Abstract
Evidence from longitudinal studies in community-dwelling elderly links complaints of urgency and urinary incontinence with structural white matter changes known as white matter hyperintensities (WMH). How WMH might lead to incontinence remains unknown, since information about how they relate to neural circuits involved in continence control is lacking. The aim of this study was to investigate the role of WMH in altered brain activity in older women with urgency incontinence. In a cross-sectional study, we measured WMH, globally and in specific white matter tracts, and correlated them with regional brain activity measured by fMRI (combined with simultaneous urodynamic monitoring) during bladder filling and reported 'urgency'. We postulated that increase in global WMH burden would be associated with changes (either attenuation or reinforcement) in responses to bladder filling in brain regions involved in bladder control. Secondly, we proposed that such apparent effects of global WMH burden might be specifically related to the burden in a few critical white matter pathways. The results showed that regional activations (e.g. medial/superior frontal gyrus adjacent to dorsal ACG) and deactivations (e.g. perigenual ACG adjacent to ventromedial prefrontal cortex) became more prominent with increased global WMH burden, suggesting that activity aimed at suppressing urgency was augmented. Secondary analyses confirmed that the apparent effect of global WMH burden might reflect the presence of WMH in specific pathways (anterior thalamic radiation and superior longitudinal fasciculus), thus affecting connections between key regions and suggesting possible mechanisms involved in continence control.
Introduction
The most prevalent and morbid form of urinary incontinence (UI) in the elderly (Resnick et al., 2007; Norton and Brubaker, 2006) is urgency incontinence: urine leakage associated with the sensation of urgency. It is conventionally thought to be caused by involuntary bladder contractions called detrusor overactivity (DO). Such contractions are observed on urodynamic testing in some (but not all) subjects with urgency incontinence and are a physiological indicator of loss of bladder control. The clinical hallmark of urgency incontinence, the urgency itself, is an abnormal sensation, defined as a sudden, compelling desire to void (Abrams et al., 2002), typically with fear of leakage (Abrams et al., 1988) and, therefore, preceding actual urine leakage).
Patients with the syndrome of geriatric urinary incontinence are usually pictured as functionally disabled, although without overt brain pathology such as stroke or dementia (Resnick et al., 2007). However, recent evidence links this syndrome with rather subtle structural changes in the brain’s white matter, known from their MRI appearance as white matter hyperintensities (WMH; synonyms: leucoaraiosis or age-related white matter changes/ARWMC). Contrary to the conventional view WMH affect many functional community-dwelling elderly (Kuo and Lipsitz 2004; Pantoni, et al., 2006; Inzitari et al., 2007; Pogessi et al., 2008). The white matter hyperintensity burden (the volume affected) progresses with age and is linked to increased prevalence of urgency (Pogessi et al., 2008). In addition, the accumulation of WMH in specific pathways is associated with the symptom of urgency UI and its severity (Kuchel et al., 2009). Elderly individuals with greater white matter hyperintensity burden also show increased prevalence of detrusor overactivity and difficulty maintaining continence on urodynamic studies (Sakakibara et al., 1999). Yet the mechanism whereby WMH might lead to incontinence remains elusive, since information about how these structural changes relate to functional brain activity and the neural circuits involved in continence control is lacking.
With the use of functional brain imaging (PET and fMRI) in the past decade, the principal brain regions involved in continence control have been identified (Blok et al., 1998; Nour et al., 2000; Athwal et al., 2001; Griffiths et al., 2005; Kavia et al., 2005; Griffiths and Tadic., 2008). Our own group has adapted urodynamic methods to the fMRI environment so as to monitor bladder pressure and brain activity in the scanner simultaneously, during bladder filling (Griffiths et al., 2005). We have used this experimental paradigm to study the neural circuits involved in regulation of the bladder storage phase (Griffiths et al., 2005; Griffiths et al., 2007; Tadic et al., 2008; Griffiths and Tadic., 2008; Griffiths et al., 2009; Tadic et al., 2009). In particular we have studied this network in older women who suffer from urgency incontinence. These patients signal a compelling need to void – mimicking the urgency that they describe in real life – when the bladder is full but there is no detrusor overactivity. Under these circumstances their brain activity is markedly different from that in age-matched controls (Griffiths et al., 2007). It displays a pattern of activations and deactivations (in response to bladder filling) in regions of the limbic system (e.g. anterior cingulate gyrus – ACG), insular cortex (e.g. right insula, involved in interoceptive awareness) and executive cortex (e.g. inferior, medial and dorsolateral parts of orbitofrontal cortex) (Griffiths et al., 2007; Griffiths and Tadic, 2008; Griffiths et al., 2009; Tadic et al., 2009). In addition, the effective connectivity between these regions differs in location and intensity, compared to subjects with normal bladder function (Tadic et al., 2008). Altogether, the abnormal brain activity in such patients seems to be aimed at suppressing urgency and maintaining continence and thus reflects clinical symptoms related to difficulty controlling the bladder (Griffiths and Tadic, 2008). Indeed, a recent analysis confirmed the association between the objective severity of incontinence and abnormal activity in similar brain regions (Tadic et al., 2010). Nevertheless, the causal link between altered activity in the gray matter of the brain and changes in the white-matter pathways that connect them is unknown.
