Abstract
Objective
CD34+ cells, present within the bone marrow, have previously been shown to possess pancreatic endocrine potential. Based on this observation, we explored the capacity of CD34+ cells derived in culture from the differentiation of human embryonic stem cells (hESC), for their in-vivo pancreatic endocrine capacity.
Methods
Sheep were transplanted with hESC-derived CD34+ cells, as well as non-sorted differentiated cultures. Transplantations were carried out with in-utero intraperitoneal injections prior to the development of the immune system in the fetus so that tolerance towards foreign antigens was acquired during gestation and persisted in the adult.
Results
All cell populations that were tested demonstrated human cellular activity and long-term presence up to 5 years. However, the in-vivo beta-cell-like activity achieved from the transplantation of the sorted CD34+ cell population was not augmented by transplanting the entire cell population from which the CD34+ cells were isolated. Human DNA and insulin mRNA were detected in sheep pancreases. An average of 1.51 ng/mL human C-peptide was detected in serum from 8 animals transplanted with differentiated cell populations and assayed up to 55 months post-transplantation. Transplantation of as few as 23,500 cells resulted in long-term sustainable beta-cell like activity. Teratomas were absent in the transplanted animals.
Conclusion
Our data suggest that hESC-derived CD34+ cells have a potential for long term in-vivo endocrine cellular activity which could prove useful in regenerative medicine. Since the same cell population has previously been shown to contain hematopoietic potential, it could be used for the induction of immunological tolerance and bone marrow chimerism prior to cellular therapy for diabetes.
Keywords: Human embryonic stem cells, Pancreas, Transplantation, Beta-cells, C-peptide
One potential therapy for diabetics is the restoration of beta-cell mass for the endogenous control of glycemia [1,2]. To this end, human embryonic stem cells (hESC) which have the capacity to form tissues of all three germ layers cells [3], are being evaluated for cellular transplantation therapy. hESC differentiation cultures have been developed for the derivation of progenitor cells that engraft and differentiate in-vivo into beta-cell-like cells [4,5]. Such research has had to occur in the absence of knowledge of a single or a group of cell-surface markers that may be used to unequivocally identify and isolate pancreatic stem cells [6]. Hence, focus has been placed on delineating culture conditions that generate endodermal germ layer cells which, during development, give rise to numerous tissues and organs, including the endocrine pancreatic cells.
Adult bone marrow (BM)-derived cells have also been evaluated for their in-vivo endocrine pancreatic potential. While a derivative of the mesodermal germ layer, BM cells have been shown to transdifferentiate into various other mesodermal derivatives as well as into cells which, during development, descend from the endodermal germ layer [7]. In light of this, a previous study demonstrated the in-vivo transdifferentiation of BM cells into beta-cell-like cells [8]. Other studies have confirmed [9,10] or refuted [11,12] this. Interestingly, the confirmations came from study designs where BM transplantations followed pancreatic injury, whereas refutations came from study designs where BM transplantations preceded pancreatic injury. It is likely that stem cells need to be mobilized or transplanted after the creation of a stem cell niche through chemical or mechanical injury in an organ in order for subsequent donor cell contribution, and that recipients transplanted preceding the creation of such a niche have no room for engraftment.
It is now commonly accepted that the CD34+ cell surface marker delineates a population of cells from the BM which encompasses hematopoietic stem cells (HSC) [13]. Recently, the CD34+ cell population differentiated in culture from hESC was also shown to contain HSC [14,15]. It must be noted however that the CD34+ marker is present on non-HSC populations in non-hematopoietic organs as well. Moreover, the CD34+ marker has been implicated in a population of cells isolated from the adult human pancreas with progenitor/stem cell characteristics of the pancreas such that these cells synthesized insulin under certain culture conditions [16].
Hence, based on previous observations that BM cells, which comprise HSC [13], may have the potential to generate beta-cell-like cells [8–10], that HSC are present in the CD34+ population derived from hESC [14,15], and that the CD34+ cell surface maker is present on a cell population in the adult human pancreas implicated with progenitor/stem characteristics [16], we hypothesized that hESC-derived CD34+ cells may have the potential for generating beta-cell-like cells. To investigate this, sheep were transplanted in-utero with hESC-derived CD34+ cells as well as the unfractionated populations of differentiated hESC, and analyzed for pancreatic endocrine function. The in-utero model provides donor cells with expanding stem cell niches that may be populated in the recipient, and a fetal microenvironment that presents cues for the ontogeny of organs and tissues.
We provide evidence in this study for the pancreatic engraftment and differentiation into endocrine cells by hESC-derived CD34+ cells as well as by the unfractionated differentiated hESC. Using a large animal model facilitated data collection long-term thereby shedding light on the in-vivo effect of progeny cells from hESC on animal health and physiology. This study did not generate teratomas in the sheep model while human C-peptide was measured in sheep serum up to 5 years post-transplantation. To the best of our knowledge, this is the first study demonstrating the long-term utility of hESC-derived CD34+ cells for pancreatic engraftment, raising the possibility for use as cellular therapy for diabetes and regenerative medicine.
Materials and Methods
Cell culture and cell differentiation
The hESC line H1 was propagated on mouse embryonic fibroblasts under conditions to maintain undifferentiated cells in serum-containing media [3]. Passages 32 to 67 were used in these studies. For differentiation by coculture, cells were plated on S17 mouse BM stromal cell line (kindly provided by Kenneth Dorshkind, University of California, Los Angeles, CA) as described previously [14]. For differentiation by embryoid body (EB) formation, undifferentiated hESC colonies were incubated with 0.5 mg/mL dispase (Invitrogen, Carlsbad, CA) at 37°C for 0.5–1 hr to allow for detachment. Cells were briefly centrifuged, washed with media, and placed in T25 culture flasks (BD Falcon, Franklin Lakes, NJ) coated with poly-2-hydroxyethyl methacrylate (Sigma, St. Louis, MO). EB differentiation media consisted of IMDM (Invitrogen) supplemented with 15% FBS (Stem Cell Technologies, Vancouver, BC, Canada), 1% MEM amino acid and 0.5 mM β-mercaptoethanol (Sigma). Media was changed every two days and flasks were tapped daily to keep EBs in suspension. Both differentiation cultures were started in Madison, WI, and shipped to Reno, NV, by overnight courier around 6–14 days of differentiation. Cells were harvested on indicated days after further culture maintenance in Reno.
