Abstract
We examined the basis for the absence in cystic fibrosis (CF) patients of opsonic antibodies to the mucoid exopolysaccharide (MEP) antigen surrounding Pseudomonas aeruginosa that infect these patients. Opsonic antibodies to MEP are found in sera of the minority of CF patients that remain noncolonized into the second to fourth decades of life and protect rodents from chronic P. aeruginosa endobronchial infections. High titers of nonopsonic antibodies to MEP are found in P. aeruginosa-infected CF patients. Immunization of mice with doses of MEP that provoke only nonopsonic antibodies elicited CD3+, CD8+, T cell receptor alpha beta receptor+, major histocompatibility complex-unrestricted cytotoxic lymphocytes specific for hybridoma cells producing opsonic but not nonopsonic antibodies. Cytotoxicity was dependent on immune complexes on the surface of the T cells. Normal murine T cells could be activated by concanavalin A and sensitized with immune complexes for cytotoxic killing of hybridoma targets. CF patients infected with P. aeruginosa had serum immune complexes that sensitized concanavalin A-activated human T cells to kill murine hybridoma cells producing opsonic but not nonopsonic antibody. These results could explain the absence in infected CF patients of MEP-specific opsonins, an occurrence that accompanies the persistence of this infectious state.
Full text
PDF

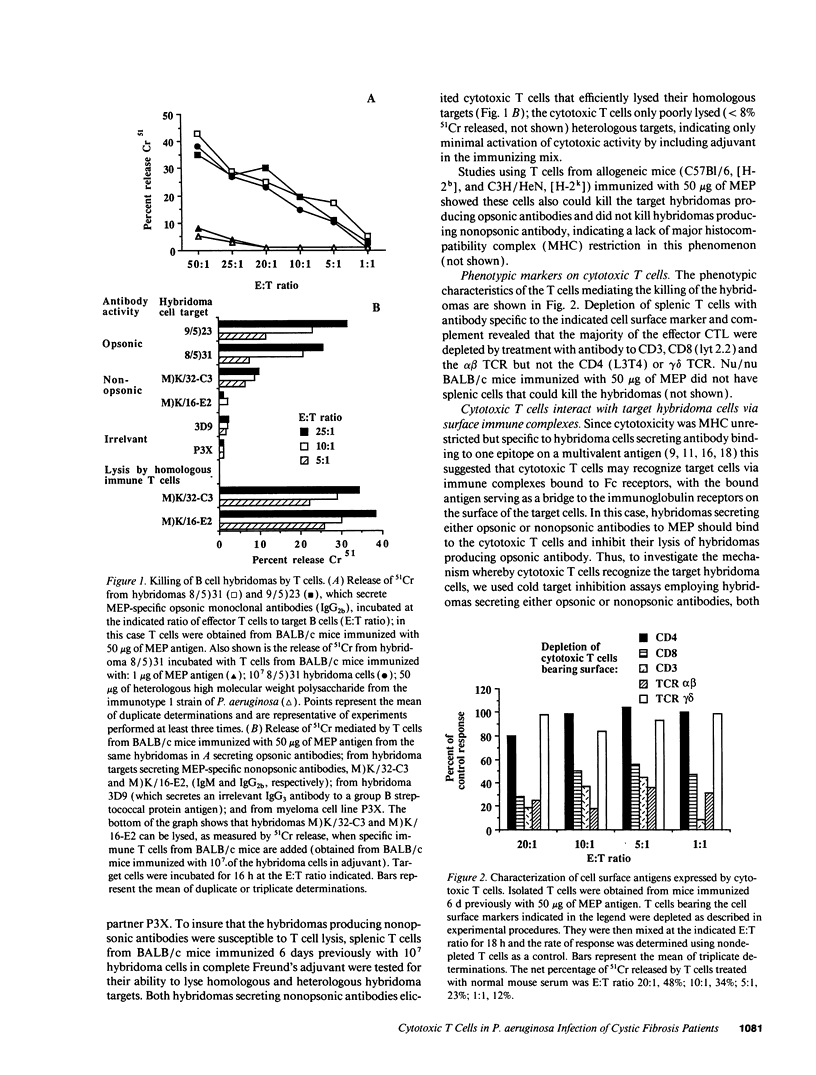
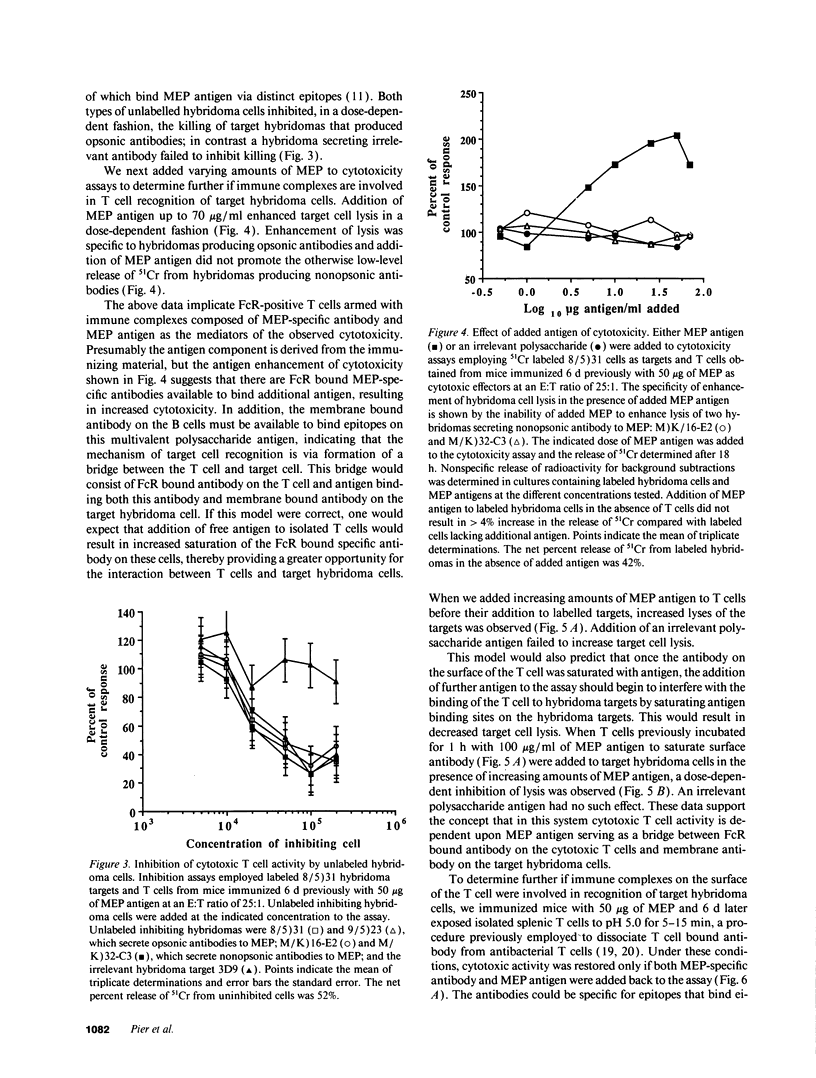
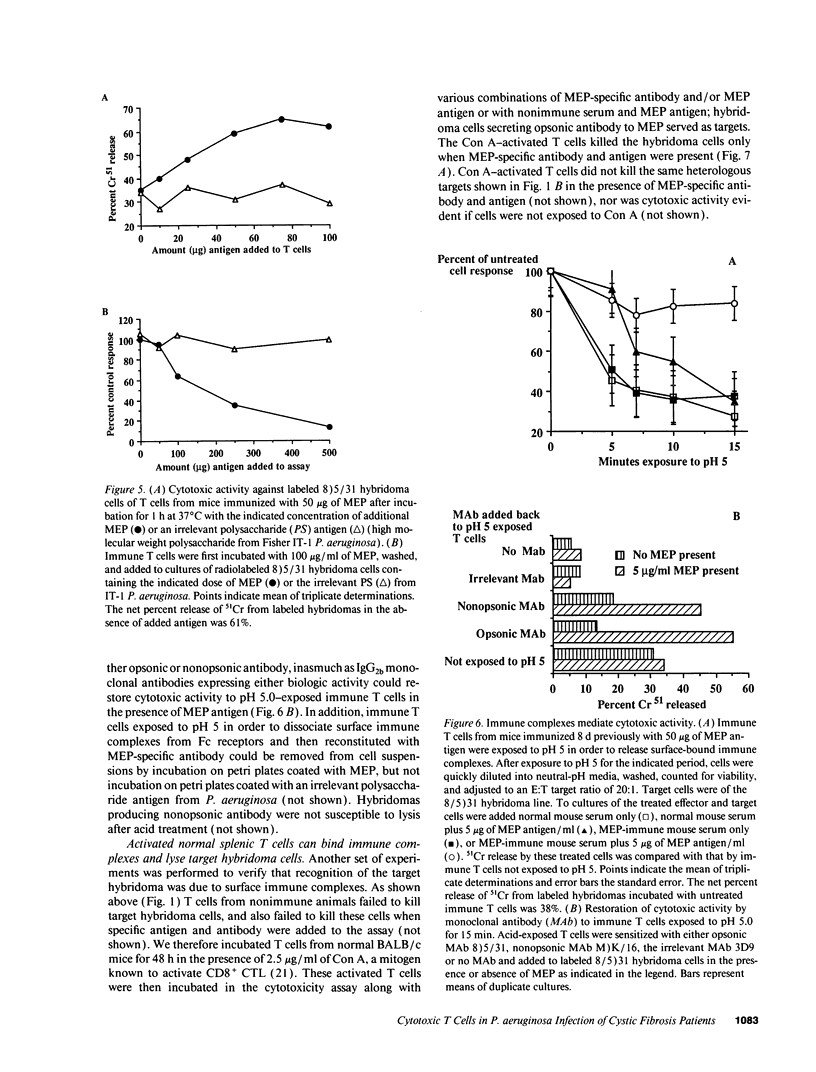
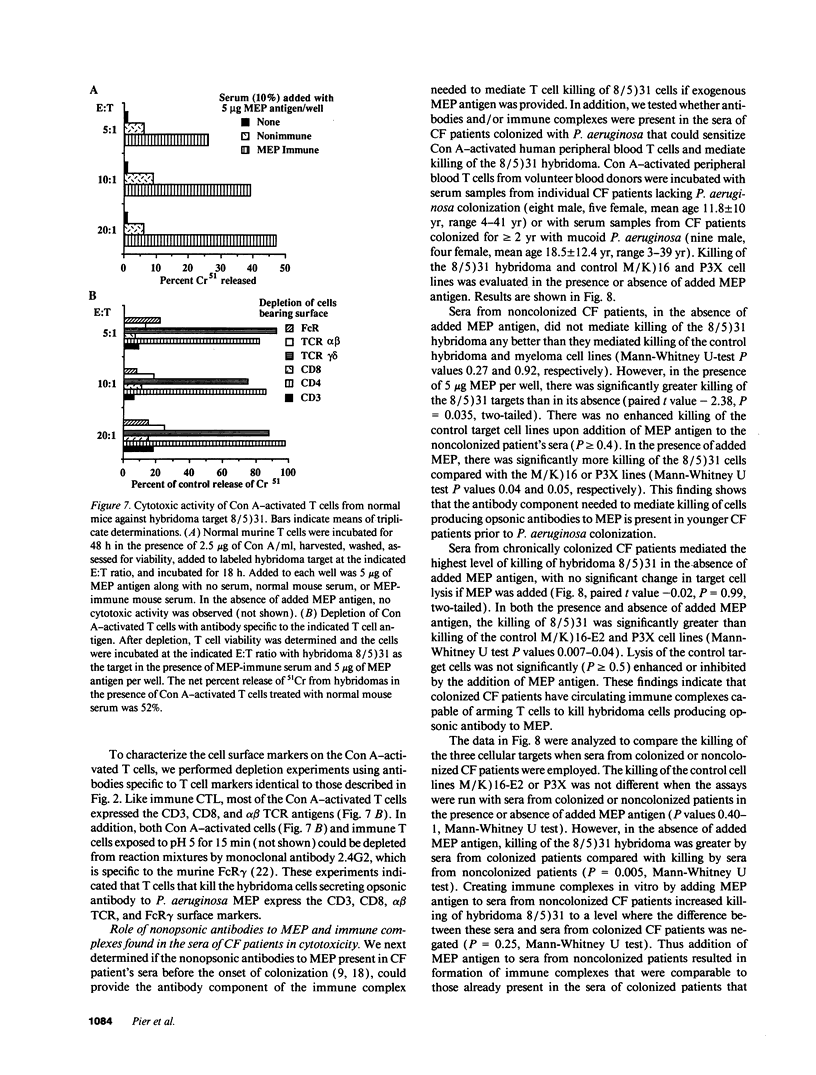


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames P., DesJardins D., Pier G. B. Opsonophagocytic killing activity of rabbit antibody to Pseudomonas aeruginosa mucoid exopolysaccharide. Infect Immun. 1985 Aug;49(2):281–285. doi: 10.1128/iai.49.2.281-285.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastassiou E. D., Mintzas A. C., Kounavis C., Dimitracopoulos G. Alginate production by clinical nonmucoid Pseudomonas aeruginosa strains. J Clin Microbiol. 