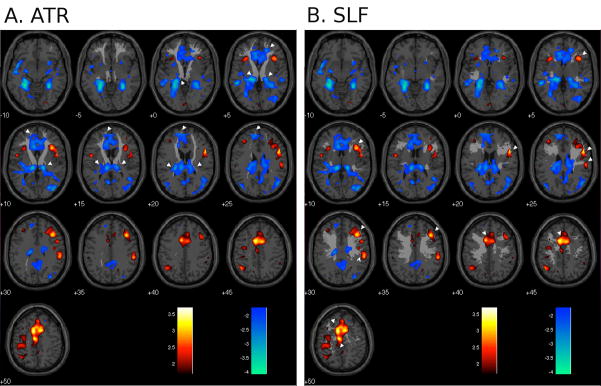
Gray matter regions involved in continence control can only function as a control system because of white matter pathways containing the neuronal axons that connect them. Pathways occupy a large part of the interior of the brain and are organized as relatively broad tracts containing fibres that proceed in generally similar directions to make contact with various gray matter regions (Schmahmann et al., 2008). The tracts have been mapped by imaging and neuroanatomical methods and displayed in atlases (e.g. Wakana et al., 2004). The effects of possible damage to these tracts (WMH) are studied in this paper. Two white matter tracts that prove to be particularly important are (1) the anterior thalamic radiation (ATR) that runs bilaterally, primarily between the thalamus (an important relay and interaction station) and the frontal and prefrontal parts of the brain; and (2) the superior longitudinal fasciculus (SLF) that interconnects lateral and some more medial parts of the cortex on each side of the superior brain (see Figure 3).
Fig. 3.
Regional brain responses to bladder filling (main effects) superimposed on selected white-matter tracts; red/yellow = activation, blue = deactivation (P < 0.01 uncorrected); numbers show z-coordinates in mm. White pointers show some of the regions where the tract appears to project to activated or deactivated areas. A. anterior thalamic radiation (ATR); B. superior longitudinal fasciculus (SLF).
The aim of this study was to investigate the potential role of WMH in altered brain activity in older women with urgency incontinence. In a cross-sectional study, we obtained measurements of WMH, globally and in specific white matter tracts, and correlated them with regional brain activity measured by fMRI during bladder filling and reported ‘urgency’, in the absence of detrusor overactivity. We postulated that increase in global WMH burden would be associated with changes in brain responses to bladder filling. We reasoned that, as for other bodily functions (Nordahl, et al., 2006), damage to white matter pathways might attenuate responses in regions involved in bladder control, thus exacerbating or causing incontinence; or alternatively that, by endangering continence, it might evoke stronger compensatory (coping) reactions and thus more prominent brain responses. We examined each possibility separately. As a secondary hypothesis, we proposed that such apparent effects of global WMH burden might in fact be specifically related to the burden in a few critical white matter pathways.
Materials and methods
Study subjects
Regional brain responses to bladder filling and white matter hyperintensities were examined in female volunteers aged > 60 years, recruited by advertising or from our other studies that used the same recruitment methods and criteria. All underwent comprehensive clinical evaluation, including detailed history, physical examination (including neurological testing and a pelvic exam), a test of cognitive status (Mini-Mental State Examination - MMSE) and comprehensive urodynamic evaluation (Tadic et al., 2007; Griffiths et al., 2007; Tadic et al., 2009). This comprehensive examination and the recruitment criteria listed below were employed to ensure a population of functionally independent, community-dwelling older women with urgency UI but without significant comorbidities that could contribute to UI (e.g. cognitive, mobility or mood impairment). All subjects had urgency incontinence, either pure or with a minor component of stress-related leakage, as determined clinically and by voiding diary and pads. Only those who had more than 2 urgency incontinence episodes in a 3-day bladder diary were recruited for further study, thus ensuring recruitment only of subjects with either moderate (between 5 and 10 episodes per week) or severe (> 10 episodes per week) incontinence as defined by Burgio et al., 1998. According to our previous experience, women with moderate to severe incontinence are clinically and phenotypically a more homogenous group than those with mild disease
Women with significant cognitive impairment (MMSE score ≤ 24/30), secondary causes of urinary incontinence (e.g. bladder cancer, spinal cord lesions, multiple sclerosis, pelvic radiation, interstitial cystitis), sphincter implant or significant neurological and vascular disease (history of strokes, neurodegenerative diseases and complicated diabetes mellitus) were excluded. We also excluded subjects with claustrophobia and the presence of implanted metal or electromagnetic devices that would preclude performing MRI. The study was approved by the University of Pittsburgh Institutional Review Board and all participants provided written informed consent.
Bladder filling protocol
All subjects underwent a brain scanning protocol that includes fMRI and simultaneous urodynamic monitoring. Details of our fMRI methods have been described previously (Griffiths et al., 2007; Tadic et al., 2008). Briefly, two 8 F urethral catheters were introduced to allow for bladder filling/emptying and bladder pressure measurement to detect detrusor overactivity (DO). Structural MRI was performed. A small volume of sterile water (10–20 ml) was introduced into the empty bladder, followed by functional MRI while fluid was repeatedly infused into and withdrawn from the bladder using an automated pump system (acquisition details: 3 T magnet; one brain scan per 2 s; 3 × 3 × 3 mm resolution, field of view 21 cm covering brainstem to near top of anterior cingulate gyrus). Each infusion/withdrawal cycle comprised: pause (12 s); infusion (22 ml in 12 s); pause (12 s); withdrawal (20 ml in 12 s). Four cycles formed one measurement block. After 2 measurement blocks, the bladder was filled quickly, without scanning, until the subject signalled compelling desire to void. With the subject’s permission, 1 to 2 further measurement blocks were performed with scanning until subjects stopped the study to void, or developed involuntary bladder muscle contraction (detrusor overactivity/DO) and urine leakage. The BOLD signal acquired in the final measurement block (or the last block before DO onset) was used for analysis. This measurement block was comparable in different subjects because it was always performed in the same situation: with full bladder and just tolerable sensation but before onset of any involuntary bladder contraction (detrusor overactivity). We refer to this situation as ‘urgency’ because it mimics most aspects of its definition (compelling desire to void, difficult to defer and associated with fear of leakage) (Abrams et al., 2002; Abrams et al., 1988).
