Abstract
The mesolimbic dopamine system plays an important role in mediating a variety of behaviors and is involved in mediating the reinforcing effects of ethanol. Genes encoding dopamine receptor subtypes are thus good candidate loci for understanding the genetic etiologies of susceptibility to alcohol dependence and its antecedent behavioral phenotypes. We tested whether variation in DRD1 influences alcohol consumption in rhesus macaques and whether its influence is mediated by sex and early rearing experience. We genotyped a single nucleotide polymorphism (-111 G/T) in the 5′ UTR of DRD1 in 96 subjects raised with their mothers until six months of age (n=43) or in peer-only groups (n=53). As young adults they underwent a seven-week voluntary ethanol consumption experiment. ANOVA revealed a significant main effect of sex (F (1,95) = 6.3, p = .014) and an interaction between genotype, sex and rearing on ethanol consumption (F (7,95) = 4.63, p =.0002). Maternally deprived males heterozygous for the T allele consumed significantly more ethanol (Prob > t = <.0001) than the other sub-groups. Maternal deprivation can produce individuals that are anxious and impulsive, both of which are known risk factors for alcohol dependence. Our work demonstrates a potential role for the dopamine D1 receptor gene in modulating alcohol consumption, especially in the context of early environmental stress.
Keywords: Alcohol consumption, Dopamine, non-human primate, Animal models, Vulnerability, Genetics
Introduction
The mesolimbic dopamine system plays an important role in mediating a variety of behaviors and is involved in mediating the reinforcing effects of ethanol (Sander et al., 1995). Dopamine mediates responses to natural rewards and is involved in the regulation of mood and affect (Bozarth, 1991). Variation in the genes encoding dopamine receptor subtypes expressed in relevant brain regions are thus good candidate loci for investigating the genetic etiologies of individual differences in alcohol dependence and its antecedent behavioral phenotypes. Five dopamine receptor genes comprise a family of G-protein-coupled receptors (Civelli et al., 1991). Among these, DRD1 has shown promise as a candidate gene for ethanol consumption or preference in rodent models. To date, only a handful of studies have been carried out to investigate the role of this gene in alcoholism and alcohol abuse in humans (Heinz et al, 1996; Comings et al., 1997; Thompson et al, 1998; Limosin et al, 2003; Kim et al., 2007; Batel et al., 2008), and none have yet investigated whether DRD1 variation moderates risk in the context of life stress or whether it increases risk for particular subtypes of alcohol dependence. Cichon et al. (1994) described four single nucleotide polymorphisms (SNPs) in the human dopamine D1 receptor gene that were detectable with simple restriction enzyme assays, two of which are located in the 5′ UTR. Three of the human amplicons are amplifiable in rhesus macaques using Cichon's human primer sequences, and several rhesus SNPs were identified with this approach (Trefilov, et al., 1999).
When some nonhuman primates are given access to a palatable ethanol solution some will consume it in quantities that produce a pharmacological response (Erwin, et al., 1979; Carroll et al., 1995; Higley and Linnoila, 1997). This has been demonstrated experimentally in rhesus (Macaca mulatta) (Kornet et al., 1990; Macenski and Meisch, 1992) and cynomolgus macaques (Macaca fascicularis) (Vivian et al., 2001) as well as in free-ranging monkeys such as the vervets (Chlorocebus aethiops) of St. Kitts (Ervin et al., 1990; Palmour et al., 1997). Ethanol consumption in monkeys, as in humans, varies considerably among individuals depending on factors such as sex, differences in temperament (e.g. impulsivity), early rearing experience and response to stress (Barr and Goldman, 2006). Although ethanol consumption varies markedly within populations, within individuals it tends to be stable over time and is generally trait-like (Higley et al., 1991). In humans, and across all animal models, heritability estimates for the propensity to consume ethanol range from 40% - 60% (Lorenz et al., 2006). Due in part to these observations, as well as the relatively close phylogenetic relationship between Old World monkeys and humans (compared to rodent models), rhesus monkeys have become a standard primate model for studying the neurobiological and behavioral effects of ethanol consumption, as well as behaviors associated with certain subtypes of alcoholism, such as aggression, stress reactivity, and impulsivity (Kraemer & McKinney, 1985; Higley et al, 1991; 1997; Barr and Goldman, 2006). Here, we examined whether variation in the 5′ UTR region of the dopamine D1 receptor gene is associated with variation in individual differences in ethanol consumption and whether such an association is influenced by early experience and sex.