Cell isolation
Single-cell suspensions of hESC differentiated on S17 were prepared as described previously [14]. Single-cell suspensions of hESC differentiated into EB were prepared by brief centrifugation of EB and digestion with 0.05% Trypsin-EDTA (Invitrogen) in the presence of 2% chicken serum (Sigma) at 37°C for 1 hr. Cells were washed and kept in media until further manipulation. CD34+ cells from differentiated cultures were concentrated with magnetic anti-CD34 microbeads (Miltenyi, Auburn, CA). CD34+/lineage− (CD34+/Lin−) or CD34+/CD38− cells were further purified on FACS Vantage (Becton Dickinson, Mountain View, CA). In this manner, the CD34+ populations were further depleted of non-stem cells. The lineage cocktail for hematopoietic depletion included CD2, CD4, CD8, CD14, CD15, CD16, CD19, and Glycophorin A. All antibodies were fluorescein isothiocyanate-labelled except CD34 which was phycoerythrin-labelled (BD BioSciences, San Jose, CA).
Reverse-transcriptase PCR (RT-PCR) for characterization of donor cells
Cells used for transplantation were characterized by RT-PCR. Gene-specific primers are described in Supplemental Table 1. Cells were harvested from culture or after isolation by FACS and stored at −20°C. mRNA was extracted with a kit according to manufacturer’s protocol (μMACS mRNA Isolation Kit, Miltenyi) and cDNA was synthesized with oligo-dT primers (SuperScript First Strand Synthesis System, Invitrogen). PCR was performed on an Eppendorf MasterCycler Gradient. Thirty-eight cycles of a three step reaction (94°C for 15s, 58°C for 15s, and 72°C for 15s) were carried out after an initial incubation at 94°C for 4 min. PCR was repeated with 50 cycles to confirm negative data and repeat positive data. Platinum Taq SuperMix PCR kit (Invitrogen) was used per manufacture’s directions with one μL of each primer from a 40 μM stock. Ten nanograms of template cDNA were used per reaction.
Sheep transplantation
Cells for transplantation were suspended in 0.5–1 mL of QBSF-60 media (Quality Biologicals, Gaithersburg, MD, USA). Twenty-three preimmune sheep fetuses (55–65 days old, term is 150 days) received transplants of 23,500 cells to 10 million cells that included sorted CD34+ populations (CD34+/Lin− or CD34+/CD38−), or whole differentiated populations (see Tables 1–3). Injections were into the peritoneal cavity as described previously [17]. Merino × Rambouillet cross breed lambs were born at term ~3 months later. This study was carried out at the University of Nevada, Reno, with approval from the Institutional Animal Care and Use Committee (IACUC). Procedures were carried out at the Agriculture Experiment Station surgical facility in accordance with Standard Operating Procedures (IACUC protocol). All animals were cared for according to standards of the U.S. Public Health Policy on the Humane Care and Use of Laboratory Animals (PHS Manual, Chap. 143).
Table 1.
Animals transplanted with cells differentiated by EB formation by hESC in suspension culture. Transplanted sheep were tested for human C-peptide. Serum was sampled at the specified time point (months) after transplantation. One animals tested positive* (see Table 3).
| Animal no. | Cell line & passage no. | Differentiated hESC | Cell type injected | Numbers injected | Time sampled (months post-transplantation) |
|---|---|---|---|---|---|
| 2136 | H1 p57 | EB, day 8 | Whole population | 5 × 106 | 20 |
| 2138 | H1 p57 | EB, day 8 | Whole population | 7.5 × 106 | 15 |
| 2139 | H1 p57 | EB, day 8 | Whole population | 7.5 × 106 | 15 |
| 2140 | H1 p57 | EB, day 8 | Whole population | 10 × 106 | 20 |
| 2141 | H1 p57 | EB, day 8 | Whole population | 10 × 106 | 20 |
| 2114 | H1 p49 | EB, day 8 | CD34+/CD38− | 33,000 | 20 |
| 2132 | H1 p54 | EB, day 8 | CD34+/CD38− | 100,000 | 19 |
| *2135 | H1 p54 | EB, day 8 | CD34+/CD38− | 100,000 | 20–39 |
Table 3.
Human C-peptide and glucose levels in serum from sheep that were transplanted with hESC-derived cells. Twenty-three transplanted animals and ten controls (non-transplanted) were sampled after fasting for 24 hr. Of these, eight transplanted animals had detectable levels of human C-peptide as reported here.
| Animal no. | Cells transplanted | When sampled (months post-transplant) | Human C-peptide (ng/mL)* | Glucose (mM) |
|---|---|---|---|---|
| 1748 | 140,000 | 38 | 0.72 ± 8% | 3.3 ± 1% |
| CD34+/CD38− on S17(day 17) | 39† | 0.77 ± 7% | 2.4 ± 1% | |
| 39† | 0.38 ± 10% | 2.8 ± 6% | ||
| 1876‡ | 23,500 | 27 | 0.75 ± 1% | 3.6 ± 4% |
| CD34+/Lin− on S17(day 17) | 29 | <0.32§ ± 14% | 3.4 ± 1% | |
| 1885 | 63,000 | 28 | 1.52 ± 5% | 2.8 ± 3% |
| CD34+/Lin− on S17(day 17) | 31 | 1.35 ± 3% | 3.6 ± 1% | |
| 32 | 0.62 ± 10% | 3.5 ± 0% | ||
| 1887 | 70,000 | 29 | 0.59 ± 3% | 2.5 ± 6% |
| CD34+/Lin− on S17(day 17) | 31 | 0.66 ± 3% | 2.6 ± 1% | |
| 55 | 0.77 ± 5% | 2.9 ± 4% | ||
| 1939 | 1,000,000 | 31 | 3.53 ± 1% | 2.8 ± 3% |
| All cells from S17(day 17) | 33 | 1.40 ± 5% | 2.9 ± 1% | |
| 52 | 2.30 ± 8% | 2.8 ± 5% | ||
| 1952 | 1,000,000 | 24 | 1.79 ± 2% | ND¶ |
| All cells from S17(day 18) | 29 | 4.04 ± 4% | 3.0 ± 1% | |
| 30 | 1.23 ± 9% | 3.1 ± 4% | ||
| 2034 | 80,000 | 20 | 1.01 ± 9% | ND¶ |
| CD34+/CD38− on S17(day 8) | 23 | 1.96 ± 8% | 2.9 ± 1% | |
| 31 | 0.85 ± 15% | 2.5 ± 6% | ||
| 2135 | 100,000 | 20 | 1.21 ± 1% | 3.2 ± 2% |
| CD34+/CD38− from EB(day 8) | 23 | 4.49 ± 2% | 2.1 ± 2% | |
| 39 | 2.62 ± 6% | 2.1 ± 5% | ||
Human C-peptide assay (ELISA) standards ranged from 0.5–20 ng/mL with a detection limit of 0.32 ng/mL. Error is reported as the coefficient of variation (%).
