Abstract
Caenorhabditis elegans senses multiple environmental stimuli through sensory systems and rapidly changes its behaviors for survival. With a simple and well-characterized nervous system, C. elegans is a suitable animal model for studying behavioral plasticity.
Previous studies have demonstrated acute neurodepressive effects of ethanol on multiple behaviors of C. elegans similar to ethanol's effect on other organisms. C. elegans also develops ethanol tolerance during continuous exposure to ethanol. In mammals, chronic ethanol exposure leads to ethanol tolerance as well as increased ethanol consumption. Ethanol preference is associated with the development of tolerance and may lead to the development of ethanol dependence.
In this study we show that C. elegans is a useful model organism for studying chronic effects of ethanol, including the development of ethanol preference. We designed a behavioral assay for testing ethanol preference after prolonged ethanol exposure. Despite baseline aversive responses to ethanol, animals show ethanol preference after 4 hours of pre-exposure to ethanol and exhibit significantly enhanced preference for ethanol after a lifetime of ethanol exposure. cat-2 and tph-1 mutant animals have defects in the synthetic enzymes for dopamine and serotonin, respectively. These mutants are deficient in the development of ethanol preference, indicating that dopamine and serotonin are required for this form of behavioral plasticity.
Keywords: ethanol, preference, olfaction, dopamine, serotonin
Introduction
Animals respond to a variety of environmental cues and modulate their behaviors. Caenorhabditis elegans can rapidly modify its behaviors based on experience. One well-studied behavior is olfactory adaptation. Olfactory preferences can be changed by adaptation caused by pre-exposure to a volatile odorant or by food signals (Colbert & Bargmann 1997; Nuttley et al. 2002; Chao et al. 2004). Despite a simple nervous system of 302 neurons, C. elegans also shows relatively complicated associative learning based on responses to chemosensory and thermosensory cues (Gomez et al. 2001; Saeki et al. 2001; Mori et al. 2008). For instance, worms modify their olfactory preferences after exposure to pathogenic bacteria to avoid toxic bacteria (Zhang et al. 2005). The switch of olfactory preference between attraction and aversion can be regulated by a single olfactory neuron (Tsunozaki et al. 2008).
We previously showed that acute ethanol exposure causes dose-dependent depressive effects on locomotory and egg-laying behaviors in C. elegans at the same internal concentration of ethanol (20mM to 30mM) that produces intoxication in humans (Davies et al. 2003). 0.1% blood alcohol, a common legal driving limit, corresponds to 21.7mM ethanol. We also demonstrated state-dependent effects of ethanol on olfactory adaptation (Bettinger & McIntire 2004). These studies revealed that C. elegans exhibits comparable acute behavioral responses to ethanol as higher organisms.
Our next approach has been to understand the effects of chronic ethanol exposure. Chronic drug use can lead to compulsive drug-seeking habits and drug addiction (Everitt & Robbins 2005). Continuous alcohol intoxication results in increased alcohol consumption, tolerance and sometimes alcohol dependency in mammals (Koob 1998; Roberts et al. 2000; Koob 2003; Rimondini et al. 2003, Rimondini et al. 2007). Ethanol tolerance also develops in Drosophila even after a single exposure to ethanol, without changes in ethanol absorption or metabolism (Scholz et al. 2000). In addition to rapid tolerance, flies develop chronic tolerance to the intoxicating effects of ethanol after prolonged exposure to a low concentration of ethanol (Berger et al. 2004). As shown in other organisms, C. elegans exibits ethanol tolerance after continuous exposure to ethanol (Davies et al. 2004).
In this study, we examined ethanol preference after pre-exposure to ethanol. Ethanol preference develops in ethanol pretreated wild-type animals within 4 hours and is enhanced by lifelong exposure to ethanol. From mutant analysis, we found that dopamine and serotonin are required for the induction of ethanol preference in C. elegans.
Materials and methods
Animals were maintained as described (Brenner, 1974). The wild-type strain was Bristol N2. The mutant strains used in this study are the following: cat-2(e1112), cat-2(tm2261), egl-4(n478), npr-1(ky13), slo-1(js118), tph-1(mg280), mod-1(n3034).