Methods
Subjects
Rhesus macaques (N = 96) were housed at the National Institutes of Health Animal Center in Poolesville, Maryland, using protocols approved by the NIH Institutional Animal Care and Use Committee in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
The set of subjects represented 5 birth cohorts comprised of both mother- and peer-reared subjects. Details of the mother- versus peer-raising paradigm have been published elsewhere (Higley, et al., 1991, 1997). In brief, peer-reared infants were removed from their mothers immediately after birth and hand-reared in a neonatal nursery for the first 30 days of life. After 30 days they were placed in a cage with three other age-mates with whom they had continuous social contact. When animals reached an average age of 8 months, mother-reared and peer-reared subjects were placed together to form larger, permanent social groups. Unlike mother-reared monkeys, peer-reared subjects interact socially and practice social skills in the absence of adult role models—a setting that results in individuals that can be aggressive, emotionally unstable and socially impaired (Higley et al, 1996). Because peer-reared animals assimilate poorly into social groups, this rearing paradigm has become a well-established model for early life stress. All subjects involved in this research lived in their respective social groups throughout the study period and received identical treatment.
Ethanol Consumption
After reaching early adulthood (approximately 4 years of age), the study animals were introduced to a procedure designed to assess individual differences in their propensity to voluntarily consume an aspartame-sweetened 8.4% ethanol solution (Higley et al.,1997; Barr et al, 2004; Barr et al, 2007). This standardized method consisted of three phases. First, subjects were trained for one hour a day to consume aspartame-sweetened water. This phase ended typically after one week, when all animals had consumed more than 50 ml. Second, in order to assure that all animals experienced the pharmacological effects of ethanol before beginning the experimental phase of the study, individuals were given simultaneous, free access for one hour each day to both an aspartame-sweetened ethanol solution (8.4% v/v final concentration) and sweetened vehicle. No special methods were employed, such as deprivation of food or water. This phase generally lasted for 2 weeks. Third, during the seven-week experimental phase, ethanol was dispensed 5 days a week, Monday through Friday from 1300 to 1400, while the animals were in their home-cage environment. In order to provide all animals with unlimited access to the ethanol solution (i.e., to prevent rank-dependent access), paired ethanol and vehicle dispensers were partitioned using Plexiglas panels.
Genotyping
We amplified a 429 base pair segment of the dopamine D1 receptor gene, encompassing 138 nucleotides of the upstream portion of exon 1 and extending 319 nucleotides into the 5′ untranslated region, using primers D1.0 5′-ATT CAG GGG CTT TCT GGT G-3′ and D1.D 5′-AGC ACA GAC CAG CGT GTT C-3′ designed from human sequence data (Cichon et al., 1994). PCR was carried out in a volume of 25 μl using 25ng template DNA, 5μM each primer, 200μM of each dNTP, 2.5mM MgCl2, 1U AmpliTaq Gold (Applied Biosystems, Foster City, CA) and manufacturer supplied 10X buffer for 30 cycles (96°C-10′ initial denature, 94°C – 15″/60°C – 15″/72°C – 20″, followed by final extension @72°C for 5′). Sequencing of the product in a subset of study subjects captured the 4 SNPs reported in Trefilov et al's (1999) study (-179 C/T; -127 G/A; -111 G/T; -81 C/T), as well as a novel SNP (-149 A/T). For the purposes of this study, we analyzed the -111 G/T SNP only, since the other SNPs exhibited low minor allele frequency and were all in full linkage disequilibrium (data not shown).