These samples were collected two weeks apart within the same month.
Animal #1876 was sampled twice only.
Numbers reported here were close to but slightly below the calculated detection limit and visibly positive on the ELISA plate.
Not done.
Sheep sampling
Sheep blood samples were collected in Vacutainer blood collection tubes (Becton Dickinson) in the mornings prior to feeding the animals. Tubes were centrifuged briefly at low speed and the separated serum layer was transferred into fresh tubes and archived at −20°C.
To obtain pancreatic tissue samples, animals were euthanized in accordance with Standard Operating Procedures (IACUC protocol). Samples were collected in RNAlater (Ambion, Austin, TX) and preserved according to manufacturer’s instructions. Samples were also collected in buffered formalin (Astral Diagnostics, West Deptford, NJ), fixed overnight, and placed in tissue cassettes (Fisher, Fair Lawn, NJ) that were embedded in paraffin wax.
Human ELISA kits
Human C-peptide ELISA kits were obtained from Alpha Diagnostic International (San Antonio, TX). Assays were carried out on sheep serum according to manufacturer’s protocols. The specificity of the kit was established by assaying the serum from 10 non-transplanted control animals which ascertained that there was no cross reaction of the kit with control sheep serum.
Serum glucose
Sheep serum samples were tested for glucose levels in order to demonstrate that the measured C-peptide levels corresponded to basal levels of glucose. Glucose assay kits were obtained from BioVision Research Products (Mountain View, CA). Samples were processed according to manufacturer’s directions.
Immunohistochemistry (IHC)
IHC and E&H staining of paraffin-embedded tissue sections were carried out according to standard protocols. For IHC, primary antibodies used were: mouse anti-human Chromogranin-A (clone DAK-A3, DAKO, Carpinteria, CA) at 1/50 dilution, mouse anti-human proinsulin (clone CPEP-01, Millipore, Temecula, CA) at 1/100 dilution, mouse anti-human C-peptide (clone M607239, Fitzgerald, Concord, MA) at 1/100 dilution, and mouse anti-CD34 (BD BioSciences) at 1/50 dilution. Host-matched secondary antibodies that were HRP-conjugated or AP-conjugated (JacksonImmuno, West Grove, PA) were used at a dilution of 1:100 after the addition of 1:1 glycerol (Sigma). Slides were developed with 3,3′ Diaminobenzidine (DAB) substrate chromogen system (DAKO) which stained positive patches a brown color or with Permanent Red (DAKO) which stained positive patches a red color while the background was counter-stained blue with Hematoxylin (Sigma). Images were acquired using an Olympus BX60 microscope (Olympus, Melville, NY) with an Olympus UPlanFI 40 × 0.75 or 20 × 0.50 numeric aperture objective lenses, an Olympus DP70 camera, and Olympus DP Controller 2.1.1.183 software. Images were processed using Adobe Photoshop CS (Adobe Systems, San Jose, CA) by adjusting curves to modify the entire color channel (RGB) of the whole image, one photomicrograph at a time.
PCR of pancreatic tissue from transplanted sheep
PCR was carried out in order to determine the presence of human DNA indicative of the presence/engraftment of human cells/tissue in sheep tissue. Tissue samples approximating 1 mm3 trimmed from sheep pancreases were preserved in RNAlater. DNA was processed using DNeasy kit according to manufacturer’s directions (Qiagen, Valencia, CA). Beta-actin primers that amplified both human and sheep templates were forward: 5′-AAA AGC CAC CCC ACT TCT CT-3′; reverse: 5′-CTC AAG TTG GGG GAC AAA AA-3′. Human-specific GAPDH primers were forward: 5′-AGT CCC TGC CAC ACT CAG TC-3′, reverse: 5′-GCA CAG GGT ACT TTA TTG ATG G-3′. PCR was carried out as described above.
Real time RT-PCR was carried out in order to detect the presence of human insulin mRNA transcripts in pancreas from the transplanted sheep. mRNA was isolated and cDNA synthesized as described above. Human-specific primers and probe for insulin were forward: 5′-TCA GAA GAG GCC ATC AAG CA-3′; reverse: 5′-TTC CCC GCA CAC TAG GTA GAG A -3′; and probe: 5′-CAG CCG CAG CCT TTG TGA ACC AAC -3′. Sheep-specific primers and probe for insulin were forward: 5′-GCA GAA GCG TGG CAT CGT-3′; reverse: 5′-GCT CGT CAG GGC TTT ATT GG-3′; and probe: 5′-CGC CGG CGT CTG CTC TCT CTA CC-3′. The probes were labeled with 6-FAM at the 5′ end and black hole quencher 1 at the 3′ end. PCR was carried out using TaqMan Universal PCR Master Mix (Applied Biosystems (AB), Foster City, CA). Real-time RT-PCR was performed on an ABI PRISM 7000 Sequence Detection System (SDS) (AB) using ABI PRISM 7000 SDS Software CTS 1.
Human tissue
Human adult pancreatic tissue for use as control was obtained through National Disease Research Interchange (NDRI), Philadelphia, PA, after submitting protocols approved by our institutional review board. Human fetal tissue for use as control was purchased from Advanced Bioscience Resources (Alameda, CA), who have their own institutional guidelines and informed consent requirements for tissue collection. Adult BM was collected from a normal, healthy volunteer donor upon approval from our institutional human subjects review board.