Preference Assays
Embryos were collected by bleaching and were grown on a standard nematode growth medium (NGM) plate at room temperature. Young adults were incubated on a control or 300mM ethanol plate with food (OP50). The ethanol pre-exposure plates were prepared as previously described (See Davies et al. 2004, for details). Pre-exposure plates were 6 cm NGM plates that had been seeded with bacteria on half of the plate and were dried for 2 hours. Ice-cold ethanol was added to the half of the plate not seeded, to a final concentration of 300mM ethanol. The plates were sealed with Parafilm and ethanol was allowed to equilibrate in the agar for 2 hours. After 4 hours pre-exposure on ethanol plates, animals were washed twice in S-basal (0.1M NaCl, 0.05M KPO4 [pH6], 5mg/lml cholesterol in 100% ethanol) and once in distilled water. 100-200 animals were placed at the origin (black spot) of an assay plate (see below, and Fig. 1) and excess water was wicked off. Animals were allowed to move and were scored after 30 min. Photographs of the quadrants were taken using a CCD camera for counting. These preference assays were conducted on assay plates, which are 10cm NGM-filled Petri plates divided into quadrants. Prior to use, assay plates were dried at room temperature without lids for 2 hours. Ice-cold ethanol was added into 9mm ethanol holes in quadrants A and B (grey circle) to achieve a final concentration of 300mM in quadrants A and B (Fig. 1). The plates were sealed with Parafilm and ethanol was allowed to diffuse into the agar for 2 hours. Tests of ethanol concentrations in the agar revealed no significant ethanol (0.31 ±0.08mM) in the control quadrants. Concentrations of ethanol in the ethanol quadrants decrease somewhat with distance from the 9mm holes, with a concentration 283±19.25 mM at the 9mm hole and 210±27.51mM ethanol at a distance of 2.5cm from the 9mm hole. A Preference Index (PI) was calculated as {[number of animals at A and B] - [number of animals at C and D]} / Total number of animals tested. Statistical differences were determined by student's t-tests. Octanol preference assays were performed by the same methods as ethanol preference. Instead of ethanol, 1% octanol was added into holes to make a 10-4 dilution.
Figure 1. Preference Assay.
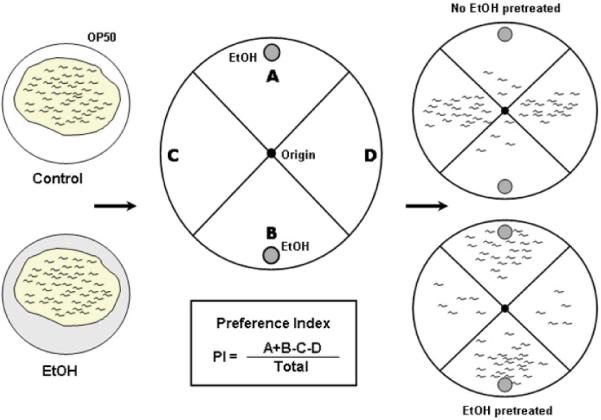
One-day adult animals were incubated on a control or ethanol plate with OP50 for defined time intervals and then placed at the origin of an assay plate (black spot). The animals remain on the assay plate for 30 minutes before scoring. EtOH pretreated animals preferentially accumulate in the ethanol quadrants (A, B). Control animals accumulate primarily to the quadrants without ethanol (C, D). A Preference Index (PI) was calculated as {[the number of animals at A and B] - [the number of animals at C and D]} / Total number of animals.
Chemotaxis Assays
Chemotaxis assay were performed by standard methods (Bargmann et al. 1993). Briefly, chemotaxis plates were prepared with 10cm Petri dishes containing 10ml of assay agar (2% agar, 5mM KPO4 [pH6], 1mM CaCl2, 1mM MgSO4). Worms were washed twice in S-basal, once in distilled water and placed onto spot on the plate. 1µl of 1:100 benzaldehyde:EtOH was placed at test spot and 1µl of ethanol was applied to the other spot. For each spot, 1µl of 1 M NaN3 was applied to immobilize the worms when they reached the spot. After one hour of chemotaxis, animals were counted, and Chemotaxis Index (CI) was calculated as {[number of animals at the test odorant] - [number of animals at the solvent]} / Total number of animals tested. Avoidance assays for 2-nonanone were performed on square plates as described previously (Troemel et al. 1997). The plates were divided into six sectors labeled A-F. 1µl each of odorant 2-nonanone (100%, 10% and 1%) and 1 M NaN3 were added in two spots in sector A, and 1µl each of control diluent (water or ethanol) and 1 M NaN3 were added in two spots in sector F. An avoidance Index (AI) was calculated as {[number of animals in sectors A and B] - [number of animals in sectors E and F]} / Total number of animals in all six sectors of the plate. Statistical differences were determined by student's t-tests.
Calculating Ethanol Concentrations
Internal or tissue ethanol concentrations following timed exposures to ethanol were determined as described previously (Davies et al. 2004). 300-600 worms were incubated on seeded plates that had ethanol added to a final concentration of 300mM. After 4 hours, worms were quickly washed in ice-cold dH2O and pelleted by centrifugation at 4°C. For a lifetime exposure to ethanol, embryos were grown on 300mM ethanol plates with bacteria. The worm pellet was resuspended in ice-cold dH2O and the worms were frozen at -80°C for 30 min before homogenization on ice. For amount of ethanol on the plate, small pieces of the agar were cut and dissolved in ice-cold dH2O for 1 hour. The concentration of ethanol was determined according to the manufacturer's instructions using an Ethanol Assay Kit (DIET-500) from BioAssay Systems. Assays of ethanol concentrations were replicated at least three times. Statistical differences were determined by student's t-tests.