Variation in the -111T/G SNP was assessed in the full dataset using restriction digest and gel visualization. A volume of 7μl of PCR product was digested with 3 units of the restriction enzyme Aci I (New England Biolabs, City, MA) for 2 hours in a 20μl reaction using the manufacturer's buffer and visualized on precast 10% TBE polyacrylamide gels (BioRad) stained with ethidium bromide. The Aci I restriction site (C′CGC) is disabled by the G to T (-111) substitution. Publicly available human sequence data for the D1 receptor gene as well as published size estimates for restriction fragments in rhesus macaques (Trefilov, et al., 1999) were used as guides for determining genotypes.
Data Analysis
We calculated allele and genotype counts and frequencies, and evaluated deviation from Hardy-Weinberg equilibrium using standard X2 tests. To determine the effects of DRD1 gene variation as well as potential interactions between genotype, sex and rearing experience, we performed analysis of variance using standard least squares, testing for main effects of genotype (DRD1 G/G vs T), sex (male vs. female), and rearing (PR vs. MR) as nominal independent variables, and all potential interactive combinations. We also introduced cohort as a variable since mean consumption values across cohorts vary. Preliminary analyses indicated that T/T subjects (n=2) did not differ from G/T subjects. Therefore, we clustered the T/G and T/T animals into a T allele carrier group. Analyses were performed using JMP™ 5.01 (SAS, Cary, NC, USA).
Results
Genotype Data
All 96 subjects yielded a readable restriction digest and were assigned to one of two genotypes. Genotype counts for the test subjects are indicated in Figure 1. The locus does not deviate significantly from Hardy-Weinberg equilibrium expectations (X2 = 1.884, df = 1, p = .17).
Figure 1. Interaction among DRD1 genotype (G/G vs. T), sex (MR vs. PR) and early rearing history (MR, mother- reared, vs. PR, peer-reared) on alcohol consumption.
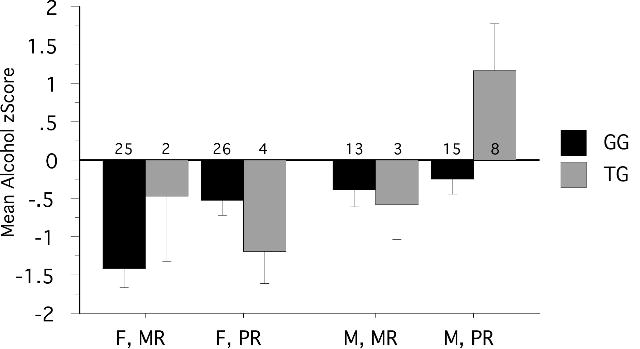
Alcohol consumption levels were statistically controlled by generating a cohort-based z-score. There was an interaction among genotype, sex and rearing. The T allele was associated with increased levels of alcohol consumption among stress-exposed male monkeys. Values shown are mean z-score ± SEM. Sample sizes for categories are indicated.
Effects of sex, rearing condition and DRD1 genotype on ethanol consumption
An analysis of variance modeling the main effects of genotype, rearing experience, sex, the interaction between these variables, and cohort, on g/kg ethanol consumption revealed robust support for the model (F (11,95)= 18.53, p = <.0001), with the combined factors explaining 71% of the variance (r2 = .708). There wasa main effect of sex (F (1,95) = 4.88, p = .029) and a marginally significant effect of rearing experience (F (1,95) = 3.22, p = .076) on g/kg ethanol consumption, but no main effect of genotype (F(1,95) = 0.085, p = .77). There was a significant 3-way interaction between sex, rearing and genotype on g/kg alcohol consumption (F = 6.43, p = 0.013), but no other interactive effects. Differences in mean g/kg ethanol consumption between test cohorts were significant (F (4,95) = 29.22, p = <.0001). Table 1 lists details of the ANOVA outputs. Therefore, to control for cohort-based differences in alcohol consumption, we repeated the analyses using cohort-corrected z scores for alcohol consumption.
