Abstract
The zebrafish has been in the forefront of developmental genetics for decades and has also been gaining attention in neurobehavioral genetics. It has been proposed to model alcohol-induced changes in human brain function and behavior. Here, adult zebrafish populations, AB and SF (short-fin wild type), were exposed to chronic treatment (several days in 0.00% or 0.50% alcohol v/v) and a subsequent acute treatment (1 h in 0.00%, 0.25%, 0.50% or 1.00% alcohol). Behavioral responses of zebrafish to computer-animated images, including a zebrafish shoal and a predator, were quantified using videotracking. Neurochemical changes in the dopaminergic and serotoninergic systems in the brain of the fish were measured using high-precision liquid chromatography with electrochemical detection. The results showed genetic differences in numerous aspects of alcohol-induced changes, including, for the first time, the behavioral effects of withdrawal from alcohol and neurochemical responses to alcohol. For example, withdrawal from alcohol abolished shoaling and increased dopamine and 3,4-dihydroxyphenylacetic acid in AB but not in SF fish. The findings show that, first, acute and chronic alcohol induced changes are quantifiable with automated behavioral paradigms; second, robust neurochemical changes are also detectable; and third, genetic factors influence both alcohol-induced behavioral and neurotransmitter level changes. Although the causal relationship underlying the alcohol-induced changes in behavior and neurochemistry is speculative at this point, the results suggest that zebrafish will be a useful tool for the analysis of the biological mechanisms of alcohol-induced functional changes in the adult brain.
Keywords: Acute alcohol, alcohol tolerance, chronic alcohol, dopamine, fear, high-precision liquid chromatography, serotonin, social behavior, withdrawal, zebrafish
Abuse of alcohol (EtOH, ethanol or ethyl alcohol) and alcoholism represent serious problems for patients and the society. Alcoholism costs more than $150 billion yearly and results in thousands of deaths in the USA alone (Harwood et al. 1998; Rice 1995). Given the high prevalence of alcohol abuse (over 30 million people afflicted only in the USA, Robins et al. 1984; Sullivan & Handley 1993) and that current treatment options are limited and inefficient (e.g. Fuller & Hiller-Sturmhöfel 1999; O'Brien et al. 1995; Vengeliene et al. 2008), the need for better understanding of alcohol's effects is clear. Among other areas of investigation, intense research is being conducted to show the mechanisms of alcohol's actions in the brain. However, the problem is that alcohol has been found to act through a large number of biochemical mechanisms (Vengeliene et al. 2008). Rodent and Drosophila models have been proposed to tackle this complexity (Browman & Crabbe 1999; Guarnieri & Heberlein 2003). In the current paper, zebrafish, a novel model organism in alcohol research, is utilized.
The zebrafish has been suggested as a tool for the analysis of the effects of alcohol on adult brain function (Gerlai et al. 2000). Its prolific nature and strong genetics lends this species to high-throughput screening, an approach that may show numerous molecular targets involved in alcohol-associated mechanisms. Behavioral effects of acute and chronic alcohol exposure on adult zebrafish have started being investigated (Gerlai 2003; Gerlai et al. 2006). The first study, conclusively showing the role of genetic factors in acute alcohol effects on zebrafish behavior, has been published (Gerlai et al. 2008; but see Dlugos & Rabin 2003).
The current paper contributes to this growing research by providing new findings on the following. First, behavioral effects of alcohol have not been tested using fully automated computerized methods. These methods are important for high-throughput screening and are scarce in zebrafish neurobehavioral genetics (Blaser & Gerlai 2006). Here, we investigate shoaling (group preference) and fear responses (antipredatory avoidance behavior) to computer-animated (moving) images of a group of zebrafish (Saverino & Gerlai 2008) and of a sympatric predator of zebrafish (Bass & Gerlai 2008) respectively. Quantification of behavior is also computerized: it utilizes videotracking (Blaser & Gerlai, 2006; Gerlai et al., 2008). Second, we now investigate the effects of chronic exposure to alcohol and study not only adaptation (tolerance) to alcohol but also alcohol withdrawal-induced changes. Third, by comparing two genetically distinct populations of zebrafish bred, raised, housed and tested under identical conditions, we investigate potential genetic differences not only in response to acute but, for the first time, also to chronic alcohol dose. Fourth, we analyze the effects of these factors not only on behavior but also on neurochemistry of the zebrafish brain focusing on the dopaminergic and serotoninergic systems, which has not been performed before. We do not claim that by studying the above we are providing mechanistic details on the actions of alcohol, but we hope that our work will open the door to future studies that will.