Results
To examine the responses of C. elegans to the presence of ethanol, we designed an assay called a Preference Assay (Fig. 1). Synchronized embryos were collected by bleaching of adult worms and were grown on nematode growth medium (NGM). Young adult animals were incubated on control or 300mM ethanol plates with bacteria for 2 or 4 hours. After pre-exposure, animals were washed and tested for their response to ethanol on ethanol containing quadrant plates. Animals were allowed to move freely for 30 minute between two control quadrants without ethanol and two ethanol quadrants. Our previous studies revealed that exogenously applied ethanol causes dose-dependent decreases in the speed of locomotion. Animals exposed to 300mM exogenous ethanol show a moderate decrease in speed (animals move at approximately 60% of untreated speed). Although moderate slowing of locomotion was observed on ethanol plates, chemotaxis to a volatile odorant benzaldehyde was not affected by ethanol treatment (Bettinger & McIntire 2004). We also found that ethanol pretreated animals were able to show normal chemotaxis to 1% benzaldehyde at an exogenous dose of ethanol of 300mM (data not shown). Because of low permeability, the internal concentration of ethanol for worms exposed to 300mM ethanol is much lower than the exogenous concentration and is comparable to the blood alcohol level in intoxicated humans (Davies et al. 2003). In our Preference Assay, a Preference Index (PI) is calculated as {[number of animals in quadrants A and B] - [number of animals in quadrants C and D]} / Total tested animals.
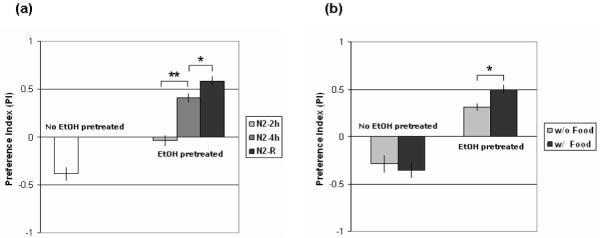
Naïve animals avoid ethanol-containing areas (A and B) at 300mM ethanol (Fig. 2a). No ethanol preference was observed in animals pretreated with ethanol for 2 hours. However, animals pre-exposed to ethanol for 4 hours developed a significant preference for the ethanol-containing quadrants, indicating that animals are able to modify their olfactory preference after pre-exposure to ethanol. These animals show ethanol preference with a positive PI (preference index) of 0.409±0.045 [n=4]. We tested whether the ethanol preference increases with more prolonged pre-exposure by testing animals that had been raised on ethanol plates. Embryos were grown on 300mM ethanol plates with bacteria OP50. Wild-type animals show a significantly higher ethanol preference (PI=0.585±0.05; t7=2.53, p<0.05 [n=5]) after a lifetime exposure to ethanol (Fig. 2a).
Figure 2. Effects of exposure time and food on Ethanol Preference.
(a) Naïve animals avoid ethanol. Animals pretreated with ethanol for 2 hours (N2-2h) do not show ethanol preference. 4 hours of pre-exposure (N2-4h) is sufficient for the development of ethanol preference (t6=6.33, p<0.001, n=4 assays). Animals (N2-R) raised on ethanol show higher ethanol preference compared to animals exposed for 4 hours (t7=2.53, p<0.05, n=5 assays). (b) EtOH pretreated animals develop ethanol preference with and without food during the pretreatment (t6=3.37, p<0.05, n=4 assays). Error bars indicate SEM. Asterisk indicates a statistical difference as tested by student's t-test (*p<0.05 and **p<0.001).
We determined internal ethanol concentrations in animals after different periods of ethanol exposure on 300mM plates. The internal concentration of ethanol was 27.54±2.52mM [n=4] after 4 hours exposure, which was not significantly different (t5=2.03, p=0.099) from the internal concentration after 30 minutes of exposure (24.24±1.26mM [n=3]). The internal concentration was 33.15±3.09mM [n=3] with the lifetime exposure.
Ethanol preference was not observed in animals after simple exposure to ethanol odor, with ethanol placed on the lid of the plate for 4 hours (PI=-0.094±0.07 [n=3]) and the internal ethanol concentration was minimal (3.78±1.26mM [n=3]) under this condition. This concentration does not result in behavioral changes or intoxication in C. elegans. These results indicate that a significant internal concentration of ethanol is required for induction of ethanol preference.
In these experiments, we examined ethanol preference after pre-exposure to ethanol with food so the animals did not starve during the pre-exposure period. This raised the question, however, of whether the animals had learned or formed an association between the food signals and ethanol. C. elegans is capable of modifying its behavior and integrating sensory signals through associative learning (Chao et al. 2004; Torayama et al. 2007). Food could act as an unconditioned stimulus and ethanol a conditioned stimulus during the pre-exposure phase of the experiment. To determine whether ethanol preference represents associative learning, we performed the ethanol preference assay in a food-deficient condition. Animals were incubated on an ethanol plate in the absence of food. Ethanol preference also developed over 4 hours in the absence of food (Fig. 2b) suggesting that the ethanol preference is not simply a learned association between the food and ethanol. The induced preference for ethanol was, however, stronger in the presence of food which could reflect an association or simply represent a nonspecific adverse effect of starvation on the animals exposed to ethanol in the absence of food.