Assessment of white matter hyperintensities
We used a fully automated method for quantifying and localizing white matter hyperintensities on MR images (Wu et al., 2006) which uses fast-FLAIR images (fast FLuid-Attenuated Inversion Recovery) and a ‘fuzzy-connected algorithm’ to identify WMH clusters in the anatomical space for each subject. The amount of white matter changes (hyperintensities) is registered in voxels and then converted to a volume (1 voxel = 4.2 mm3). For regression analyses, to account for individual differences in brain size, we normalized the global WMH burden by dividing each individual’s total WMH volume by her total brain volume. Regional WMH burdens were calculated as follows: the 20 white-matter tracts identified by the Johns Hopkins white matter atlas [Wakana et al, 2004] were warped into the anatomical space and the normalized WMH burden was calculated for each tract by dividing the volume of WMH in the tract by the whole-brain volume.
Selection of white matter tracts for regression analysis
The distribution of WHM burden over the 25 subjects was similar for many of the tracts. Consequently, the correlations between brain activity and regional WMH burden in these tracts were also similar and could not be used to distinguish between them. A factor analysis (see Appendix) revealed only two distinct underlying patterns (factors) in the distribution of WMH burden over subjects. We therefore examined correlations between brain responses and regional WMH burden for the tract showing pattern 2 (SLF, superior longitudinal fasciculus) and the four tracts showing pattern 1 (anterior thalamic radiation, ATR; uncinate fasciculus, UNC; inferior fronto-occipital fasciculus, IFO; and inferior longitudinal fasciculus, ILF). We paid particular attention to ATR and SLF (see Appendix).
fMRI analyses
After image acquisition, pre-processing and further analyses were done using Statistical Parametric Mapping (SPM5; Wellcome Department of Imaging Neuroscience, 2003, http://www.fil.ion.ucl.ac.uk/spm/spm2.html). As previously, first-level analysis was performed in each subject to determine regional brain responses to bladder filling by comparing the fMRI signal during fluid infusion with the baseline during fluid withdrawal (see Griffiths et al., 2007; Tadic et al., 2008; Griffiths et al., 2009 and Griffiths et al., 2005 at www.learncontinence.com/griffiths_suppl_material). To determine the main effects of bladder filling we combined the single-subject results for the selected measurement block in a standard second-level (random-effects) analysis and thresholded statistical maps at P < 0.01 (uncorrected for multiple comparisons). For second-level correlation analyses, the first-level image for this measurement block was selected for each individual, together with the covariate of interest (normalized white matter burden: global, or in ATR or SLF). A voxel-by-voxel correlation between the images and the covariate was then performed. The statistical maps showing voxels with a significant correlation with each of the covariates were thresholded at P < 0.01 (uncorrected for multiple comparisons) which corresponds to correlation coefficient r ≥6.
Results
Study participants
As outlined in Table 1, the study population represented functional, community-dwelling, older women with moderate to severe urgency urinary incontinence (Burgio et al., 1998). 14 subjects (56%) had detrusor overactivity present during urodynamic evaluation. Severity of incontinence (number of daytime urgency incontinence episodes on bladder diary) was significantly correlated with global white matter burden (r = 0.5; P < 0.01).
Table 1.
Characteristics of study participants (n=25).
| Variable | Mean (SD) |
|---|---|
| Age (years) | 71.5 (7.5) |
| Chronic conditions | 2.3 (1.3) |
| Depression history (n and %) | 8 (32) |
| MMSE (0–30) | 29.2 (1.04) |
| Urge Incontinence Episodes/Day | 2.5 (1.3) |
| Urine Leakage/Day (mL) | 29.2 (44.3) |
| White Matter Hyperintensities (cm3) | 3.3 (4.5) |
Functional brain activity: main effect of bladder filling
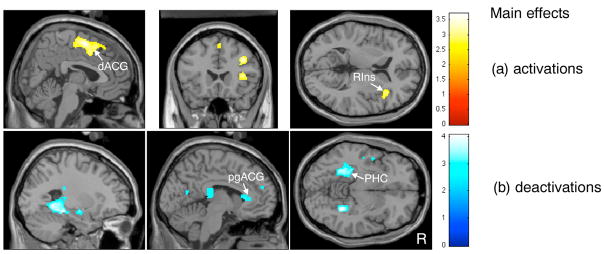
Brain response to bladder filling during self-reported ‘urgency’ showed significant activations in a cluster of frontal regions, including medial and superior frontal gyrus (BA 6 and BA 9), adjacent to dorsal anterior cingulate gyrus (dACG) (BA24/32) (see Fig. 1(a)); as well as right inferior frontal gyrus (BA 44 and BA45) adjacent to right insula (BA 13); dorsolateral prefrontal cortex (BA 9) and cerebellum (Fig. 1(a) and Table 2). eactivations were significant in the parahippocampal complex (PHC) and pre/subgenual ACG (BA24/32) as shown in Fig. 1(b), as well as other regions listed in Table 2.
Fig 1.
Images related to main effects (regions activated or deactivated on bladder filling): (a) activations (P < 0.01 uncorrected); (b) deactivations (P < 0.01 uncorrected). dACG = dorsal anterior cingulate gyrus; RIns = right anterior insula; pgACG = perigenual anterior cingulate gyrus; PHC = parahippocampal complex.
Table 2.