Methods
Animals and housing
Fully mature, 1-year-old zebrafish (Danio rerio, 50−50% males and females) of the AB strain and of the short-fin wild-type (SF) population (AB and SF referred to as populations from here onward) bred and raised in our facility (University of Toronto Mississauga Vivarium, Mississauga ON, Canada) under identical conditions were used. These populations were chosen because AB is frequently utilized in forward genetic studies (e.g. Guryev et al. 2006; Lockwood et al. 2004) and SF is an outbred population readily available from most pet stores (Bass & Gerlai 2008). The origin, breeding and maintenance of our experimental fish and additional rationale for their choice are described in detail elsewhere (e.g. Gerlai et al. 2008; also see Appendix S1).
Experimental design for behavioral analysis
We employed a 2 × 4 × 2 between-subject experimental design for the behavioral analysis: two chronic alcohol doses (0.00% or 0.50% alcohol, v/v percentage), four acute alcohol doses (0.00%, 0.25%, 0.50%, or 1.00% alcohol), and two populations of zebrafish (AB or SF). The dosing regimen employed (concentrations, timing and length of alcohol exposure) was based on previous findings (Gerlai et al. 2000, 2006) and on our pilot dose-escalation studies. During chronic treatment the holding tank water was replaced with the appropriate alcohol solution once a day. The chronic alcohol dose of 0.50% was achieved using a dose-escalation procedure, i.e. by increasing the alcohol concentration of the holding tank water by 0.125% increments once every 4 days (12 days of dose escalation) and subsequently maintaining the concentration at 0.50% for additional 10 days. No increased mortality or morbidity was observed in our alcohol-exposed fish. The holding conditions were identical for the 0.00% and the 0.50% chronic dose groups. At the conclusion of the chronic treatment, experimental fish were placed in the appropriate acute alcohol dose for 1 h immediately before and during behavioral testing (for further details see Appendix S1).
Quantification of alcohol content of holding tank during chronic exposure
The exact alcohol concentration of the water of the holding tanks during chronic treatment was quantified by sampling five tanks three times a day for 16 days (for 4 days at each dose step including the final 0.50% concentration). The alcohol concentration of the samples was analyzed using the AM1 Alcohol Analyzer (Analox Instruments, London, UK) as described in Taylor et al. (2002) and in Appendix S1.
Behavioral apparatus
The experimental set-up was a 37-l tank (50 × 25 × 30 cm, length × width × height) with a flat LCD computer screen on its left and right sides that could present different animated images (Saverino & Gerlai 2008). Two identical experimental set-ups ran in parallel. The order of the recording sessions of experimental fish was randomized across population, chronic treatment condition and acute alcohol dose. The behavior of experimental fish was digitally recorded and later replayed for analysis by Ethovision Color Pro Videotracking (Version 3, Noldus Info Tech, Wageningen, the Netherlands) as described in Appendix S1.
Behavioral test procedure
Previously we found zebrafish to prefer swimming close to an animated zebrafish group presented on a computer screen, a typical shoaling response (Saverino & Gerlai 2008). Alcohol is known to affect shoaling responses in zebrafish (e.g. Gerlai et al. 2000) and to influence social behavior in other species including our own (e.g. Shively et al. 2002). Alcohol is also known to alter fear responses (Kushner et al. 2000), which can be quantified in zebrafish using a predator stimulus paradigm (Gerlai et al. 2000). Last, the natural predator of zebrafish, the Indian leaf fish (Nandus nandus) has been shown to induce antipredatory responses in zebrafish (Bass & Gerlai 2008). Here, we present computer-animated images of a shoal (a group of five zebrafish) or of a single Indian leaf fish, allowing precise stimulus control and consistent stimulus delivery across sessions. Experimental zebrafish were placed in the test tank singly and were monitored for 24 min. The sequence of stimulus presentations was as follows (expressed as 1-min intervals): 1st to 8th no stimulus; 9th to 16th image of a shoal (five zebrafish moving independently); 17th no stimulus; 18th predator image (Indian leaf fish image moving slowly); 19th to 20th no stimulus; 21st predator image; 22nd to 23rd no stimulus; 24th predator. All stimuli were presented on one side for each experimental fish but the side varied randomly across experimental fish as described by Fernandes & Gerlai (2009) and in Appendix S1.