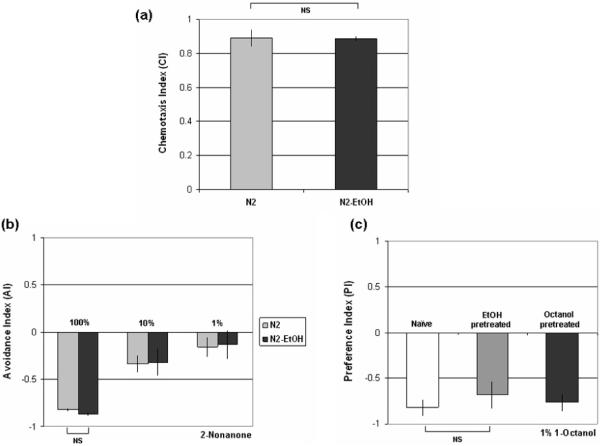
We next tested whether sustained ethanol exposure generally disrupts chemotactic responses to volatile odorants. We analyzed chemotaxis of ethanol-pretreated animals to the volatile attractant odorant benzaldehyde and the repulsive odorant 2-nonanone. The ethanol-pretreated animals showed normal chemotaxis to 1% benzaldehyde compared with control animals (Fig. 3a) and avoided 2-nonanone normally at 100%, 10% and 1% concentrations (Fig. 3b) indicating that prolonged exposure to ethanol does not generally disrupt odorant sensing or the ability to chemotax appropriately. It had previously been demonstrated that pre-exposure to ethanol for a shorter period does not cause a significant decrease in chemotaxis to benzaldehyde (Bettinger & McIntire 2004). We also tested the effect of ethanol pre-exposure on the response to another alcohol, octanol. Octanol is also a repellant for C. elegans (Bargmann et al. 1993). There was no significant difference in chemotaxis to octanol between naïve and the ethanol-pretreated animals (data not shown). Ethanol pre-exposure also did not cause any change in octanol preference (Fig. 3c). Hence, the change in ethanol responses after ethanol preconditioning does not generalize to another alcohol and ethanol-pretreated animals perform well in the quadrant assay with an aversive signal other than ethanol.
Figure 3. Chemotaxis to benzaldehyde and 2-nonanone.
(a) EtOH pretreated worms show normal chemotaxis to 1% benzaldehyde (t6=0.058, p=0.96, n=4 assays). (b) EtOH pretreated animals exhibit normal responses to 100%, 10% and 1% of 2-nonanone (t12=1.96, p=0.073, n=7 assays). (c) Ethanol pre-exposure does not affect the response to octanol, and preference to octanol is not induced by octanol pretreatment (t4=0.872, p=0.43, n=3 assays). Error bars indicate SEM. NS, not significantly different (p>0.05).
We also wanted to determine whether C. elegans would develop a preference for octanol as a result of pre-exposure to octanol. Animals were pretreated with octanol instead of ethanol to determine whether this would result in a switch from octanol repulsion to attraction as had been observed with ethanol. Octanol pretreated animals did not exhibit any change in octanol responses in the preference assay (Fig. 3c). Preference does not therefore generally develop for alcohols that act as repellants for C. elegans.
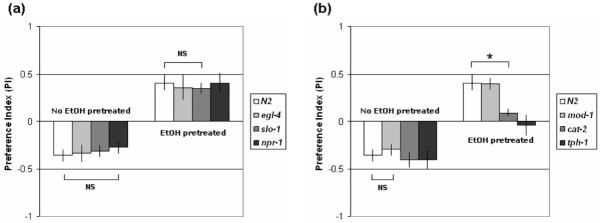
C. elegans adapts to continuous olfactory stimuli and the adaptation is reversible and odor-selective (Colbert & Bargmann 1995). Although adaptation occurs rapidly by adjustment of sensitivity to various odors, adaptation does not result in an opposite response as we observed in our ethanol preference assays. To determine if ethanol preference is related to olfactory adaptation, we analyzed ethanol preference in the olfactory adaptation defective mutant, egl-4(n478). egl-4 encodes cGMP-dependent protein kinase that functions in AWC olfactory neurons and is required for adaptation after prolonged exposure to AWC-sensed odors. egl-4 mutants display defective adaptation to benzaldehyde, butanone and isoamyl alcohol (L'Etoile et al. 2002). egl-4(n478) mutant animals showed normal sensitivity to ethanol and also exhibited ethanol preference after ethanol pre-exposure (Fig. 4a) suggesting that ethanol preference is a distinct behavior from olfactory adaptation. It is nevertheless possible that there is some superimposed adaptation that occurs to ethanol during the assay.