Regional brain activity in response to bladder filling (main effects) (n=25, threshold P<0.01 uncorrected). Coordinates are given in MNI space.
| Region | Coordinates | Z-Value | Voxel Level Significance (uncorrected) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Activations | |||||
| Cluster 1: SMA, superior/medial frontal gyrus adjacent to dorsal ACG; P<0.01 (corrected) | |||||
| Medial Frontal Gyrus (BA 6) | 2,−8 | 0, −8 | 58,56 | 3.27,3.21 | |
| Superior Frontal Gyrus (BA 8) | −2,2 | 16,30 | 52,52 | 2.86,2.48 | |
| Anterior Cingulate Gyrus (BA 24/32) | −10, −10 | 4,8 | 50,48 | 3.17,3.15 | |
| Other regions: | |||||
| Right Middle Frontal Gyrus (BA 9) | 36 | 22 | 34 | 3.07 | 0.001 |
| Right Insula (BA 13) | 34 | 24 | 4 | 2.79 | 0.003 |
| Right Inferior Frontal Gyrus (BA 45) | 44 | 18 | 6 | 2.53 | 0.006 |
| Right Inferior Frontal Gyrus (BA 44) | 50 | 12 | 22 | 2.99 | 0.001 |
| Inferior Parietal Lobule (BA 40) | −44,54 | −58, −32 | 44,34 | 2.59,2.75 | 0.005 |
| Postcentral Gyrus (BA 2/3) | 54, −36 | −22, −34 | 28,60 | 2.59,2.79 | 0.005 |
| Left cerebellum (posterior lobe) | −38 | −52 | −34 | 2.69 | 0.004 |
| Deactivations | |||||
| Cluster 1: parahippocampus, hippocampus, thalamic nuclei; P < 0.04 (corrected) | |||||
| Parahippocampal Gyrus (BA 19) | −26,28 | −44, −48 | −2, −8 | 3.41,3.51 | |
| Left Parahippocampal Gyrus (BA 36) | −28 | −32 | −14 | 3.43 | |
| Left Hippocampus | −24 | −38 | −2 | 3.18 | |
| Thalamus | −22,14 | −28, −28 | 2,10 | 3.17,3.09 | |
| Caudate | −18,14 | −18, −22 | 30,24 | 2.55,2.53 | |
| Other regions: | |||||
| Left Anterior Cingulate Gyrus (BA 24) | −8, −10 | 26,34 | 8,6 | 2.80,2.61 | 0.003 |
| Anterior Cingulate Gyrus (BA 32) | −16,16 | 26,42 | 20,2 | 2.47,2.5 | 0.007 |
| Left Medial/Superior Frontal Gyrus (BA 9) | −8, −14 | 52,48 | 22,26 | 2.46,2.39 | 0.007 |
| Left Middle/Superior Temporal Gyrus (BA 22) | −54, −48 −38, −32 | 4,2 | 3.01,2.92 | 0.001 | |
| Right Middle Occipital Gyrus (BA 19) | 38 | −82 | 10 | 2.70 | 0.004 |
| Right Cuneus (BA 30) | 28 | −78 | 10 | 2.61 | 0.005 |
Functional brain activity: association with global white matter burden
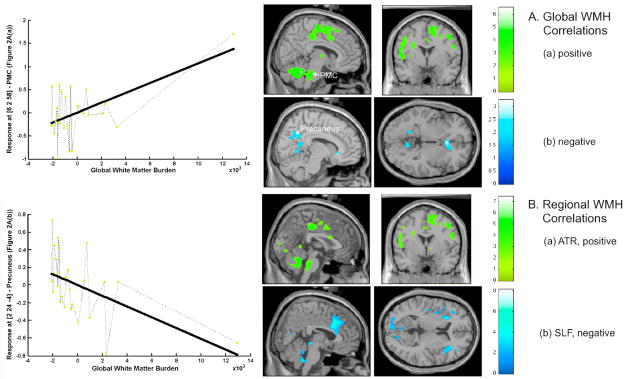
Brain activity (in response to bladder filling) was positively correlated with global WMH burden in cerebellum and brainstem at the level of the pontine micturition center (PMC), as shown in Fig. 2A(a), and a cluster of frontal regions adjacent to dorsal ACG as listed in Table 3. Thus not only did brain activity change in concert with WMH burden, but it changed in regions specifically concerned with bladder control (medial/superior frontal gyrus adjacent to dorsal ACG and PMC). Frontal cortex adjacent to dorsal ACG in particular was activated in response to bladder filling (Fig. 1(a)) and this response became more pronounced with increasing WMH burden (Fig. 2A(a), picture and graph). Note that one subject with WMH burden greater than the rest (but still only moderate) also showed more prominent brain (de)activation, thus confirming the trend indicated by the subjects with smaller burden (see Fig. 2).
Figure 2. Images related to correlations.
A. Correlations between regional brain activity on fMRI and global WMH burden: (a) positive correlations (P < 0.01, uncorrected; r ≥ 0.46); upper left graph: correlations in PMC; (b) negative correlations (P < 0.05* uncorrected; r ≥ 0.34); lower left graph: correlations in Precuneus.
B. Correlations between regional brain activity on fMRI and WMH burden in selected white-matter tracts: (a) positive correlations with ATR burden (P < 0.01 uncorrected; r ≥ 0.46); (b) negative correlations with SLF burden (P < 0.05* uncorrected; r ≥ 0.34). *threshold chosen for image display only.
Table 3.
Regions where brain activity correlated with white matter hyperintensities (WMH):
A. correlations with global WMH burden (n=25, threshold P<0.01 uncorrected); B. correlations with WMH burden in superior longitudinal fasciculus (SLF) (n=10, threshold P < 0.01 uncorrected). Coordinates are given in MNI space.