Results
Genetic profile in differentiation cultures used for CD34+ cell isolation
The cell populations used for transplantation were characterized for genes that signified pluripotency (NANOG, POU51, and NODAL), differentiation into germ layers (TBX1, MEOX1, SOX17, GSC, ZIC1, CXCR4), and commitment to the pancreatic lineage (REG1B, PAX4, PDX1, and INS). While caution must be taken to not interpret data from a heterogeneous cell population to represent each individual cell within the population, or to associate the expression of a single gene to a single event, a scheme for the genes tested is none-the-less provided (Supplemental Table 2). Samples analyzed included undifferentiated hESC (hESC), hESC differentiated on S17 for 17 days (S17/day 17) and CD34+ cells isolated from these (S17/CD34+), and hESC differentiated into EB for 8 days (EB/day 8) and CD34+ cells isolated from these (EB/CD34+). Adult BM-derived CD34+ cells (BM/CD34+) and fetal human pancreas (22–24 week) were included as controls.
NANOG, POU5F1 (OCT4), and NODAL, transcription factors required for pluripotency [18,19], were present in hESC, and did not completely disappear from the differentiation cultures on the days sampled (S17/day 17 and EB/day 8), although they were absent in the CD34+ fractions isolated from these (Fig. 1). TBX1 (Brachyury) and MEOX1 (mesenchyme homeobox 1), associated with the mesoderm [20], were present in EB/day 8 while only MEOX1 was present in S17/day 17. SOX17 and GSC, expressed in the endoderm [20], were present in EB/day 8 while only SOX17 was present in S17/day 17. The faint expression of SOX17 in hESC may be an indication of some peripheral differentiation in the culture. GSC is also expressed in the mesoderm, and ZIC1 (zinc finger protein of the cerebellum 1) is expressed by the mesoderm and ectoderm [20]; both these genes were present in the EB/day 8 sample. CXCR4 (chemokine receptor 4), expressed in the mesoderm and endoderm [20], was present in both the differentiated cultures but absent in the CD34+ cells isolated from these.
Figure 1.
Characterization of donor cells by RT-PCR: Presence of genes pertinent to germ layers and differentiation, and commitment to the pancreatic lineage. hESC was differentiated by co-culture on S17 stromal layer for 17 days (S17/Day 17) or by EB formation for 8 days (EB/day 8). CD34+ cells were isolated from these: (S17/CD34+) and (EB/CD34+). RT-PCR was performed for genes indicated. Lane 1: water; 2: hESC; 3: S17/day 17, 4: S17/CD34+, 5: EB/day 8, 6: EB/CD34+; 7: human fetal pancreas, 8: water, 9: CD34+ from adult BM (BM/CD34+), and 10: human fetal pancreas (22–24 wk).
REG1B (reg I beta protein), expressed by acinar cells of the pancreas [21], was absent in all test samples. The transcription factors PAX4 (paired box gene 4) and PDX1 (pancreatic and duodenal homeobox 1), important in beta-cell differentiation [22], were present in EB/day 8 sample but only PAX4 was present in the S17/day 17 sample. INS (insulin), indicative of beta-cells of the pancreas, was present in both differentiation cultures but absent in the CD34+ cells fractionated from these. NES (Nestin), a broadly expressed intermediate filament protein, was expressed in all samples except in the hESC-derived CD34+ fractions. KRT19 (keratin 19), a broadly expressed gene during gastrulation which is also expressed by pancreatic ductal cells [23], was present in all samples except EB/CD34+ and BM/CD34+. B2M (beta-2-microglobulin) and ACTB (beta-actin), house-keeping genes, were present in all samples.
Hence, the genetic profile of hESC and cells derived from it indicated that the pluripotent nature of hESC is progressively lost upon differentiation such that the CD34+ cells fractionated from culture were totally devoid of pluripotent genes. Further, while the differentiation cultures presented markers for the three germ layers as well as markers specific for the pancreatic lineage, the CD34+ cells did not present these genes. Therefore, the isolated CD34+ cell populations were not committed to the pancreatic lineage or even more broadly to any of the germ layers. We demonstrated by flow cytometry that culture systems that generated CD34+ cells used for transplantation supported the commitment and/or differentiation of various hematopoietic lineages (Supplemental Fig. 1), concurrent with the commitment and/or differentiation of insulin-positive cells (Fig. 1). Cells for transplantation were sorted as CD34+/CD38− population which in essence included the entire CD34+ population as CD38 was not expressed on any of the cells (Supplemental Fig. 1), and CD34+/Lin− population where CD34+ cells expressing other hematopoietic lineage markers were depleted (see methods). We also transplanted unfractionated EB/day 8 and S17/day 17 populations, both of which encompassed CD34+ cells, hematopoietic cells, and insulin-positive cells.
The profiles of CD34+ cells isolated from S17 co-culture (specific differentiation, hematopoietic microenvironment [24]) or by EB formation (non-specific differentiation) differed from BM-derived CD34+ cells (Fig. 1) in that they were devoid of numerous markers expressed by BM/CD34+ cells. BM-derived CD34+ cell populations have previously been demonstrated to express many genes not restricted to the hematopoietic lineage including NANOG, POU5F1, PDX1, CXCR4, NES, and KRT19 [25].
Presence of human C-peptide in chimeric sheep serum
Twenty-three sheep were transplanted with 23,500 to 10,000,000 cells of various types (see Tables 1–2). Serum samples were collected from the sheep and tested for the presence of human C-peptide by ELISA at times indicated (Tables 1–3). Seven out of fifteen (47%) of the S17 transplants and one out of eight (13%) of the EB transplants had measurable levels of human C-peptide in serum sampled up to 55 months post-transplantation. Chimeric animals were weighed in order to gain a perspective on the number of cells transplanted (Tables 1–2) and the level of human beta cell activity achieved (Table 3) vs. the size of the animals which weighed between 81–125 kg (Supplemental Table 3). The highest serum level of human C-peptide was 4.49 ng/mL (1.5 nM) and the average was 1.51 ng/mL (0.5 nM) (Table 3). In lactating ewes, the basal concentration of C-peptide is 1.5–2.0 nM [26].
Table 2.