1987 Apr;25(4):656–659. doi: 10.1128/jcm.25.4.656-659.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J. Regulation of magnitude of antibody response to bacterial polysaccharide antigens by thymus-derived lymphocytes. Infect Immun. 1990 Nov;58(11):3465–3468. doi: 10.1128/iai.58.11.3465-3468.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnaba V., Franco A., Alberti A., Benvenuto R., Balsano F. Selective killing of hepatitis B envelope antigen-specific B cells by class I-restricted, exogenous antigen-specific T lymphocytes. Nature. 1990 May 17;345(6272):258–260. doi: 10.1038/345258a0. [DOI] [PubMed] [Google Scholar]
- Braley-Mullen H. Distinct populations of antigen-presenting cells are required for activation of suppressor and contrasuppressor T cells by type III pneumococcal polysaccharide. Cell Immunol. 1990 Jul;128(2):528–541. doi: 10.1016/0008-8749(90)90046-t. [DOI] [PubMed] [Google Scholar]
- Brown P. B., Köhler H., Rowley D. A. Specific suppression of the antibody response in vitro by serum from paralyzed mice. J Immunol. 1975 Aug;115(2):419–424. [PubMed] [Google Scholar]
- Brunati S., Moncuit J., Fridman W. H., Teillaud J. L. Regulation of IgG production by suppressor Fc gamma RII+ T hybridomas. Eur J Immunol. 1990 Jan;20(1):55–61. doi: 10.1002/eji.1830200109. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., Kureishi A., Rabin H. R. Detection of antibodies to Pseudomonas aeruginosa alginate extracellular polysaccharide in animals and cystic fibrosis patients by enzyme-linked immunosorbent assay. J Clin Microbiol. 1983 Aug;18(2):276–282. doi: 10.1128/jcm.18.2.276-282.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield M. J., Shaffer D. Immunoregulation by antigen/antibody complexes. I. Specific immunosuppression induced in vivo with immune complexes formed in antibody excess. J Immunol. 1987 Jun 1;138(11):3680–3683. [PubMed] [Google Scholar]