| Region | Coordinates | Z-Value | Voxel Level Significance (uncorrected) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| A. Global WMH burden | |||||
| Positive Correlations: | |||||
| Cluster 1: Cerebellum and Brainstem; P<0.000 (corrected) | |||||
| Cerebellum, Anterior Lobe | −6,12 | −50, −46 | −24, −26 | 4.86,4.84* | |
| Cerebellum, Anterior Lobe | −24 | −44 | −26 | 3.25 | |
| Cerebellum, Posterior Lobe | 24,38 | −40, −60 | −32, −30 | 4.27,4.25 | |
| Cerebellum, Posterior Lobe | 10 | −60 | −38 | 4.02 | |
| Pons (PMC) | 8,0 | −28, −30 | −30, −32 | 3.58,3.48 | |
| Left Pons | −16 | −34 | −28 | 3.70 | |
| Midbrain (PAG) | −2 | −28 | −18 | 2.44 | |
| Cluster 2: SMA, Superior/Medial Frontal Gyrus adjacent to Dorsal ACG; P<0.000 (corrected) | |||||
| Postcentral Gyrus (BA3) | −56, −24 | −14, −32 | 22,64 | 4.08,3.60 | |
| Precentral Gyrus (BA 4) | −16, −32 | −20, −16 | 60,54 | 3.96,3.20 | |
| Precentral Gyrus (BA 6) | −48 | −6 | 22 | 3.19 | |
| Precentral Gyrus | 48,54 | 2,0 | 46,26 | 3.84,3.30 | |
| Medial Frontal Gyrus (BA 6) | 14, −10 | −20, −14 | 54,48 | 3.68,3.64 | |
| Medial Frontal Gyrus (BA 32) | 12 | 14 | 44 | 3.48 | |
| Superior Frontal Gyrus (BA 6) | 8,8 | 2,24 | 66,58 | 3.53,3.09 | |
| Anterior Cingulate Gyrus (BA24) | −20 | −12 | 44 | 3.77 | |
| Inferior Frontal Gyrus (BA 9) | −54 | 4 | 28 | 3.37 | |
| Left Insula (BA13) | −38 | −8 | 6 | 3.12 | |
| Paracentral Lobule (BA31) | 0 | −22 | 42 | 3.59 | |
| Superior Temporal Gyrus (BA 22) | −50 | −2 | 6 | 3.57 | |
| Other Regions: | |||||
| Middle frontal gyrus (BA 8) | 30 | 20 | 42 | 2.83 | 0.002 |
| Thalamus (ventral posterior lateral nucleus) | 18 | −20 | 6 | 2.99 | 0.001 |
| Midbrain, Red nucleus | 8 | −18 | −4 | 2.84 | 0.002 |
| Hippocampus/parahipocampus | 28 | −20 | −14 | 3.12 | 0.001 |
| Cuneus (BA18) | −2 | −94 | 10 | 3.15 | 0.001 |
| Negative Correlations: | |||||
| Anterior Cingulate Gyrus (BA 24) | 4 | 24 | −4 | 2.93 | 0.002 |
| Caudate | 12 | 26 | −4 | 2.72 | 0.002 |
| Precuneus (BA 7) | 4 | −62 | 32 | 2.66 | 0.004 |
| B. Superior Longitudinal Fasciculus (Entire Pathway) | |||||
| Negative Correlations: | |||||
| Cluster 1: Dorsal ACG/Medial Frontal Gyrus; P < 0.006 (uncorrected) | |||||
| Anterior Cingulate Gyrus (BA24/32) | 6, −4 | 22,24 | 24,36 | 3.53,3.44 | |
| Medial Frontal Gyrus (BA 9/8) | 10,4 | 38,26 | 28,38 | 3.15,3.06 | |
| ACG/BA 32 | −2,2 | 20,20 | 28,28 | 3.12,3.12 | |
| Other Regions: | |||||
| Left Parietal Lobe (Angular Gyrus/BA 39) | −42 | −58 | 36 | 4.21 | 0.000 |
| Inferior Frontal Gyrus/BA13 | 40 | 20 | 8 | 2.80 | 0.003 |
Significant after corrections for multiple comparisons (FWE, P < 0.05)
In pre-/subgenualACG, precuneus (PCun), caudate and lingual gyrus, brain response was negatively associated with global WMH burden (Fig. 2A(b) and Table 3). In perigenual ACG and precuneus in particular there was deactivation in response to bladder filling (Fig. 1(b)) and this deactivation became more pronounced with increasing WMH burden (Fig. 2A(b), picture and graph). Thus increasingly prominent deactivation in brain regions specifically involved in bladder control was associated with increasing WMH burden.
WMH burden in specific pathways
The factor analysis described in the Appendix showed that, in each of 4 white matter tracts (ATR, UNC, IFO, ILF), the distribution of WMH burden over the 25 subjects was similar to the global distribution. The distribution was entirely different in one other tract (SLF). Consistent with this finding, for each of the first 4 tracts, brain activity was positively correlated with WMH burden in a regional pattern similar to that for global WMH burden (data shown for ATR in Fig. 2B(a); compare with Fig. 2A(a)). Negative correlation with ATR burden at threshold level of P < 0.01 was scarce, but there was evidence for it in the ventromedial prefrontal cortex similar to global burden analyses (data not shown). For SLF, however, there was a negative correlation with WMH burden in dorsal ACG (BA24/32) and right inferior frontal gyrus adjacent to insula, as well as other regions shown in Fig. 2B(b) and Table 3.
In order to locate the brain responses with respect to the tracts containing WMH, the main-effects activations and deactivations were superimposed on diagrams showing ATR and SLF in standard MNI space (Figs. 3A and B). The anatomical projections of ATR appeared to connect several regions that were deactivated by bladder filling (Fig. 1(b)). In contrast, projections of SLF appeared to connect mainly to regions that were activated by bladder filling (Fig. 1(a)).
Discussion
Regional brain activity and global white matter hyperintensity burden
The results confirmed our initial postulate that, in older women with urgency incontinence, responses to bladder filling in many brain regions correlated significantly with global WMH burden. The correlation was positive in some of the regions that were activated during bladder filling (e.g. medial/superior frontal gyrus adjacent to dorsal ACG) (Figs. 1(a) and 2A (a)), implying increasing activity with increasing white matter burden. As indicated in the Introduction, this behaviour suggests that the activity is a reaction to a threat to continence engendered by the white matter burden. Negative correlations were observed also, and some of them occurred in regions that were deactivated during bladder filling (e.g. perigenual ACG adjacent to ventromedial prefrontal cortex) (Figs. 1(b) and 2A (b)), implying that brain deactivations became more pronounced with increasing WMH burden. Again this suggests that the deactivation is a reaction to threatened incontinence, aimed at suppressing it. These effects of WMH on the function of the cerebral control system have clinical consequences: incontinence, as assessed by the number of daytime urgency incontinent episodes on bladder diary was more severe in those with greater WMH burden.