Animals transplanted with cells differentiated by co-culture of hESC on S17 stroma layer. Transplanted sheep were tested for human C-peptide. Serum was sampled at the specified time point (months) after transplantation. Seven animals tested positive* (see Table 3).
| Animal no. | Cell line & passage no. | Differentiated hESC | Cell type injected | Numbers injected | Time sampled (months post-transplantation) |
|---|---|---|---|---|---|
| 1916 | H1 p32 | S17, day 17 | Whole population | 1.5 × 106 | 25 |
| *1939 | H1 p59 | S17, day 17 | Whole population | 1.0 × 106 | 31–52 |
| *1952 | H1 p67 | S17, day 18 | Whole population | 1.0 × 106 | 24–30 |
| *1748 | H1.1 p56 | S17, day 17 | CD34+/CD38− | 140,000 | 38–39 |
| 1749 | H1.1 p56 | S17, day 17 | CD34+/CD38− | 140,000 | 33 |
| 2032 | H1 p55 | S17, day 8 | CD34+/CD38− | 200,000 | 19 |
| 2033 | H1 p55 | S17, day 8 | CD34+/CD38− | 80,000 | 20 |
| *2034 | H1 p55 | S17, day 8 | CD34+/CD38− | 80,000 | 20–31 |
| 1838 | H1 p35 | S17, day 17 | CD34+/Lin− | 95,000 | 30 |
| 1869 | H1 p43 | S17, day 17 | CD34+/Lin− | 60,000 | 30 |
| 1871 | H1 p45 | S17, day 17 | CD34+/Lin− | 26,500 | 29 |
| *1876 | H1 p44 | S17, day 17 | CD34+/Lin− | 23,500 | 27–29 |
| *1885 | H1 p48 | S17, day 17 | CD34+/Lin− | 63,000 | 28–32 |
| 1886 | H1 p49 | S17, day 17 | CD34+/Lin− | 70,000 | 28 |
| *1887 | H1 p49 | S17, day 17 | CD34+/Lin− | 70,000 | 29–55 |
Presence of human DNA and human insulin mRNA transcripts in sheep pancreas
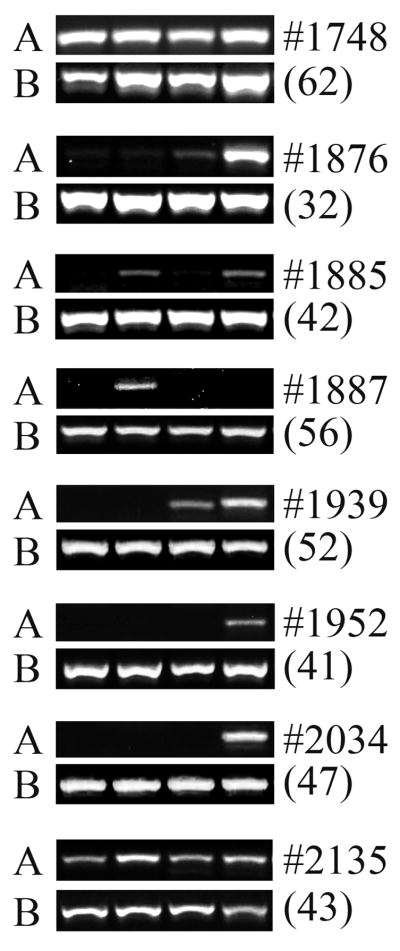
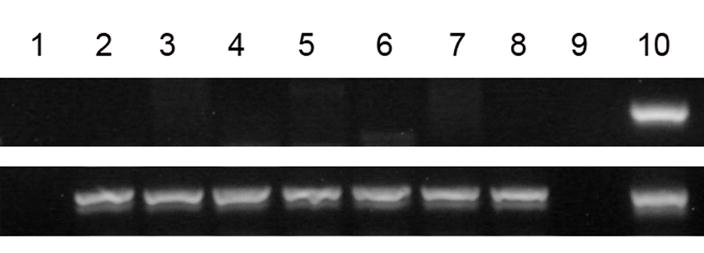
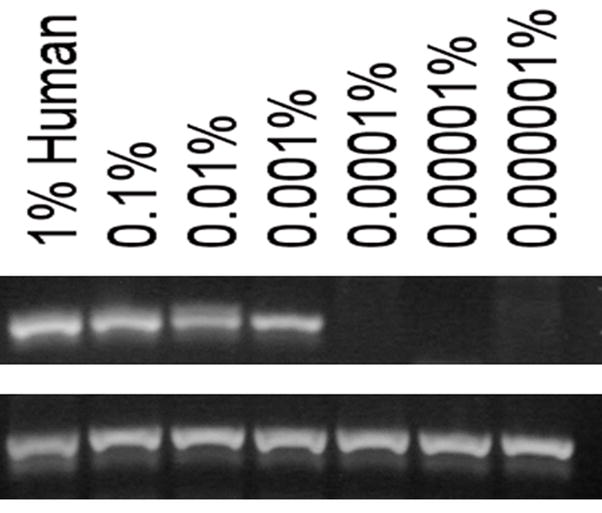
Sheep that were chimeric by ELISA were euthanized at 32 to 62 months after transplantation. Beta-islets encompass only 1–2% of the pancreatic mass, and are distributed mainly in the tail of the pancreas. Since a 1 mm3 sized tissue sample was used to prepare DNA and mRNA from our large animal, we tested 4 samples with end-point PCR, and 5 samples with real-time RT-PCR. We found human DNA in at least 1 and sometimes in all 4 samples from each animal by end point PCR (Fig. 2A). All seven control sheep consistently tested negative for human DNA (Fig. 2B), demonstrating the specificity of the assay. The limit of detection for human DNA in sheep DNA was 0.001% (Fig. 2C). Our real-time RT-PCR data for human insulin was a qualitative assay as we were only interested in the presence or absence of the transcript. Here, we found 1–2 samples scored positive out of the 5 tested from each chimeric sheep (Table 4). We also tested these same 5 samples using primers specific for sheep insulin and some samples were negative, indicative of the necessity to include numerous 1 mm3 tissue samples in order to ensure the inclusion of beta-islets and amplifiable mRNA transcripts in our samples.
Figure 2. Detection of human DNA in sheep pancreas: Engraftment and presence of human cells.
(A) Sheep were transplanted in-utero with hESC-derived cells as noted in Tables 1–3. Pancreatic tissue was harvested from each animal (identified by animal no. on top right) at various periods after transplant (months, indicated in parentheses, bottom right). Four samples from each animal were amplified. Primers for human-specific GAPDH gene (A, top row) were for the detection of human DNA. Primers for beta-actin (B, bottom row) indicated the presence of amplifiable DNA from both human and sheep.