Table 1. Test I: Effects of Genotype, Sex, Rearing, and Cohort on g/kg Alc consumption.
| F | df | p | r2 | |
|---|---|---|---|---|
| Whole Model | 18.53 | 11 | <.0001 | 0.708 |
| Main effects | ||||
| Genotype | 0.09 | 1 | 0.771 | |
| Sex | 4.88 | 1 | 0.029 | |
| Rearing | 3.22 | 1 | 0.076 | |
| Cohort | 29.22 | 4 | <.0001 | |
| Interaction Effects | ||||
| Genotype × Sex | 0.77 | 1 | 0.382 | |
| Genotype × Rearing | 0.04 | 1 | 0.844 | |
| Sex × Rearing | 2.75 | 1 | 0.101 | |
| Genotype × Sex × Rearing | 6.43 | 4 | 0.013 |
After this correction, we tested for main effects of genotype, sex and rearing, and interactions as above, but without cohort. The ANOVA model (genotype, sex, rearing) was statistically significant (F (7,95) = 4.63, p = .0002, R2 = .27), with significant main effects of sex (F (1,95) = 6.31, p = .014), but no main effect of genotype (F (1,95) = 1.09, p = 0.299) or rearing (F(1,95) = 2.12, p = .149. There was a significant (F (1,95) = 5.12, p = .026) sex by rearing by genotype interaction (Figure 1). Table 2 lists the ANOVA outputs. Post-hoc tests revealed a number of groups, clustered by sex, rearing and genotype, between which there were statistically significant differences in mean alcohol z scores. The largest difference in mean alcohol consumption z scores occurred between peer-reared males carrying the T allele (1.16 ± .42) and mother-reared G/G females (-1.40 ± .24, mean diff. = 2.57, Prob > t = <.0001)., with peer reared subjects drinking significantly more than mother reared G/G females Indeed, this group of males consumed significantly more alcohol than all other groups.
Table 2. Test II: Effects of Genotype, Sex and Rearing on Cohort-controlled Alc consumption.
| F | df | p | r2 | |
|---|---|---|---|---|
| Whole Model | 4.63 | 7 | 0.0002 | 0.269 |
| Main effects | ||||
| Genotype | 1.09 | 1 | 0.299 | |
| Sex | 6.31 | 1 | 0.014 | |
| Rearing | 2.12 | 1 | 0.149 | |
| Interaction Effects | ||||
| Genotype × Sex | 0.47 | 1 | 0.493 | |
| Genotype × Rearing | 0.00 | 1 | 0.974 | |
| Sex × Rearing | 1.53 | 1 | 0.220 | |
| Genotype × Sex × Rearing | 5.12 | 1 | 0.026 |
Discussion
Previous investigations into genetic factors that contribute to individual differences in alcohol consumption in our test animals have established that both sex and early rearing experience are important variables for explaining inter-individual differences in alcohol consumption. In the present study, we found an interaction among genotype, sex and the “environmental” variable of early infant rearing experience. Our results suggest that the influence imparted by the DRD1 locus on alcohol consumption is subtle, and is phenotypically measurable only in individuals with specific combinations of sex and rearing condition variables. Specifically, the minor allele (T) exerts its influence primarily in maternally deprived males, and therefore functions as a vulnerability marker for the propensity, or risk, to consume alcohol in this subset of individuals. Several studies in this population of rhesus have examined the effects of sex, rearing, and other candidate genes such as the serotonin transporter (Barr et al, 2004), mu opioid (Barr et al, 2007) and CRH (Barr et al, 2008). These studies are not directly comparable, however, as they are not based on the identical set of study subjects. Although the debate over correcting for multiple testing is not resolved, we address it here. The statistical significance of the three-factor model is p = .0002 which allows for 250 multiple tests before correction (.05/.0002 = 250). On the other hand, the three-way interaction between sex, rearing and genotype on alcohol consumption (p = .026) would be questionable if multiple testing is considered a confounding element.