- Chesnut R. W., Grey H. M. Antigen presentation by B cells and its significance in T-B interactions. Adv Immunol. 1986;39:51–94. doi: 10.1016/s0065-2776(08)60348-x. [DOI] [PubMed] [Google Scholar]
- Fomsgaard A., Høiby N., Shand G. H., Conrad R. S., Galanos C. Longitudinal study of antibody response to lipopolysaccharides during chronic Pseudomonas aeruginosa lung infection in cystic fibrosis. Infect Immun. 1988 Sep;56(9):2270–2278. doi: 10.1128/iai.56.9.2270-2278.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner C. V., DesJardins D., Pier G. B. Immunogenic properties of Pseudomonas aeruginosa mucoid exopolysaccharide. Infect Immun. 1990 Jun;58(6):1835–1842. doi: 10.1128/iai.58.6.1835-1842.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Hoiby N., Axelsen N. H. Identification and quantitation of precipitins against Pseudomonas aeruginosa in patients with cystic fibrosis by means of crossed immunoelectrophoresis with intermediate gel. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Jun;81(3):298–308. doi: 10.1111/j.1699-0463.1973.tb02207.x. [DOI] [PubMed] [Google Scholar]
- Hoover R. G., Gebel H. M., Dieckgraefe B. K., Hickman S., Rebbe N. F., Hirayama N., Ovary Z., Lynch R. G. Occurrence and potential significance of increased numbers of T cells with Fc receptors in myeloma. Immunol Rev. 1981;56:115–139. doi: 10.1111/j.1600-065x.1981.tb01049.x. [DOI] [PubMed] [Google Scholar]
- Kumagai K., Abo T., Sekizawa T., Sasaki M. Studies of surface immunoglobulins on human B lymphocytes. I. Dissociation of cell-bound immunoglobulins with acid pH or at 37 degrees C. J Immunol. 1975 Oct;115(4):982–987. [PubMed] [Google Scholar]
- Lynch R. G., Sandor M., Waldschmidt T. J., Mathur A., Schaiff W. T., Berg D. J., Snapp K., Mueller A., Robinson M. G., Noben N. Lymphocyte Fc receptors: expression, regulation and function. Mol Immunol. 1990 Dec;27(12):1167–1179. doi: 10.1016/0161-5890(90)90019-v. [DOI] [PubMed] [Google Scholar]
- Markham R. B., Pier G. B., Powderly W. G. Suppressor T cells regulating the cell-mediated immune response to Pseudomonas aeruginosa can be generated by immunization with anti-bacterial T cells. J Immunol. 1988 Dec 1;141(11):3975–3979. [PubMed] [Google Scholar]