Although this is the first study of the effect of WMH on brain responses to bladder filling, other studies have confirmed that the area of the subgenual ACG and ventromedial prefrontal cortex is less active during withholding of urine than during micturition (Blok et al., 1997; Athwal et al., 2001; Nour et al., 2000; Griffiths and Tadic, 2008; Tadic et al., 2009), consistent with the deactivation during bladder filling reported here. Similarly, regions activated during scanning experiments that required urine withholding or control of voiding (Blok et al., 1997; Blok et al., 1998; Nour et al., 2000; Athwal et al., 2001; Yin et al., 2006; Kuhtz-Buschbeck et al., 2005; Seseke et al., 2006; Zhang et al., 2005) include the anterior insula/lateral prefrontal cortex and the area adjacent to dorsal ACG, as reported in this study, further confirming their role in executive control of continence.
The responses to bladder filling observed in the brainstem at the level of the pontine micturition center (PMC) and adjacent cerebellum were puzzling. They were not clearly seen in main-effects analyses but showed strong positive correlations with global WMH burden. One possibility is that inhibitory activity in the PMC became stronger with increasing WMH in order to maintain continence. Another is that PMC excitation became stronger as inhibition began to fail, threatening incontinence.
Some areas in the posterior cortex (e.g. precuneus) became less active with increasing WMH burden. In our recent study (Tadic et al., 2009) activity in these regions was negatively correlated with the reported severity of incontinence-related psychological burden. Thus the present findings may suggest that brain activity related to coping with incontinence psychologically is also affected by increased WMH burden.
Regional brain activity and WMH burden in white matter tracts
According to our secondary hypothesis the apparent effect of global WMH burden might in fact be attributable to the presence of WMH in specific white matter pathways critical to continence. The white-matter changes in the four tracts with WMH distribution similar to that of the global burden were more ubiquitous and more severe than those in other white matter pathways. For these four tracts (ATR, UNC, IFO and ILF) the correlations of brain activity with WMH burden showed a regional pattern similar to that of global burden (e.g. compare Figs. 2A(a) and 2B (a)). This is mathematically inevitable, but suggests that, if there are a few critical pathways whose integrity is critical for continence, at least one of these four is among them. The anterior thalamic radiation (ATR) is a likely candidate, firstly because it accounts for about 55% of global WMH, secondly because its anterior terminus in the medial prefrontal cortex is in the area identified by stroke and trauma studies as critical to long-term continence (Andrew and Nathan, 1964; Fowler and Griffiths., 2009; Fowler et al., 2008), and thirdly because, as shown in Fig. 3A, its anatomical projections appear to connect several regions that are deactivated by bladder filling (Kahle and Frotscher, 2002; Schmahmann, et al., 2008). Thus the ATR seems to carry a signal to or from all these regions that is associated with their deactivation and presumably maintains continence by inhibiting the PAG or PMC. The pathway through which this occurs is still unclear however.
The other white matter tract for which there is information, the SLF, appears on the contrary to connect regions that are activated by bladder filling (Fig. 3B), including those adjoining dorsal ACG. The SLF carries only 8% of the global burden and shows changes in 40% of subjects. Nevertheless, increased WMH burden in SLF caused some of the activations that it carried to become less prominent (e.g. dorsal ACG and right inferior frontal gyrus adjacent to insula). One might speculate that WMH in this pathway interfere directly with afferent and efferent signals to and from the regions it serves, including insula/inferior frontal gyrus and dorsal ACG that are critical to bladder control (Griffiths and Tadic, 2008). In contrast, increased WMH burden in ATR caused activations apparently carried by SLF to become stronger (e.g. near dorsal ACG). Perhaps ATR damage leads to compensatory strengthening of the SLF pathway, so maintaining continence. Thus there may be two continence mechanisms, one involving the SLF and the dorsal ACG and the other involving the ATR and the medial frontal cortex and perigenual ACG. Additional areas apparently deactivated by the ATR mechanism include hippocampus and parahippocampal complex, suggesting reaction to an emotionally unpleasant event (e.g. retrieval of the memory of an incontinence episode).
Our findings related to ATR and SLF corroborate those of Kuchel et al. (2009), who showed that accumulation of WMH in similar locations (the corona radiata and the frontal-occipital zone) predicted urgency incontinence and was associated with its severity. It is important to note that the burden of white matter disease was only moderate in our group of ‘functional’ community-dwelling older women (Wu et al., 2006; Vannorsdall et al., 2009; Schmidt et al., 2007; Chen et al., 2006). There was no significant impairment of cognition, mobility or mood, all of which might contribute to worsening of continence control (Resnick et al., 2007). Hajjar et al. (2009) have suggested that damage to frontal white-matter pathways might lead to poor executive function, slow gait speed, and depressive symptoms in older non-demented individuals, but they did not report continence status. Possibly, the effects of WMH on brain activity related to continence control in our group may represent an early stage (or variant) of this phenotype, the changes in brain activity that we observe being compensatory to a relatively small increase in the burden of WMH in critical pathways. Such behaviour would be consistent with a general principle governing functional impairment in the elderly, that increased effort is required to maintain homeostasis (Kuchel, 2009).
Clinical implications
Unlike in younger subjects, urgency UI is multifactorial in the elderly (Resnick and Yalla, 2007) and white matter damage might have multiple implications for continence control. Firstly, by affecting critical cerebral pathways it may lead directly to poor bladder control and contribute to the presence of urge symptoms or DO. Secondly, it may also exacerbate incontinence by affecting potential compensatory factors such as the ability to suppress urgency and DO, or the ability to reach a bathroom in time to prevent leakage. Clinically therefore there are compelling reasons to believe that prevention or perhaps treatment of white matter damage at an early stage would avert incontinence in some older adults.
