W., Feingold K. R. Search for mediators of the lipogenic effects of tumor necrosis factor: potential role for interleukin 6. Cancer Res. 1990 Jul 15;50(14):4233–4238. [PubMed] [Google Scholar]
- Grunfeld C., Feingold K. R. The metabolic effects of tumor necrosis factor and other cytokines. Biotherapy. 1991;3(2):143–158. doi: 10.1007/BF02172087. [DOI] [PubMed] [Google Scholar]
- Guckian J. C. Role of metabolism in pathogenesis of bacteremia due to Diplococcus pneumoniae in rabbits. J Infect Dis. 1973 Jan;127(1):1–8. doi: 10.1093/infdis/127.1.1. [DOI] [PubMed] [Google Scholar]
- Hajduk S. L., Moore D. R., Vasudevacharya J., Siqueira H., Torri A. F., Tytler E. M., Esko J. D. Lysis of Trypanosoma brucei by a toxic subspecies of human high density lipoprotein. J Biol Chem. 1989 Mar 25;264(9):5210–5217. [PubMed] [Google Scholar]
- Harris H. W., Eichbaum E. B., Kane J. P., Rapp J. H. Detection of endotoxin in triglyceride-rich lipoproteins in vitro. J Lab Clin Med. 1991 Aug;118(2):186–193. [PubMed] [Google Scholar]
- Harris H. W., Grunfeld C., Feingold K. R., Rapp J. H. Human very low density lipoproteins and chylomicrons can protect against endotoxin-induced death in mice. J Clin Invest. 1990 Sep;86(3):696–702. doi: 10.1172/JCI114765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf U., Ramadori G., Möller B., Galanos C. Hepatocellular clearance function of bacterial lipopolysaccharides and free lipid A in mice with endotoxic shock. Am J Emerg Med. 1984 Jan;2(1):13–19. doi: 10.1016/0735-6757(84)90105-0. [DOI] [PubMed] [Google Scholar]
- Kaufmann R. L., Matson C. F., Beisel W. R. Hypertriglyceridemia produced by endotoxin: role of impaired triglyceride disposal mechanisms. J Infect Dis. 1976 May;133(5):548–555. doi: 10.1093/infdis/133.5.548. [DOI] [PubMed] [Google Scholar]
- Marinkovic S., Jahreis G. P., Wong G. G., Baumann H. IL-6 modulates the synthesis of a specific set of acute phase plasma proteins in vivo. J Immunol. 1989 Feb 1;142(3):808–812. [PubMed] [Google Scholar]
- Masoro E. J. Fat metabolism in normal and abnormal states. Am J Clin Nutr. 1977 Aug;30(8):1311–1320. doi: 10.1093/ajcn/30.8.1311. [DOI] [PubMed] [Google Scholar]
- Mathison J. C., Ulevitch R. J. In vivo interaction of bacterial lipopolysaccharide (LPS) with rabbit platelets: modulation by C3 and high density lipoproteins. J Immunol. 1981 Apr;126(4):1575–1580. [PubMed] [Google Scholar]
- Mathison J. C., Ulevitch R. J. The clearance, tissue distribution, and cellular localization of intravenously injected lipopolysaccharide in rabbits. J Immunol. 1979 Nov;123(5):2133–2143. [PubMed] [Google Scholar]
- Munford R. S., Andersen J. M., Dietschy J. M. Sites of tissue binding and uptake in vivo of bacterial lipopolysaccharide-high density lipoprotein complexes: studies in the rat and squirrel monkey. J Clin Invest. 1981 Dec;68(6):1503–1513. doi: 10.1172/JCI110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Lipton J. M., Dietschy J. M. Biological activity, lipoprotein-binding behavior, and in vivo disposition of extracted and native forms of Salmonella typhimurium lipopolysaccharides. J Clin Invest. 1982 Oct;70(4):877–888. doi: 10.1172/JCI110684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M., Hough G. P., Van Lenten B. J., Berliner J. A., Fogelman A. M. Low density lipoproteins transfer bacterial lipopolysaccharides across endothelial monolayers in a biologically active form. J Clin Invest. 1988 Feb;81(2):601–605. doi: 10.1172/JCI113359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens B. J., Anantharamaiah G. M., Kahlon J. B., Srinivas R. V., Compans R. W., Segrest J. P. Apolipoprotein A-I and its amphipathic helix peptide analogues inhibit human immunodeficiency virus-induced syncytium formation. J Clin Invest. 1990 Oct;86(4):1142–1150. doi: 10.1172/JCI114819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. S., Shepard H. M., Wilking H., Lewis G., Aggarwal B. B., Eessalu T. E., Gavin L. A., Grunfeld C. Interferons and tumor necrosis factors have similar catabolic effects on 3T3 L1 cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8313–8317. doi: 10.1073/pnas.83.21.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekala P. H., Kawakami M., Angus C. W., Lane M. D., Cerami A. Selective inhibition of synthesis of enzymes for de novo fatty acid biosynthesis by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2743–2747. doi: 10.1073/pnas.80.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Dinarello C. A., Punsal P. I., Colten H. R. Cachectin/tumor necrosis factor regulates hepatic acute-phase gene expression. J Clin Invest. 1986 Nov;78(5):1349–1354. doi: 10.1172/JCI112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prioli R. P., Rosenberg I., Shivakumar S., Pereira M. E. Specific binding of human plasma high density lipoprotein (cruzin) to Trypanosoma cruzi. Mol Biochem Parasitol. 1988 Apr;28(3):257–263. doi: 10.1016/0166-6851(88)90010-2. [DOI] [PubMed] [Google Scholar]