- Markham R. B., Pier G. B., Schreiber J. R. The role of cytophilic IgG3 antibody in T cell-mediated resistance to infection with the extracellular bacterium, Pseudomonas aeruginosa. J Immunol. 1991 Jan 1;146(1):316–320. [PubMed] [Google Scholar]
- Mongini P. K., Blessinger C. A., Dalton J. P. Affinity requirements for induction of sequential phases of human B cell activation by membrane IgM-cross-linking ligands. J Immunol. 1991 Mar 15;146(6):1791–1800. [PubMed] [Google Scholar]
- Muller E., Apicella M. A. T-cell modulation of the murine antibody response to Neisseria meningitidis group A capsular polysaccharide. Infect Immun. 1988 Jan;56(1):259–266. doi: 10.1128/iai.56.1.259-266.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noelle R. J., Snow E. C. Cognate interactions between helper T cells and B cells. Immunol Today. 1990 Oct;11(10):361–368. doi: 10.1016/0167-5699(90)90142-v. [DOI] [PubMed] [Google Scholar]
- Pedersen S. S., Espersen F., Høiby N., Jensen T. Immunoglobulin A and immunoglobulin G antibody responses to alginates from Pseudomonas aeruginosa in patients with cystic fibrosis. J Clin Microbiol. 1990 Apr;28(4):747–755. doi: 10.1128/jcm.28.4.747-755.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S. S., Kharazmi A., Espersen F., Høiby N. Pseudomonas aeruginosa alginate in cystic fibrosis sputum and the inflammatory response. Infect Immun. 1990 Oct;58(10):3363–3368. doi: 10.1128/iai.58.10.3363-3368.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Desjardins D., Aguilar T., Barnard M., Speert D. P. Polysaccharide surface antigens expressed by nonmucoid isolates of Pseudomonas aeruginosa from cystic fibrosis patients. J Clin Microbiol. 1986 Aug;24(2):189–196. doi: 10.1128/jcm.24.2.189-196.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Grout M., Desjardins D. Complement deposition by antibodies to Pseudomonas aeruginosa mucoid exopolysaccharide (MEP) and by non-MEP specific opsonins. J Immunol. 1991 Sep 15;147(6):1869–1876. [PubMed] [Google Scholar]