Naturally, the question arises as to the factors behind the motivation to consume alcohol in non alcohol-dependent rhesus macaques. Our findings suggest that individuals inheriting a gene variant that potentially affects dopamine transmission are primed for the life-altering effects of parental deprivation. Early life stress in the form of peer rearing can induce anxious, impulsive, or novelty-seeking phenotypes. However, individual differences in these phenotypic variables have been described, suggesting that other factors, such as genetic variation, be involved. Given the role of dopamine in mediating the rewarding properties of alcohol, it is tempting to speculate that macaques carrying the T allele exhibit increased levels of consumption because they experience more reward. The fact that it is only among males with a history of stress that the effect of genotype is observed is consistent with the fact that males exhibit higher levels of alcohol-induced stimulation and that they are more likely to initially consume alcohol for it's rewarding properties. Oh the other hand, the dopamine system is also important in emotion regulation, and, in humans, amygdalar dopamine activity predicts anxious responding (Keinast et al, 2008), and DRD1 variation modulates risk for anxiety-related disorders (Maron et al., 2005). Therefore, it may be that the DRD1 locus could increase an individual's vulnerability to seek out alcohol for its anxiolytic properties as well. The observation that rearing experience plays an important role in mediating the effects of DRD1 genetic variation in alcohol consumption, specifically that maternally deprived individuals tend to drink more than mother-reared individuals, suggests that our subjects may be consuming alcohol for its anxiolytic properties instead of—or in addition to—the euphoric, sedative, and rewarding experience of alcohol.
Several studies have shown convincing evidence that the mesolimbic dopamine system is directly involved in the reward or reinforcement pathway of alcohol and other μ-opioid receptors in animal models. Cohen et al., (1999), showed that D1 receptor agonists reduced self-administered oral ethanol consumption in rats whereas Eiler et al. (2003) showed that antagonists targeting D1 receptors in the bed nucleus of stria terminalis in alcohol-preferring rats reduced alcohol-motivated behaviors. Moreover, in mice in which expression of the D1 receptor was disrupted, D1 deficient homozygotes showed reduced voluntary alcohol consumption and preference compared to heterozygotes and wild-type controls (El-Ghundi, 1998). In humans, Kim et al., (2007) found a non-functional SNP (rs4532, -48A>G) in DRD1 to be associated with the severity of alcohol related behavioral deficits in Korean alcohol dependent subjects compared to control, measured using the Alcohol Use Disorders Identification Test (AUDIT). In a study of French Caucasian patients with alcohol dependence versus controls, Batel et al., (2008) found an association of another non-functional SNP (rs686, +1403T>C) in patients with severe alcohol dependence, but not with rs4532 as in the Kim et al., study. All of these studies, translated across primate and non-primate species, support a role for the D1 receptor in modulating alcohol-consuming behavior. It is not unexpected, then, that mutations in the D1 receptor gene may very well play an important role in mediating alcohol consumption and associated motivational behaviors in our rhesus model.
The DRD1 restriction site polymorphism reported in this study occurs in proximity to the transcription start site, but it is unknown at present whether the polymorphism has functional effects. Lee et al. (1996) presented evidence that the DRD1 gene possesses two possible transcription initiation sites giving way to two splice variants, one with and one without the non-coding first exon. Since exon 1 is non-coding in humans, it is also likely to be non-coding in rhesus, and most mutations in and around exon 1 may have no effect on the receptor protein. On the other hand, the SNP we analyzed may be a tagging SNP for a functional haplotype. We have previously demonstrated that patterns of LD are similar in rhesus and in humans, even when the variants themselves are not conserved (Barr et al, 2008), and, in humans, there is a high degree of LD around DRD1, according to HAPMAP and NCBI databases.