(B) Human GAPDH primers do not amplify sheep DNA. Seven control sheep were amplified with GAPDH (top row) and beta-actin (bottom row), lanes 2–8. Lane 1: water. Lane 10: Human positive control.
(C) Detection limit for human DNA present in sheep. Human DNA was spiked into sheep DNA at levels indicated to determine the detection limit of this assay. Ten ng of total DNA was used per reaction. Top row: GAPDH gene. Bottom row: Beta-actin.
Table 4.
Presence of human insulin transcripts in chimeric sheep. Sheep were transplanted with hESC-derived cells as indicated in Tables 1–2. Chimeric animals were euthanized at periods (months) after transplantation indicated in Fig. 2A. mRNA was prepared with 1mm3 tissue samples. RT-PCR was carried out as described under methods for the detection of human and sheep insulin transcripts.
| Animal no. | No. of tissue samples analyzed | No. positive for sheep insulin | No. positive for human insulin |
|---|---|---|---|
| 1748 | 5 | 4 | 1 |
| 1876 | 5 | 5 | 1 |
| 1885 | 5 | 5 | 1 |
| 1887 | 5 | 5 | 2 |
| 1939 | 5 | 5 | 1 |
| 1952 | 5 | 5 | 2 |
| 2034 | 5 | 5 | 2 |
| 2135 | 5 | 4 | 1 |
| Control sheep | 5 | 5 | 0 |
| Control human | 5 | 0 | 5 |
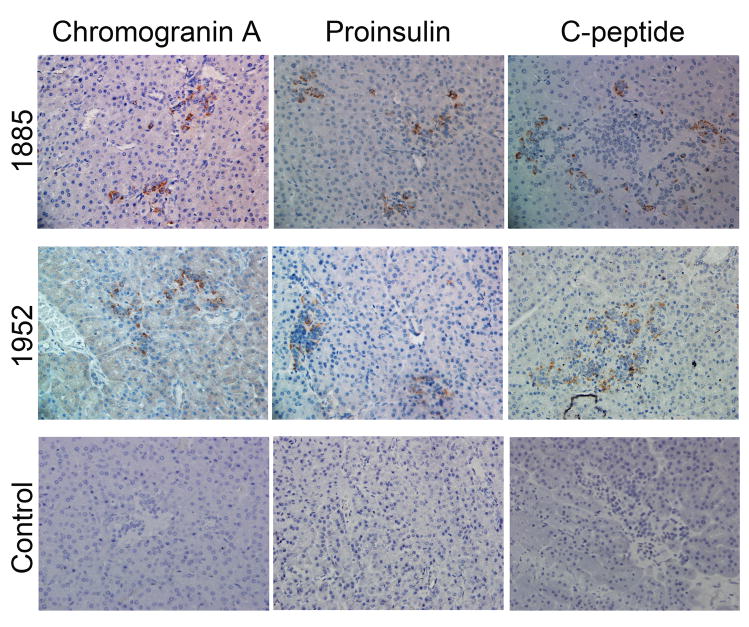
Absence of CD34+, presence of human chromogranin A, proinsulin, and C-peptide by IHC
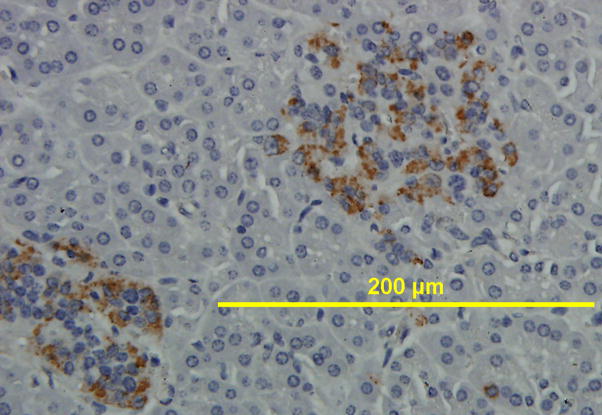
Of the 8 animals that were chimeric by ELISA for human C-peptide, and were positive for human DNA and human insulin mRNA transcripts, we further tested 2 animals by IHC (Fig. 3A). Tissue sections were stained with human-specific antibodies for proinsulin and C-peptide, the precursor and break-down product of insulin, respectively. An antibody against chromogranin A (Chrom A), present predominantly in alpha cells [27], was also used. Islets could be distinguished by their morphology as the clusters of nuclei were a denser population within islets than in the parenchyma, as evident by H&E stain (Fig. 4). Islets could also be distinguished by staining with antibodies that bind islet proteins, including ChromA, proinsulin, and C-peptide. We found human Chrom A, human proinsulin, and human C-peptide to be present on tissue sections from chimeric sheep, but not on non-transplanted control sheep tissue (Fig. 3B). We also tested additional control sheep which all scored negative (Supplemental Fig. 2). An anti-CD34 antibody that reacts with HSC and endothelial cells did not detect any CD34 markers on pancreatic tissue (Supplemental Fig. 3) indicating loss of the CD34+ marker on engrafted cells.
Figure 3.
IHC of pancreatic tissue from chimeric sheep. Sheep were transplanted with hESC-derived cells as indicated in Tables 1–2. Chimeric animals were euthanized at periods after transplantation indicated in Fig. 2A. Pancreatic tissue was processed for IHC as described in methods. (A)Two representative animals were analyzed with antibodies that specifically recognized human proteins. The proteins were: chromogranin A, proinsulin, and C-peptide. A control sheep (not transplanted) stained negative for all these antibodies. (Original magnification × 400). (B) Image enlarged for detail.
Figure 4.
H&E staining of human and sheep pancreas for comparison of morphology. Human (top) 200× magnification (A) and 400× (B). Sheep (bottom) 200× magnification (C) and 400× (D). Islets stained lighter and revealed a denser cluster of nuclei as also evident in the IHC stains.