- Pier G. B., Matthews W. J., Jr, Eardley D. D. Immunochemical characterization of the mucoid exopolysaccharide of Pseudomonas aeruginosa. J Infect Dis. 1983 Mar;147(3):494–503. doi: 10.1093/infdis/147.3.494. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Pollack M. Isolation, structure, and immunogenicity of Pseudomonas aeruginosa immunotype 4 high-molecular-weight polysaccharide. Infect Immun. 1989 Feb;57(2):426–431. doi: 10.1128/iai.57.2.426-431.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Saunders J. M., Ames P., Edwards M. S., Auerbach H., Goldfarb J., Speert D. P., Hurwitch S. Opsonophagocytic killing antibody to Pseudomonas aeruginosa mucoid exopolysaccharide in older noncolonized patients with cystic fibrosis. N Engl J Med. 1987 Sep 24;317(13):793–798. doi: 10.1056/NEJM198709243171303. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Small G. J., Warren H. B. Protection against mucoid Pseudomonas aeruginosa in rodent models of endobronchial infections. Science. 1990 Aug 3;249(4968):537–540. doi: 10.1126/science.2116663. [DOI] [PubMed] [Google Scholar]
- Russell N. J., Gacesa P. Chemistry and biology of the alginate of mucoid strains of Pseudomonas aeruginosa in cystic fibrosis. Mol Aspects Med. 1988;10(1):1–91. doi: 10.1016/0098-2997(88)90002-7. [DOI] [PubMed] [Google Scholar]
- Sabourin L. A., Hawley R. G. Suppression of programmed death and G1 arrest in B-cell hybridomas by interleukin-6 is not accompanied by altered expression of immediate early response genes. J Cell Physiol. 1990 Dec;145(3):564–574. doi: 10.1002/jcp.1041450325. [DOI] [PubMed] [Google Scholar]
- Shand G. H., Pedersen S. S., Brown M. R., Høiby N. Serum antibodies to Pseudomonas aeruginosa outer-membrane proteins and iron-regulated membrane proteins at different stages of chronic cystic fibrosis lung infection. J Med Microbiol. 1991 Apr;34(4):203–212. doi: 10.1099/00222615-34-4-203. [DOI] [PubMed] [Google Scholar]