Our work demonstrates a potential role for the dopamine D1 receptor gene in modulating alcohol consumption in male rhesus macaques, especially in the context of early environmental stress. We have shown an interaction of sex and early stress exposure with genotype in a classic example of how genetically predisposing factors often require a combination of additional environmental variables in order to produce effects. Finally, the similarity between humans and rhesus monkeys in genetic variation of the dopamine D1 receptor gene, as well as dopamine-mediated behavioral deficits, suggests that the nonhuman primate model is valuable for determining whether genetic variation in the DRD1 locus may be used to identify, and eventually develop, appropriate pharmacotherapies for the treatment of dopamine-related disorders, including alcohol dependence and abuse.
Acknowledgments
This research was funded by the NICHD and NIAAA intramural research programs.
Footnotes
None of the authors have conflicts of interest to declare.
References
- Barr CS, Dvoskin RL, Yuan Q, Lipsky RH, Gupte M, Hu X, Zhou Z, Schwandt ML, Lindell SG, McKee M, Becker ML, Kling MA, Gold PW, Higley JD, Heilig M, Suomi SJ, Goldman D. CRH haplotype as a factor influencing cerebrospinal fluid levels of corticotropin-releasing hormone, hypothalamic-pituitary-adrenal axis activity, temperament, and alcohol consumption in rhesus macaques. Arch Gen Psychiatry. 2008;65(8):934–44. doi: 10.1001/archpsyc.65.8.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Goldman D. Nonhuman primate models of inheritance of vulnerability to alcohol abuse and addiction. Addiction Biol. 2007;11:374–385. doi: 10.1111/j.1369-1600.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Lindell S, Parker CC, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD. Interaction Between Serotonin Transporter Gene Variation and Rearing Condition in Alcohol Preference and Consumption in Female Primates. Arch Gen Psychiatry. 2004;61(11):1146–1152. doi: 10.1001/archpsyc.61.11.1146. [DOI] [PubMed] [Google Scholar]
- Barr CS, Schwandt ML, Lindell SG, Chen SA, Goldman D, Suomi SJ, Higley JD, Heilig M. Association of a functional polymorphism in the mu-opioid receptor gene with alcohol response and consumption in male rhesus macaques. Arch Gen Psychiatry. 2007;64(3):369–76. doi: 10.1001/archpsyc.64.3.369. [DOI] [PubMed] [Google Scholar]
- Batel P, Houchi H, Daoust M, Ramoz N, Naassila M, Goodwood P. A Haplotype of the DRD1 Gene is Associated with Alcohol Dependence. Alcohol: Clin Exp Res. 2008;32(4):567–572. doi: 10.1111/j.1530-0277.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- Bozarth MA. The mesolimbic dopamine system as a model brain reward system. In: Willner P, Scheel-Krüger J, editors. The mesolimbic dopamine system: From motivation to action. London: John Wiley & Sons; 1991. pp. 301–330. [Google Scholar]
- Cichon S, Nothen MM, Erdmann J, Propping P. Detection of four polymorphic sites in the human dopamine D1 receptor gene (DRD1) Hum Mol Genet. 1994;3:209. doi: 10.1093/hmg/3.1.209. [DOI] [PubMed] [Google Scholar]