Significance and conclusion
Overall, this study is the first to demonstrate a link between structural changes in cerebral white matter and brain activity in neural circuits involved in bladder control and, thus, provides intriguing clues to the possible role of white-matter damage in the genesis of urgency incontinence and furthermore to the cerebral mechanisms of bladder control in older women. Although it remains possible that global white-matter changes are responsible for the development of urgency incontinence, it seems plausible that two specific white-matter pathways are involved, probably the anterior thalamic radiation and the superior longitudinal fasciculus. Damage in either of these pathways appears to affect connections between the cortical regions involved in processing bladder signals, consistent with the observed connectivity differences between urgency-incontinent women and normal controls (Tadic et al., 2008). Thus, the findings of this study underline the importance of white-matter tracts as targets for further investigations to reveal the mechanism of impaired control of continence in the elderly. This new area of research, coupled with other promising methods of assessing white-matter damage (e.g. diffusion tensor imaging) will improve our understanding of the syndrome of geriatric urinary incontinence and may change our clinical approach to incontinence in the elderly.
Supplementary Material
Acknowledgments
NIH: K23AG031916-01; Kl2RR024154-02; 2R01AG020629-06; John A. Hartford Center of Excellence in Geriatric Medicine. We are especially thankful to Megan Nable for her technical assistance in white matter assessment methodology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams P, Blaivas JG, Stanton S, et al. The standardization of terminology of lower urinary tract function. Neurourol Urodyn. 1988;7:403–426. [Google Scholar]
- Abrams P, Cardozo L, Fall M, et al. The standardization of terminology of lower urinary tract function: report from the Standardization Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- Andrew J, Nathan PW. Lesions of the anterior frontal lobes and disturbances of micturition and defaecation. Brain. 1964;87:233–262. doi: 10.1093/brain/87.2.233. [DOI] [PubMed] [Google Scholar]
- Athwal BS, Berkley KJ, Hussain I, et al. Brain responses to changes in bladder volume and urge to void in healthy men. Brain. 2001;124:369–377. doi: 10.1093/brain/124.2.369. [DOI] [PubMed] [Google Scholar]
- Blok BF, Willemsen AT, Holstege G. A PET study on brain control of micturition in humans. Brain. 1997;120:111–21. doi: 10.1093/brain/120.1.111. [DOI] [PubMed] [Google Scholar]
- Blok BF, Sturms LM, Holstege G. Brain activation during micturition in women. Brain. 1998;121:2033–2042. doi: 10.1093/brain/121.11.2033. [DOI] [PubMed] [Google Scholar]
- Burgio KL, Locher JL, Goode PS, Hardin JM, McDowell BJ, Dombrowski M, Candib D. Behavioral vs drug treatment for urge urinary incontinence in older women: a randomized controlled trial. JAMA. 1998;280:1995–2000. doi: 10.1001/jama.280.23.1995. [DOI] [PubMed] [Google Scholar]
- Chen PS, McQuoid DR, Payne ME, Steffens DC. White matter and subcortical gray matter lesion volume changes and late-life depression outcome: a 4-year magnetic resonance imaging study. Int Psychogeriatr. 2006;18:445–56. doi: 10.1017/S1041610205002796. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–66. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ, Griffiths DJ. A decade of functional brain imaging applied to bladder control. Neurourol Urodyn. 2010;29:49–55. doi: 10.1002/nau.20740. [DOI] [PubMed] [Google Scholar]
- Griffiths D, Derbyshire S, Stenger A, et al. Brain control of normal and overactive bladder. J Urol. 2005;174:1862–1867. doi: 10.1097/01.ju.0000177450.34451.97. [DOI] [PubMed] [Google Scholar]
- Griffiths D, Tadic SD, Schaefer W, Resnick NM. Cerebral control of the bladder in normal and urge-incontinent women. NeuroImage. 2007;37:1–7. doi: 10.1016/j.neuroimage.2007.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths D, Tadic SD. Bladder control, urgency and urge incontinence: evidence from functional brain imaging. Neurourol Urodyn. 2008;27:466–474. doi: 10.1002/nau.20549. [DOI] [PubMed] [Google Scholar]
- Griffiths DJ, Tadic SD, Schaefer W, Resnick NM. Cerebral control of the lower urinary tract: how age-related changes might predispose to urge incontinence. Neuroimage. 2009;47:981–6. doi: 10.1016/j.neuroimage.2009.04.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar I, Yang F, Sorond F, Jones RN, Milberg W, Cupples LA, Lipsitz LA. A novel aging phenotype of slow gait, impaired executive function, and depressive symptoms: relationship to blood pressure and other cardiovascular risks. J Gerontol A Biol Sci Med Sci. 2009;64:994–1001. doi: 10.1093/gerona/glp075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzitari D, Simoni M, Pracucci G, Poggesi A, Basile AM, Chabriat H, Erkinjuntti T, Fazekas F, Ferro JM, Hennerici M, Langhorne P, O’Brien J, Barkhof F, Visser MC, Wahlund LO, Waldemar G, Wallin A, Pantoni L LADIS Study Group. Risk of rapid global functional decline in elderly patients with severe cerebral age-related white matter changes: the LADIS study. Arch Intern Med. 2007;167:81–88. doi: 10.1001/archinte.167.1.81. [DOI] [PubMed] [Google Scholar]
- Kahle W, Frotscher M. Color atlas of human anatomy. Vol. 3. Thieme Medical Publishers, Incorporated; 2002. pp. 178–189. [Google Scholar]