Figure 4. Ethanol Preference in wild type and mutant animals.
(a) egl-4, slo-1 and npr-1 mutants show normal ethanol preference compared to wild type. NS, not significantly different (t6=0.835, p=0.44 / t6=0.569, p=0.59). (b) mod-1 mutant animals show normal ethanol preference. cat-2 and tph-1 mutants are defective in ethanol preference. All strains were pretreated with ethanol in the presence of food for 4 hours. Error bars indicate SEM; n=4 assays for all data. Asterisk indicates a statistical difference as tested by student's t-test (t6=3.62, p<0.05). NS, not significantly different (t6=0.626, p=0.55).
In order to determine whether mutations that alter responses to the sedative effects of ethanol or the development of acute tolerance also disrupt ethanol preference, we analyzed slo-1(js118) and npr-1(ky13) mutants for ethanol preference. slo-1 encodes the BK potassium channel and has a central role in certain acute ethanol responses (Davies et al. 2003). npr-1, a neuropeptide Y (NPY) receptor like protein, negatively regulates the development of acute ethanol tolerance (Davies et al. 2004). Both slo-1(js118) and npr-1(ky13) mutant animals show normal ethanol preference compared to wild type (Fig. 4a), suggesting that these genes do not contribute to the development of ethanol preference in C. elegans.
It has been reported that dopamine signaling is important for drug-dependent behaviors in other organisms (Berke & Hyman 2000). Previous studies in our lab have shown that dopamine has a role in state-dependency in C. elegans (Bettinger & McIntire 2004). To determine whether dopamine is required for the development of ethanol preference, we tested cat-2(e1112) mutant animals which have reduced dopamine levels. cat-2(e1112) animals are deficient in the enzyme which synthesizes dopamine, tyrosine hydroxylase (Lint & Emmons 1999). We have previously shown that cat-2(e1112) has wild-type sensitivity to ethanol and metabolizes ethanol normally. cat-2 animals exhibit normal chemotaxis and adaptation to benzaldehyde (Bettinger & McIntire 2004). In the preference assay, untreated cat-2(e1112) mutant animals show normal avoidance to ethanol (negative PI). Ethanol pretreated cat-2(e1112) animals do not show as strong aversion to ethanol, but fail to develop a preference for ethanol (Fig. 4b) indicating that cat-2 animals have defects in induction of ethanol preference or show a significantly reduced rate in the development of ethanol preference. Similar results were observed with another loss-of-function allele, cat-2(tm2261) (PI=0.054±0.09 [n=3]). The results suggest that normal ethanol preference in C. elegans requires dopamine.
Serotonin has a role in experience-dependent behavioral plasticity of C. elegans that is modulated by food signals (Sawin et al. 2000; Nuttley et al. 2002). We therefore analyzed tph-1(mg280) mutant animals which are deficient in the enzyme for serotonin synthesis. tph-1(mg280) animals were defective in ethanol preference (Fig. 4b) indicating that serotonin is required for normal ethanol preference. We then tested the 5-HT receptor-deficient mutant, mod-1, in the preference assay (Ranganathan et al. 2000). mod-1 is required in ADF neurons for learned responses to pathogenic bacteria (Zhang et al. 2005). mod-1(n3034) animals show normal ethanol preference compared with wild type (Fig. 4b) suggesting that ethanol preference is not regulated by the mod-1/ADF serotonergic pathway.
Discussion
Our results demonstrate that C. elegans develops a preference for ethanol or attraction to ethanol as a result of prolonged pre-exposure to the drug. Previously, acute ethanol exposure was shown to cause a dose-dependent depression in locomotion and egg-laying behavior of C. elegans (Davies et al. 2003). Tolerance was found to be induced by continuous exposure to ethanol (Davies et al. 2004). In Drosophila, attraction of larvae to ethanol correlates with enzyme activity of alcohol dehydrogenase (ADH). Adult naïve flies slightly prefer ethanol-containing media and the ethanol response is enhanced by pre-exposure to ethanol (Cadieu et al. 1999). Genetic differences in ethanol preference have been documented in rodents such as preferring (P) and non-preferring (NP) lines (Li et al. 1994; Murphy et al. 2002). 2-bottle choice experiments are widely used to determine voluntary ethanol intake relative to control solutions (Blizard and McClearn 2000; Blizard et al. 2008). The preference assay that we have developed measures the attractiveness of ethanol verses control media.
C. elegans senses various environmental cues and rapidly changes its behaviors based on previous experience. C. elegans is able to modify its innate chemosensory preferences. It has been observed that preference can change from an attractive to an aversive response when animals are raised on pathogenic bacteria (Zhang et al. 2005). Here, we show a novel example of behavioral plasticity in the opposite direction; ethanol normally acts as a repellant but becomes an attractant after prolonged pre-exposure. It is not uncommon to observe a baseline aversive response to ethanol that changes to a preference response after pre-exposure in mammalian systems (Samson 1986; Carrillo et al. 2008; Simms et al. 2008).