Discussion
Using an in-vivo model to assess the capacity of stem cells requires that transplanted cells be capable of homing with the innate potential to differentiate into cells of the available local niche. The fetal sheep model provided an ideal system for evaluating the potential of donor cells to differentiate into specialized cells. Transplantation was carried out at a point early in gestation when the fetus was immunologically immature. In this manner, others have shown that tolerance is developed during gestation towards foreign antigens that persist in the immune-competent large animal [28,29]. Organs, tissues, and stem cell compartments are expanding rapidly at this stage and donor cells could readily populate the available niches allowing for their systemic distribution. Theoretically, all organs may be engrafted by intra-peritoneal transplantation in the in-utero model. As such, others have used the in-utero intraperitoneal injection of human stem cells in the mouse and demonstrated multi-organ engraftment [30].
hESC-derived CD34+ cells were chosen to be evaluated for their in-vivo beta-cell-like capacity based on prior observations that BM cell populations comprising CD34+ cells had pancreatic potential [8–10], as well as the later implication of the CD34+ marker in a pancreatic progenitor population [16]. As previously mentioned, there is controversy over the plasticity and trans-differentiation of HSC, thought to be the most pertinent component of the BM CD34+ population. It has recently been hypothesized that BM serves as a niche for rare, versatile, tissue-committed stem cells, and that these cells are CD34+ but CD45−, and that it is this non-HSC albeit BM-derived population which contributes to plasticity and trans-differentiation attributed to HSC [25]. In the present studies we show the differentiation cultures to express hematopoietic markers (Supplemental Fig. 1) and previously we have shown the S17/CD34+ cells to have in-vivo hematopoietic potential [15]. Further, we have demonstrated here that CD34+ cells isolated from these same cultures have pancreatic potential, while they were absent for markers that tested positive in the BM/CD34+ cells which comprise HSC as well as non-HSC (Fig. 1). We cannot conclude that the CD34+ cells that contributed to beta-cell-like activity in the current studies are the same cells that previously were shown to have in-vivo hematopoietic potential, but we can say that cells with HSC and pancreatic endocrine potential are present in the same hESC-derived CD34+ cell population. We expect the hESC-derived CD34+ cell population to have an even more diverse potential than BM/CD34+ cells, based on being derived from pluripotent embryonic stem cells.
On a practical basis, hESC-derived CD34+ cells provide ease of differentiation with the recent development of conditions that generate up to 20% of CD34+ cells after 8–9 days in culture [31]. In addition, the widespread use of CD34+ cells has led to the development of commercial kits for their rapid isolation. We derived CD34+ cells from hESC differentiated by co-culture on S17 – a cell layer that provides a hematopoietic environment [24], or by spontaneous differentiation into EB. Whole differentiated cultures (S17/day 17 and EB/day 8) containing insulin mRNA (Fig. 1) and albumin mRNA (data not shown) depicting the inclusion of terminally differentiated cells, were also evaluated. Cell numbers used for transplantation were variable (Tables 1–3) with larger numbers transplanted when whole cultures were used whereas CD34+ populations were smaller as they constituted ~5% of the culture.
Other hESC-derived cell populations characterized for in-vivo beta-cell-like activity thus far have included endoderm cells from 12-day cultures that successfully generated functional endocrine cells in immune-compromised mice upon the transplantation of 5–10 million cells [4]. Another group conducted the spontaneous differentiation of hESC over 14–19 days that generated cells rich in PDX1 which successfully generated insulin-producing cells upon the transplantation of 100,00–150,000 cells beneath the kidney capsules of immune-deficient mice when co-transplanted with embryonic mouse pancreases [5]. And in a third study, the directed differentiation of hESC for 20 days produced cells which upon transplantation (1 million cells) under the kidney capsule of mice with injured pancreases normalized their blood sugar [32]. The human C-peptide levels reported in the 3 studies were 625 pM (or 1.89 ng/mL) [4], 1.81 ng/mL [5], and 0.5 ng/mL [32], respectively. While these studies effectively demonstrated the in-vivo potential of other cells, the efficacy of using CD34+ cells becomes interesting when the number of cells injected, the size of the animal model used, the human C-peptide levels achieved, and the duration of the studies are compared. We demonstrated that 23,500 CD34+ cells were sufficient for engraftment (Table 3), study animals averaged over 90 kg (Supplemental Table 2) and plasma volume in sheep can be approximated to 37 mL/kg [33], and human C-peptide in the 8 chimeric sheep averaged 1.51 ng/mL (or 500 pM) measured up to 5 years post-transplantation (Table 3). In humans, fasting levels of C-peptide measure up to 5.0 ng/mL in the >16 year age group [34].
To summarize, we carried out transplantations into a large animal model using CD34+ cells isolated from differentiated cultures of hESC. Transplanting whole populations of unfractionated differentiated cells did not augment the level of beta-cell-like activity achieved from transplanting the lower numbers of CD34+ cells isolated from these. Levels of human C-peptide in this study rivaled that from hESC-derived endodermal progenitor cells in the mouse model [4,5,32]. We found that CD34+ cells derived from hESC in the longer-term culture (17 days) to be the most suited for in-vivo beta-cell-like activity. This is the first long-term demonstration of hESC-derived CD34+ cell engraftment in a large animal model where donor endocrine activity was present and teratomas were absent for over 5 years. This study provides compelling evidence that the hESC-derived CD34+ cell population may be a suitable candidate for diabetic cellular therapy.
Supplementary Material
Acknowledgments
We gratefully acknowledge Dr James Thomson (University of Wisconsin, Madison) and his lab (Rachel Lewis and Dr. Dan Kaufman) for providing us with cells used in this study. Our sincere appreciation to our technicians: Eileen Meredith for performing sheep surgeries, and Donna Colón for sorting cells. This work was supported by National Institutes of Health grant numbers HL66058, HL052955, and HL49042.
Footnotes
Conflict of interest disclosure
No financial interest/relationships with financial interest relating to the topic of this article have been declared.