- Kavia RB, Dasgupta R, Fowler CJ. Functional imaging and the central control of the bladder. J Comp Neurol. 2005;493:27–32. doi: 10.1002/cne.20753. [DOI] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck JP, van der Horst C, Pott C, Wolff S, Nabavi A, Jansen O, Jünemann KP. Cortical representation of the urge to void: a functional magnetic resonance imaging study. J Urol. 2005;174:1477–81. doi: 10.1097/01.ju.0000173007.84102.7c. [DOI] [PubMed] [Google Scholar]
- Kuchel GA, Moscufo N, Guttman CR, Zeeve N, Wakefield D, Schmidt J, DuBeau CE, Wolfson L. Localization of brain white matter hyperintensities and urinary incontinence in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2009;64:902–909. doi: 10.1093/gerona/glp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchel GA. Aging and homeostatic regulation. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Astana S, editors. Hazzard’s Geriatric Medicine & Gerontology. 6. McGraw-Hill Companies; 2009. [Google Scholar]
- Kuo HK, Lipsitz LA. Cerebral white matter changes and geriatric syndromes: is there a link? J Gerontol A Biol Sci Med Sci. 2004;59:818–826. doi: 10.1093/gerona/59.8.m818. [DOI] [PubMed] [Google Scholar]
- Nordahl CW, Ranganath C, Yonelinas AP, DeCarli C, Fletcher E, Jagust WJ. White matter changes comprise prefrontal cortex function in healthy elderly individuals. J Cogn Neurosci. 2006;18:418–429. doi: 10.1162/089892906775990552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton P, Brubaker L. Urinary incontinence in women. Lancet. 2006;367:57–67. doi: 10.1016/S0140-6736(06)67925-7. [DOI] [PubMed] [Google Scholar]
- Nour S, Svarer C, Kristensen JK, et al. Cerebral activation during micturition in normal men. Brain. 2000;123:781–789. doi: 10.1093/brain/123.4.781. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Poggesi A, Basile AM, Pracucci G, Barkhof F, Chabriat H, Erkinjuntti T, Ferro JM, Hennerici M, O’Brien J, Schmidt R, Visser MC, Wahlund LO, Waldemar G, Wallin A, Inzitari D LADIS Study Group. Leukoaraiosis predicts hidden global functioning impairment in nondisabled older people: the LADIS (Leukoaraiosis and Disability in the Elderly) Study. J Am Geriatr Soc. 2006;54:1095–101. doi: 10.1111/j.1532-5415.2006.00798.x. [DOI] [PubMed] [Google Scholar]
- Poggesi A, Pracucci G, Chabriat H, Erkinjuntti T, Fazekas F, Verdelho A, Hennerici M, Langhorne P, O’Brien J, Scheltens P, Visser MC, Crisby M, Waldemar G, Wallin A, Inzitari D, Pantoni L Leukoaraiosis And DISability Study Group. Urinary Complaints in nondisabled elderly people with age-related white matter changes: The Leucoaraiosis And DISability (LADIS) Study. J Am Geriatr Soc. 2008;56:1638–1643. doi: 10.1111/j.1532-5415.2008.01832.x. [DOI] [PubMed] [Google Scholar]
- Resnick NM, Yalla SV. Geriatric incontinence and voiding dysfunction. In: Wein AJ, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 9. WB Saunders Co; 2007. [Google Scholar]
- Sakakibara R, Hattori T, Uchiyama T, Yamanishi T. Urinary function in elderly people with and without leuokoaraiosis: relation to cognitive and gait function. J Neurol Neurosurg Psychiatry. 1999;67:658–660. doi: 10.1136/jnnp.67.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter. Neuroanatomy, clinical neurology and neurobehavioral correlates. Ann NY Acad Sci. 2008;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Petrovic K, Ropele S, Enzinger C, Fazekas F. Progression of leukoaraiosis and cognition. Stroke. 2007;38:2619–25. doi: 10.1161/STROKEAHA.107.489112. [DOI] [PubMed] [Google Scholar]
- Seseke S, Baudewig J, Kallenberg K, Ringert RH, Seseke F, Dechent P. Voluntary pelvic floor muscle control--an fMRI study. Neuroimage. 2006;31:1399–407. doi: 10.1016/j.neuroimage.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Tadic SD, Zdaniuk B, Griffiths D, Rosenberg L, Schafer W, Resnick NM. Effect of biofeedback on psychological burden and symptoms in older women with urge urinary incontinence. J Am Geriatr Soc. 2007;55:2010–2015. doi: 10.1111/j.1532-5415.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- Tadic SD, Griffiths D, Schaefer W, Resnick NM. Abnormal connections in the supraspinal bladder control network in women with urge urinary incontinence. NeuroImage. 2008;39:1647–1653. doi: 10.1016/j.neuroimage.2007.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadic SD, Griffiths D, Schaefer W, Cheng CI, Resnick NM. Brain activity measured by functional magnetic resonance imaging (fMRI) is related to patient-reported severity of urgency urinary incontinence. J Urol. 2010;183:221–228. doi: 10.1016/j.juro.2009.08.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannorsdall TD, Waldstein SR, Kraut M, Pearlson GD, Schretlen DJ. White matter abnormalities and cognition in a community sample. Arch Clin Neuropsychol. 2009;24:209–17. doi: 10.1093/arclin/acp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Rosano C, Butters M, Whyte E, Nable M, Crooks R, Meltzer CC, Reynolds CF, Aizenstein HJ. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Research: Neuroimaging. 2006;148:133–142. doi: 10.1016/j.pscychresns.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PCM, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Yin Y, Shuke N, Okizaki A, Sato J, Aburano T, Li Y, Kaneko S, Mizunaga M, Yachiku S. Cerebral activation during withholding urine with full bladder in healthy men using 99mTc-HMPAO SPECT. J Nucl Med. 2006;47:1093–8. [PubMed] [Google Scholar]
- Zhang H, Reitz A, Kollias S, Summers P, Curt A, Schurch B. An fMRI study of the role of suprapontine brain structures in the voluntary voiding control induced by pelvic floor contraction. Neuroimage. 2005;24:174–80. doi: 10.1016/j.neuroimage.2004.08.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.