Food modulates chemosensory responses in C. elegans. Avoidance of octanol is modulated by food (Chao et al. 2004) and butanone chemotaxis is enhanced after pre-exposure to butanone in the presence of food (Torayama et al. 2007). We examined the effect of food availability on the development of ethanol preference. Our results reveal that food is not required for the development of ethanol preference, but the development of ethanol preference is enhanced by the presence of food. The results indicate that the development of ethanol preference is a distinct behavior from associative learning, but food-associative learning might modulate the development of ethanol preference.
We also show that prolonged exposure to ethanol doesn't alter the olfactory preference to other volatile odorants. Ethanol pretreated animals exhibit normal chemotaxis to an attractant (benzadehyde) and repellents (2-nonanone and 1-octanol) suggesting that ethanol preference is an ethanol-specific phenomenon. C. elegans shows olfactory adaptation as a result of prolonged pre-exposure to an odorant. To address whether ethanol preference requires the acquisition of olfactory adaptation, we examined the olfactory adaptation defective mutant, egl-4 and we observed normal preference to ethanol in egl-4 mutant animals. This result suggests that olfactory adaptation is not necessary for the induction of ethanol preference and confirms that ethanol preference is different from simple olfactory adaptation. However, it is impossible to exclude the possibility of some olfactory adaptation occurring during the preference assay.
Previously, we demonstrated that slo-1 plays an important role in acute behavioral responses to ethanol. slo-1 mutants are strongly resistant to the sedative effects of the drug (Davies et al. 2003). npr-1 negatively regulates acute ethanol tolerance; acute tolerance develops more rapidly in npr-1 loss-of-function mutants (Davies et al. 2004). We confirmed that ethanol preference after prolonged pre-exposure in both slo-1 and npr-1 mutants was not significantly different from wild type (Fig. 4a). The results suggest that different behavioral responses to ethanol are regulated by different genes in C. elegans.
It has been shown that dopamine modulates diverse forms of behavioral plasticity including appetitive learning, ethanol withdrawal and drug addiction in mammals (Schultz et al. 1998; Uzbay et al. 1998; Berke & Hyman 2000). Dopamine may also alter neural circuits that enhance synaptic plasticity and result in drug seeking and consuming behaviors (Maldve et al. 2002). Reduction of dopamine synthesis causes a decrease of ethanol-induced locomotor activity in Drosophila (Bainton et al. 2000). Dopamine mediates context-dependent touch responses, associative learning and state-dependency in C. elegans (Bettinger & McIntire 2004; Kindt et al. 2007; Voglis et al. 2008). Therefore, we sought to determine whether dopamine may have a function in ethanol preference. cat-2 mutant animals which are deficient in the synthetic enzyme for dopamine failed to develop a preference for ethanol suggesting that dopamine is required for ethanol preference.
Serotonin signaling has also been implicated in the regulation of ethanol intake, preference and dependence (LeMarquand et al. 1994; Uzbay et al. 1998; Uzbay et al. 2004). The serotonergic system plays a key role in impulsiveness and mood disorders including anxiety and depression that are associated with drug craving (Koob 2000; Dolan et al. 2001). Serotonin also mediates food-modulated behaviors such as pharyngeal pumping, locomotion and egg-laying behavior and food-associated learning (Sawin et al. 2000; Nuttley et al. 2002; Zhang et al. 2005) in C. elegans. Thus, we tested ethanol preference changes in tph-1 mutant animals. tph-1 encodes the enzyme for serotonin biosynthesis, tryptophan hydroxylase. tph-1 in the serotonergic ADF neurons has a role in olfactory learning (Zhang et al. 2005). tph-1 mutant animals were defective in ethanol preference indicating that serotonin has a role in the development of ethanol preference. It would be interesting to determine if the ethanol preference defects of cat-2 and tph-1 are additive.
In aversive learning to pathogenic bacteria, mod-1 seems to be a downstream target of serotonin signaling from ADF neurons (Zhang et al. 2005). mod-1 is a serotonin-gated chloride channel, which also regulates food responses in C. elegans (Sawin et al. 2000; Ranganathan et al. 2000). We examined mod-1 mutant animals for ethanol preference. mod-1 showed normal development of ethanol preference suggesting that ethanol preference requires serotonin but is not dependent on serotonergic signaling through mod-1/ADF neurons.
Further genetic studies of ethanol preference in C. elegans may lead to the identification of novel regulators of this form of behavioral plasticity.