- Rifkin M. R. Identification of the trypanocidal factor in normal human serum: high density lipoprotein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3450–3454. doi: 10.1073/pnas.75.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer C. A., Cerami A. Hypertriglyceridemia associated with Trypanosoma brucei brucei infection in rabbits: role of defective triglyceride removal. Mol Biochem Parasitol. 1980 Oct;2(1):31–38. doi: 10.1016/0166-6851(80)90046-8. [DOI] [PubMed] [Google Scholar]
- Seganti L., Grassi M., Mastromarino P., Panà A., Superti F., Orsi N. Activity of human serum lipoproteins on the infectivity of rhabdoviruses. Microbiologica. 1983 Apr;6(2):91–99. [PubMed] [Google Scholar]
- Sernatinger J., Hoffman A., Hardman D., Kane J. P., Levy J. A. Neutralization of mouse xenotropic virus by lipoproteins involves binding to the virions. J Gen Virol. 1988 Oct;69(Pt 10):2657–2661. doi: 10.1099/0022-1317-69-10-2657. [DOI] [PubMed] [Google Scholar]
- Shortridge K. F., Ho W. K., Oya A., Kobayashi M. Studies on the inhibitory activities of human serum lipoproteins for Japanese encephalitis virus. Southeast Asian J Trop Med Public Health. 1975 Dec;6(4):461–466. [PubMed] [Google Scholar]
- St Hilaire R. J., Hradek G. T., Jones A. L. Hepatic sequestration and biliary secretion of epidermal growth factor: evidence for a high-capacity uptake system. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3797–3801. doi: 10.1073/pnas.80.12.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalenhoef A. F., Malloy M. J., Kane J. P., Havel R. J. Metabolism of apolipoproteins B-48 and B-100 of triglyceride-rich lipoproteins in normal and lipoprotein lipase-deficient humans. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1839–1843. doi: 10.1073/pnas.81.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terkeltaub R., Curtiss L. K., Tenner A. J., Ginsberg M. H. Lipoproteins containing apoprotein B are a major regulator of neutrophil responses to monosodium urate crystals. J Clin Invest. 1984 Jun;73(6):1719–1730. doi: 10.1172/JCI111380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Ulevitch R. J., Cochrane C. G. Role of complement in lethal bacterial lipopolysaccharide-induced hypotensive and coagulative changes. Infect Immun. 1978 Jan;19(1):204–211. doi: 10.1128/iai.19.1.204-211.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Isolation and characterization of a bacterial lipopolysaccharide-high density lipoprotein complex formed in rabbit plasma. J Clin Invest. 1981 Mar;67(3):827–837. doi: 10.1172/JCI110100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Their participation in intravascular reactions of bacterial lipopolysaccharides. J Clin Invest. 1979 Nov;64(5):1516–1524. doi: 10.1172/JCI109610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J. The preparation and characterization of a radioiodinated bacterial lipopolysaccharide. Immunochemistry. 1978 Mar;15(3):157–164. doi: 10.1016/0161-5890(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Van Bossuyt H., Wisse E. Cultured Kupffer cells, isolated from human and rat liver biopsies, ingest endotoxin. J Hepatol. 1988 Aug;7(1):45–56. doi: 10.1016/s0168-8278(88)80505-1. [DOI] [PubMed] [Google Scholar]
- Van Lenten B. J., Fogelman A. M., Haberland M. E., Edwards P. A. The role of lipoproteins and receptor-mediated endocytosis in the transport of bacterial lipopolysaccharide. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2704–2708. doi: 10.1073/pnas.83.8.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi G., Gelfand J. A., Burke J. F., Thompson R. C., Dinarello C. A. A specific receptor antagonist for interleukin 1 prevents Escherichia coli-induced shock in rabbits. FASEB J. 1991 Mar 1;5(3):338–343. doi: 10.1096/fasebj.5.3.1825816. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Shaw J. H., Durkot M. J. Effect of sepsis on VLDL kinetics: responses in basal state and during glucose infusion. Am J Physiol. 1985 Jun;248(6 Pt 1):E732–E740. doi: 10.1152/ajpendo.1985.248.6.E732. [DOI] [PubMed] [Google Scholar]
- Zlydaszyk J. C., Moon R. J. Fate of 51Cr-labeled lipopolysaccharide in tissue culture cells and livers of normal mice. Infect Immun. 1976 Jul;14(1):100–105. doi: 10.1128/iai.14.1.100-105.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]