Support and Financial Disclosure Statement: This work was supported by NIH grant numbers HL66058, HL052955, and HL49042. No potential conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanafusa T, Miyazaki A, Miyagawa J, et al. Examination of islets in the pancreas biopsy specimens from newly diagnosed type 1 (insulin-dependent) diabetic patients. Diabetologia. 1990;33:105–111. doi: 10.1007/BF00401048. [DOI] [PubMed] [Google Scholar]
- 2.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. {beta}-Cell Deficit and Increased {beta}-Cell Apoptosis in Humans With Type 2 Diabetes. Diabetes. 52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 3.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. 2003. [DOI] [PubMed] [Google Scholar]
- 4.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 5.Brolen GK, Heins N, Edsbagge J, Semb H. Signals from the embryonic mouse pancreas induce differentiation of human embryonic stem cells into insulin-producing beta-cell-like cells. Diabetes. 2005;54:2867–2874. doi: 10.2337/diabetes.54.10.2867. [DOI] [PubMed] [Google Scholar]
- 6.Ku HT. Minireview: pancreatic progenitor cells--recent studies. Endocrinology. 2008;149:4312–4316. doi: 10.1210/en.2008-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almeida-Porada G, Porada CD, Chamberlain J, Torabi A, Zanjani ED. Formation of human hepatocytes by human hematopoietic stem cells in sheep. Blood. 2004;104:2582–2590. doi: 10.1182/blood-2004-01-0259. [DOI] [PubMed] [Google Scholar]
- 8.Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest. 2003;111:843–850. doi: 10.1172/JCI16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasegawa Y, Ogihara T, Yamada T, et al. Bone Marrow (BM) Transplantation Promotes {beta}-Cell Regeneration after Acute Injury through BM Cell Mobilization. Endocrinology. 2007;148:2006–2015. doi: 10.1210/en.2006-1351. [DOI] [PubMed] [Google Scholar]
- 10.Hess D, Li L, Martin M, et al. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol. 2003;21:763–770. doi: 10.1038/nbt841. [DOI] [PubMed] [Google Scholar]
- 11.Choi JB, Uchino H, Azuma K, et al. Little evidence of transdifferentiation of bone marrow-derived cells into pancreatic beta cells. Diabetologia. 2003;46:1366–1374. doi: 10.1007/s00125-003-1182-9. [DOI] [PubMed] [Google Scholar]
- 12.Lechner A, Yang Y-G, Blacken RA, Wang L, Nolan AL, Habener JF. No Evidence for Significant Transdifferentiation of Bone Marrow Into Pancreatic {beta}-Cells In Vivo. Diabetes. 2004;53:616–623. doi: 10.2337/diabetes.53.3.616. [DOI] [PubMed] [Google Scholar]
- 13.Berenson RJ, Andrews RG, Bensinger WI, et al. Antigen CD34+ marrow cells engraft lethally irradiated baboons. J Clin Invest. 1988;81:951–955. doi: 10.1172/JCI113409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proceedings of the National Academy of Sciences. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narayan AD, Chase JL, Lewis RL, et al. Human embryonic stem cell-derived hematopoietic cells are capable of engrafting primary as well as secondary fetal sheep recipients. Blood. 2006;107:2180–2183. doi: 10.1182/blood-2005-05-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puglisi MA, Giuliani L, Fierabracci A. Identification and characterization of a novel expandable adult stem/progenitor cell population in the human exocrine pancreas. J Endocrinol Invest. 2008;31:563–572. doi: 10.1007/BF03346409. [DOI] [PubMed] [Google Scholar]
- 17.Porada CD, Park PJ, Almeida-Porada G, et al. Gestational age of recipient determines pattern and level of transgene expression following in utero retroviral gene transfer. Mol Ther. 2005;11:284–293. doi: 10.1016/j.ymthe.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James D, Levine AJ, Besser D, Hemmati-Brivanlou A. TGF{beta}/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- 20.D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez D, Figarella C, Marchand-Pinatel S, Bruneau N, Guy-Crotte O. Preferential expression of reg I beta gene in human adult pancreas. Biochem Biophys Res Commun. 2001;284:729–737. doi: 10.1006/bbrc.2001.5033. [DOI] [PubMed] [Google Scholar]
- 22.Schroeder IS, Kania G, Blyszczuk P, Wobus AM. Insulin-producing cells. Methods Enzymol. 2006;418:315–333. doi: 10.1016/S0076-6879(06)18019-2. [DOI] [PubMed] [Google Scholar]
- 23.Marselli L, Thorne J, Ahn YB, et al. Gene expression of purified beta-cell tissue obtained from human pancreas with laser capture microdissection. J Clin Endocrinol Metab. 2008;93:1046–1053. doi: 10.1210/jc.2007-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins LS, Dorshkind K. A stromal cell line from myeloid long-term bone marrow cultures can support myelopoiesis and B lymphopoiesis. J Immunol. 1987;138:1082–1087. [PubMed] [Google Scholar]
- 25.Kucia M, Ratajczak J, Ratajczak MZ. Are bone marrow stem cells plastic or heterogenous--that is the question. Exp Hematol. 2005;33:613–623. doi: 10.1016/j.exphem.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Leenanuruksa D, McDowell GH. Development of a radioimmunoassay for plasma C-peptide in sheep: kinetics of C-peptide and effects of exogenous growth hormone and glucose on insulin and C-peptide. Aust J Biol Sci. 1988;41:517–525. doi: 10.1071/bi9880517. [DOI] [PubMed] [Google Scholar]
- 27.Lukinius A, Wilander E, Eriksson B, Oberg K. A chromogranin peptide is co-stored with insulin in the human pancreatic islet B-cell granules. Histochem J. 1992;24:679–684. doi: 10.1007/BF01047589. [DOI] [PubMed] [Google Scholar]
- 28.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 29.Zanjani ED, Flake AW, Rice H, Hedrick M, Tavassoli M. Long-term repopulating ability of xenogeneic transplanted human fetal liver hematopoietic stem cells in sheep. J Clin Invest. 1994;93:1051–1055. doi: 10.1172/JCI117054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan J, Waddington SN, O’Donoghue K, et al. Widespread distribution and muscle differentiation of human fetal mesenchymal stem cells after intrauterine transplantation in dystrophic mdx mouse. Stem Cells. 2007;25:875–884. doi: 10.1634/stemcells.2006-0694. [DOI] [PubMed] [Google Scholar]
- 31.Vodyanik MA, Bork JA, Thomson JA, Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105:617–626. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- 32.Jiang W, Shi Y, Zhao D, et al. In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Res. 2007;17:333–344. doi: 10.1038/cr.2007.28. [DOI] [PubMed] [Google Scholar]
- 33.Adams D, McKinley M, Colditz I, Dart C ANZCCART. Fact sheets about animals: The University of Adelaide. Australia: ANZCCART; 2009. The Sheep. Fact Sheet A9. [Google Scholar]
- 34.Ratzmann KP, Strese J, Kohnert KD, Jahr D, Michaelis D. Age-dependent relationship of fasting C-peptide concentration and insulin secretion in non-obese subjects with normal glucose tolerance. Exp Clin Endocrinol. 1986;88:57–63. doi: 10.1055/s-0029-1210575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.