- Shinohara N., Huang Y. Y., Muroyama A. Specific suppression of antibody responses by soluble protein-specific, class II-restricted cytolytic T lymphocyte clones. Eur J Immunol. 1991 Jan;21(1):23–27. doi: 10.1002/eji.1830210105. [DOI] [PubMed] [Google Scholar]
- Shinohara N., Watanabe M., Sachs D. H., Hozumi N. Killing of antigen-reactive B cells by class II-restricted, soluble antigen-specific CD8+ cytolytic T lymphocytes. Nature. 1988 Dec 1;336(6198):481–484. doi: 10.1038/336481a0. [DOI] [PubMed] [Google Scholar]
- Smith M. J., Morris L., Stead R. J., Hodson M. E., Batten J. C. Lymphocyte subpopulations and function in cystic fibrosis. Eur J Respir Dis. 1987 May;70(5):300–308. [PubMed] [Google Scholar]
- Speert D. P., Lawton D., Mutharia L. M. Antibody to Pseudomonas aeruginosa mucoid exopolysaccharide and to sodium alginate in cystic fibrosis serum. Pediatr Res. 1984 May;18(5):431–433. doi: 10.1203/00006450-198405000-00008. [DOI] [PubMed] [Google Scholar]
- Tarkowski A., Lue C., Moldoveanu Z., Kiyono H., McGhee J. R., Mestecky J. Immunization of humans with polysaccharide vaccines induces systemic, predominantly polymeric IgA2-subclass antibody responses. J Immunol. 1990 May 15;144(10):3770–3778. [PubMed] [Google Scholar]
- Taylor C. E., Bright R. T-cell modulation of the antibody response to bacterial polysaccharide antigens. Infect Immun. 1989 Jan;57(1):180–185. doi: 10.1128/iai.57.1.180-185.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. E., Fauntleroy M. B., Stashak P. W., Baker P. J. Antigen-specific suppressor T cells respond to recombinant interleukin-2 and other lymphokines. Infect Immun. 1991 Feb;59(2):575–579. doi: 10.1128/iai.59.2.575-579.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- di Sant'agnese P. A., Davis P. B. Cystic fibrosis in adults. 75 cases and a review of 232 cases in the literature. Am J Med. 1979 Jan;66(1):121–132. doi: 10.1016/0002-9343(79)90491-1. [DOI] [PubMed] [Google Scholar]