Acknowledgments
This work was supported by funds provided by the State of California for medical research on alcohol and substance abuse through the University of California, San Francisco. Strains used were provided by the C. elegans Genetics Center, which is funded by a grant from the National Institutes of Health National Center for Research Support. We would like to thank Dr. Shohei Mitani for providing the cat-2(tm2261) strain. We thank members of the McIntire laboratory for helpful discussions and comments on the manuscript.
References
- Bainton RJ, Tsai LT-Y, Singh CM, Moore MS, Neckameyer WS, Heberlein U. (2000) Dopamine modulates acute responses to cocaine, nicotine, and ethanol in Drosophila Curr Biol 10187-194 [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Hartieg E, Horvitz HR. (1993) Odorant selective genes and neurons mediate olfaction in C. elegans Cell 74515-527 [DOI] [PubMed] [Google Scholar]
- Berger KH, Heberlein U, Moore MS. (2004) Rapid and chronic: two distinct forms of ethanol tolerance in Drosophila Alcohol Clin Exp Res 101469-1480 [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. (2000) Addiction, dopamine, and the molecular mechanisms of memory Neuron 25515-532 [DOI] [PubMed] [Google Scholar]
- Bettinger JC, McIntire SL. (2004) State-depenency in C. elegans Genes Brain Behav 3266-272 [DOI] [PubMed] [Google Scholar]
- Blizard DA, McClearn GE. (2000) Association between ethanol and sucrose intake in the laboratory mouse: exploration via congenic strains and conditioned taste aversion Alcohol Clin Exp Res 24253-258 [PubMed] [Google Scholar]
- Blizard DA, Vandenbergh DJ, Lionikas A, McClearn GE. (2008) Learning in the 2-bottle alcohol preference test Alcohol Clin Exp Res 322041-2046 [DOI] [PubMed] [Google Scholar]
- Brenner S. (1974) The genetics of Caenorhabditis elegans Genetics 7771-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadieu N, Cadieu J-C, El Ghadraoui L, Grimal A, Lambouf Y. (1999) Conditioning to ethanol in the fruit fly-a study using and inhibitor of ADH J Insect Physiol 45579-586 [DOI] [PubMed] [Google Scholar]
- Carrillo J, Howard EC, Moten M, Houck BD, Czachowski CL, Gonzales RA. (2008) A three-day exposure to 10% ethanol with 10% sucrose successfully initiates ethanol self-administration Alcohol 42171-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MY, Komatsu H, Fukuto HS, Dionne HM, Hart AC. (2004) Feeding status and serotonin rapidly and reversibly modulate a Caenorhabditis elegans chemosensory circuit Proc Natl Acad Sci USA 10115512-15517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert HA, Bargmann CI. (1995) Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans Neuron 4803-812 [DOI] [PubMed] [Google Scholar]
- Colbert HA, Bargmann CI. (1997) Environmental signals modulate olfactory acuity, discrimination, and memory in Caenorhabditis elegans Learning Mem 4179-191 [DOI] [PubMed] [Google Scholar]
- Davies AG, Pierce-Shimomura JT, Kim H, VanHoven MK, Thiele TR, Bonci A, Bargmann CI, McIntire SL. (2003) A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans Cell 115655-66 [DOI] [PubMed] [Google Scholar]
- Davies AG, Bettinger JC, Thiele TR, Judy ME, McIntire SL. (2004) Natural variation in the npr-1 gene modifies ethanol responses of wild strains of C. elegans Neuron 42731-743 [DOI] [PubMed] [Google Scholar]
- Davies AG, McIntire SL. (2004) Using C. elegans to screen for targets of ethanol and behavior-altering drugs Bio. Proced. Online 6113-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan M, Anderson IM, Deakin JF. (2001) Relationship between 5-HT function and impulsivity and aggression in male offenders with personality disorders Br J Psychiatry 178352-359 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. (2005) Neural systems of reinforcement for drug addiction: from actions to habits to compulsion Nat Neurosci 81481-1489 [DOI] [PubMed] [Google Scholar]
- Gomez M, De Castro E, Guarin E, Sasakura H, Kuhara A, Mori I, Bartfai T, Bargmann CI, Nef P. (2001) Ca2+ signaling via the neuronal calcium sensor-1 regulates associative learning and memory in C. elegans Neuron 30241-248 [DOI] [PubMed] [Google Scholar]
- Kindt KS, Quast KB, Giles AC, De S, Hendrey D, Nicastro I, Rankin CH, Schafer WR. (2007) Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans Neuron 55662-676 [DOI] [PubMed] [Google Scholar]
- Koob GF. (1998) Drug abuse and alcoholism overview Adv Pharmacol 42969-977 [DOI] [PubMed] [Google Scholar]
- Koob GF. (2000) Neurobiology of addiction. Toward the development of new therapies Ann NY Acad Sci 909170-185 [DOI] [PubMed] [Google Scholar]