- Civelli O, Bunzow JR, Grandy DC, Zhou QY, Van Tol HHM. Molecular biology of the dopamine receptors. Eur J Pharmacol. 1991;207:277–86. doi: 10.1016/0922-4106(91)90001-x. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Sanger DJ. Effects of D1 dopamine receptor agonists on oral ethanol self-administration in rats: comparison with their efficacy to produce grooming and hyperactivity. Psychopharmacol. 1999;142(1):102–10. doi: 10.1007/s002130050868. [DOI] [PubMed] [Google Scholar]
- Comings DE, Gade R, Wu S, Chiu C, Dietz G, Muhleman D, Saucier G, Ferry L, Rosenthal RJ, Lesieur HR, Rugle LJ, MacMurray P. Studies of the potential role of the dopamine D1 receptor gene in addictive behaviors. Mol Psychiatry. 1997;2:44–56. doi: 10.1038/sj.mp.4000207. [DOI] [PubMed] [Google Scholar]
- Eiler WJA, Seyoum R, Foster KL, Mailey C, June HL. D1 dopamine receptor regulates alcohol-motivated behaviors in the bed nucleus of stria terminalis in alcohol preferring rats. Synapse. 2003;48:45–56. doi: 10.1002/syn.10181. [DOI] [PubMed] [Google Scholar]
- El-Ghundi M, George SR, Drago J, Fletcher PJ, Fan T, Nguyen T, Liu C, Sibley DR, Westphal H, O'Dowd BF. Disruption of dopamine D1 receptor gene expression attenuates alcohol-seeking behavior. Eur J Pharmacol. 1998;353(2-3):149–58. doi: 10.1016/s0014-2999(98)00414-2. [DOI] [PubMed] [Google Scholar]
- Erwin J, Drake D, Kassorla E, Deni R. Drinking of ethanol by adult and infant rhesus monkeys (Macaca mulatta) J Stud Alcohol. 1979;40(3):301–6. doi: 10.15288/jsa.1979.40.301. [DOI] [PubMed] [Google Scholar]
- Ervin FR, Palmour RM, Young SN, Guzman-Flores C, Juarez J. Voluntary consumption of beverage alcohol by vervet monkeys: population screening, descriptive behavior and biochemical measures. Pharmacology Biochemistry & Behavior. 1990;36:367–73. doi: 10.1016/0091-3057(90)90417-g. [DOI] [PubMed] [Google Scholar]
- Higley JD, Linnoila M. A nonhuman primate model of excessive alcohol intake: personality and neurobiological parallels of type-I and type-II-like alcoholism. Recent Dev Alcohol. 1997;13:191–219. [PubMed] [Google Scholar]
- Higley JD, Hasert MF, Suomi SJ, Linnoila M. Nonhuman primate model of alcohol abuse: effects of early experience, personality and stress on alcohol consumption. Proc Natl Acad Sci USA. 1991;88(16):7261–5. doi: 10.1073/pnas.88.16.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze A, Sander T, Harms H, Finckh U, Kuhn S, Dufeu P, Dettling M, Graf K, Rolfs A, Rommelspacher H, Schmidt LG. Lack of allelic association of dopamine D1 and D2 (TaqIA) receptor gene polymorphisms with reduced dopaminergic sensitivity to alcoholism. Alcohol Clin Exp Res. 1996;20(6):1109–13. doi: 10.1111/j.1530-0277.1996.tb01954.x. [DOI] [PubMed] [Google Scholar]
- Huang W, Ma JZ, Payne TJ, Beuten J, Dupont RT, Li MD. Significant association of DRD1 with nicotine dependence. Hum Genet. 2008;123:133–140. doi: 10.1007/s00439-007-0453-9. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Park BL, Yoon S, Lee HK, Joe KH, Choen YH, Gwon DH, Cho SN, Lee HW, NamGung S, Shin HD. 5′ UTR polymorphism of dopamine recepter D1 (DRD1) associated with severity and temperament of alcoholism. Biochem Biophys Res Comm. 2007;357:1135–1141. doi: 10.1016/j.bbrc.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Keinast T, Hariri AR, Schlagenhauf F, Wrase J, Sterzer P, Bucholz HG, Smolka MH, Grunder G, Cumming P, Kumakura Y, Bartenstein P, Dolan RJ, Heinz A. Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nat Neurosci. 2008;11:1381–1382. doi: 10.1038/nn.2222. [DOI] [PubMed] [Google Scholar]