- Koob GF. (2003) Alcoholism: allostasis and beyond Alcohol Clin Exp Res 27232-243 [DOI] [PubMed] [Google Scholar]
- LeMarquand D, Pihl RO, Benkelfat C. (1994) Serotonin and alcohol intake, abuse, and dependence: findings of animal studies Biological Psychiatry 36395-421 [DOI] [PubMed] [Google Scholar]
- L'Etoile ND, Coburn CM, Eastham J, Kistler A, Gallegos G, Bargmann CI. (2002) The cyclic GMP-dependent protein kinase EGL-4 regulates olfactory adaptation in C. elegans Neuron 361079-1089 [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, Murphy JM. (1994) Genetic and neurobiological basis of alcohol-seeking behavior Alcohol Alcoholism 29697-700 [PubMed] [Google Scholar]
- Lint R, Emmons SW. (1999) Patterning of dopaminergic sensory neurons by a TGFβ family signaling pathway and a Hox gene Development 1265819-5831 [DOI] [PubMed] [Google Scholar]
- Maldve RE, Zhang TA, Ferrani-Kile K, Schreiber SS, Lippmann MJ, Snyder GL, Fienberg AA, Leslie SW, Gonzales RA, Morrisett RA. (2002) DARPP-32 and regulation of the ethanol sensitivity of NMDA receptors in the nucleus accumbens Nat Neurosci 5641-648 [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. (2002) Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference Behav Genet 32363-388 [DOI] [PubMed] [Google Scholar]
- Nuttley WM, Atkinson-Leadbeater KP, van der Kooy D. (2002) Serotonin mediates food-odor associative learning in the nematode Caenorhabditis elegans Proc Natl Acad Sci USA 9912449-12454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan R, Cannon SC, Horvitz HR. (2000) Mod-1 is a serotonin-gated chloride channel that modulates locomotory behaviour in C. elegans Nature 408470-475 [DOI] [PubMed] [Google Scholar]
- Rimondini R, Sommer WH, Heilig M. (2003) A temporal threshold for induction of persistent alcohol preference: behavioral evidence in a rat model of intermittent intoxication J Stud Alcohol 64445-449 [DOI] [PubMed] [Google Scholar]
- Rimondini R, Sommer WH, Dall'Olio R, Heilig M. (2007) Long-lasting tolerance to alcohol following a history of dependence Addiction Biol 1326-30 [DOI] [PubMed] [Google Scholar]
- Robert AJ, Heyser CJ, Cole M, Griffin P, Koob GF. (2000) Excessive ethanol drinking following a history of dependence: animal model of allostasis Neuropsychopharmacology 22581-594 [DOI] [PubMed] [Google Scholar]
- Saeki S, Yamamoto M, Iino Y. (2001) Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans J Exp Biol 2041757-1764 [DOI] [PubMed] [Google Scholar]
- Samson HH. (1986) Initiation of ethanol reinforcement using a sucrose substitution procedure in food-and water-sated rats Alcohol Clin Exp Res 10436-442 [DOI] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR. (2000) C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway Neuron 26619-631 [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. (2008) Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats Alcohol Clin Exp Res 321816-1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz H, Ramond J, Singh CM, Heberlein U. (2000) Functional ethanol tolerance in Drosophila Neuron 28261-271 [DOI] [PubMed] [Google Scholar]
- Schultz W. (1998) Predictive reward signal of dopamine neurons J Neurophysiol 801-27 [DOI] [PubMed] [Google Scholar]
- Tomioka M, Adachi T, Suzuki H, Kunitomo H, Schafer WR, Iino Y. (2006) The insulin/PI3-kinase pathway regulates salt chemotaxis learning in Caenorhabditis elegans Neuron 51613-625 [DOI] [PubMed] [Google Scholar]
- Troemel ER, Kimmel BE, Bargmann CI. (1997) Reprogramming chemotaxis responses: sensory neurons define olfactory preference in C. elegans Cell 91161-169 [DOI] [PubMed] [Google Scholar]
- Tsunozaki M, Chalasani SH, Bargmann CI. (2008) A behavioral switch: cGMP and PKC signaling in olfactory neurons reverses odor preference in C. elegans Neuron 59959-971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzbay IT, Usanmaz S, Tapanyidit EE, Aynacioglu S, Akarsu ES. (1998) Dopaminergic and serotonergic alterations in the rate brain during ethanol withdrawal: association with behavioral signs Drug Alcohol Dependence 5339-47 [DOI] [PubMed] [Google Scholar]
- Uzbay IT, Saglam E, Kayir H, Celik T, Beyazyürek M. (2004) Effects of fluoxetine on ethanol withdrawal syndrome in rats J Psychiatric Research 38445-450 [DOI] [PubMed] [Google Scholar]
- Voglis G, Tavernarakis N. (2008) A synaptic DEG/ENaC ion channel mediates learning in C. elegans by facilitating dopamine signaling EMBO J 273288-3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyang Y, Lu H, Bargmann CI. (2005) Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans Nature 438179-184 [DOI] [PubMed] [Google Scholar]