- Kornet M, Goosen C, Ribbens LG, van Ree JM. Analysis of spontaneous alcohol drinking in rhesus monkeys. Physiol Behav. 1990a;47(4):679–84. doi: 10.1016/0031-9384(90)90077-h. [DOI] [PubMed] [Google Scholar]
- Kornet M, Goosen C, van Ree JM. The effect of interrupted alcohol supply on spontaneous alcohol consumption by rhesus monkeys. Alcohol Alcohol. 1990b;25(4):407–12. [PubMed] [Google Scholar]
- Kraemer GW, McKinney WT. Social separation increases alcohol consumption in rhesus monkeys. Psychopharmacol. 1985;86:182–189. doi: 10.1007/BF00431706. [DOI] [PubMed] [Google Scholar]
- Lee SH, Minowa MT, Mouradian MM. Two distinct promoters drive transcription of the human D1a dopamine receptor gene. J Biol Chem. 1996;271(41):25292–99. doi: 10.1074/jbc.271.41.25292. [DOI] [PubMed] [Google Scholar]
- Limosin F, Loze JY, Rouillon F, Ades J, Gorwood P. Association between dopamine receptor D1 gene DdeI polymorphism and sensation seeking in alcohol-dependent men. Alcohol Clin Exp Res. 2003;27(8):1226–8. doi: 10.1097/01.ALC.0000081624.57507.87. [DOI] [PubMed] [Google Scholar]
- Lorenz JG, Long JC, Linnoila M, Goldman D, Suomi SJ, Higley JD. Genetic and other contributions t alcohol intake in rhesus macaques (Macaca mulatta) Alcohol Clin Exp Res. 2006;30(3):1–10. doi: 10.1111/j.1530-0277.2006.00044.x. [DOI] [PubMed] [Google Scholar]
- Macenski MJ, Meisch RA. Ethanol-reinforced responding of naïve rhesus monkeys: acquisition without induction procedures. Alcohol. 1992;9:547–554. doi: 10.1016/0741-8329(92)90095-r. [DOI] [PubMed] [Google Scholar]
- Maron E, Nikopensius T, Koks S, Altmae S, Heinaste E, Vabrit K, Tammekivi V, Hallast P, Koido K, Kurg A, Mespalu A, Vasar E, Vasar V, Shlik J. Psychiatr Genet. 2005;15:17–24. doi: 10.1097/00041444-200503000-00004. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. National Academies Press; Washington: 1996. p. 140. [Google Scholar]
- Newman TK, Syagailo Y, Barr CS, Wentland J, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch KP. MAOA Gene Promoter Variation and Rearing Experience Influences Aggressive Behavior in Rhesus Monkeys. Biol Psychiatry. 2005;57:167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Palmour RM, Mulligan J, Howbert JJ, Ervin F. Insights from model systems. Of monkeys and men: Vervets and the genetics of human-like behaviors. Am J Hum Genet. 1997;61:481–88. doi: 10.1086/515526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander T, Harms H, Podschus J, Finckh U, Nickel B, Rolfs A, Rommelspacher H, Schmidt LG. Psychistr Genet. 1995;5(4):171–6. doi: 10.1097/00041444-199524000-00004. [DOI] [PubMed] [Google Scholar]
- Thompson M, Comings DE, Feder L, George SR, O'Dowd BF. Mutation screening of the dopamine D1 receptor gene in Tourette's syndrome and alcohol dependent patients. Am J Med Genet. 1998;81(3):241–4. [PubMed] [Google Scholar]
- Trefilov A, Krawczak M, Berard J, Schmidtke J. DNA sequence polymorphisms in genes involved in the regulation of dopamine and serotonin metabolism in rhesus macaques. Electrophoresis. 1999;20:1771–1777. doi: 10.1002/(SICI)1522-2683(19990101)20:8<1771::AID-ELPS1771>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25(8):1087–97. [PubMed] [Google Scholar]