Quantification of behavior
The digital video files (AVI format), recorded during the experimental sessions, were later replayed and analyzed using Ethovision as described by Gerlai et al. (2008). We quantified the distance the experimental fish maintained from the stimulus presentation computer screen (expressed in centimetres), a measure expected to reflect preference of conspecifics (shoaling) or avoidance of the predator (fear).
Experimental design and quantification of neurotransmitter levels in the zebrafish brain
For the analysis of potential neurochemical changes induced by alcohol treatment in the two populations of zebrafish we conducted a separate acute and chronic study instead of employing the fully randomized 2 × 4 × 2 experimental design. The reason for this was practical: limited time and resources. In the acute alcohol administration study, the exposure methods and alcohol concentrations were as described above with acute dose groups indicated on the figures as C000A000, C000A025, C000A050, C000A100 (where C stands for ‘chronic’ exposure, which equals 0.00% alcohol, and A stands for ‘acute’ dosing with numbers corresponding to the employed alcohol concentrations without the decimal point). Following the acute alcohol study, we conducted a chronic alcohol exposure study in which we compared four alcohol treatment groups of zebrafish C000A000, C050A000, C050A050 and C050A100 (group designations are as explained above, for further details see Appendix S1). Alcohol treatment, tissue sampling and high-precision liquid chromatography (HPLC) analysis were conducted in both the acute and the chronic analysis in a manner that the order of zebrafish was randomized according to alcohol treatment and population origin. Sample sizes are indicated in the figure legends. We analyzed dopamine, its metabolite 3,4-dihydroxyphenylacetic acid (DOPAC), serotonin, and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) because, among other neuro-transmitters, the dopaminergic and serotoninergic neurotransmitter systems have been implicated in mediating alcohol's actions in the brain (Crabbe et al. 1996, 2006; Di Chiara & Imperato 1988; Gessa et al. 2004; Lovinger 1999; Lovinger & Zhou 1994; Rodd-Henricks et al. 2000; Sari et al. 2006; Spanagel & Weiss 1999; Thielen et al. 2004). The rationale for the choice of techniques, their superiority compared with other methods and all pertinent methodological details are described by Chatterjee and Gerlai (2009) and are found in Appendix S1. Briefly, zebrafish were decapitated and their brains were dissected and sonicated. The sonicates were centrifuged and the supernatant was analyzed with HPLC using a BAS 460 MICRO-BORE-HPLC system with electrochemical detection (Bio-analytical Systems Inc., West Lafayette, IN, USA) together with a Uniget C-18 reverse phase microbore column as the stationary phase (BASi, Cat. No. 8949). Standard dopamine, DOPAC (Sigma Chemicals, St. Louis, MO, USA), serotonin and 5-HIAA (Sigma) were used to quantify and identify the peaks on the chromatographs.
Statistical analysis
Data were analyzed using spss (14.1) for the PC. Using repeated measures variance analysis (anova) we analyzed the effect of interval (the within-subject factor), and the effect of between-subject factors acute alcohol dose, chronic alcohol dose and population. Composite measures of shoaling (decrease of distance from stimulus screen in response to the shoal image) and of predator avoidance (increase of distance from stimulus screen in response to the predator image) were subsequently calculated and analyzed with univariate non-repeated measures anovas. Tukey honestly significant difference (HSD) post hoc multiple comparison tests were employed where appropriate. One-sample t-tests with Holm correction were also performed to investigate whether the stimulus-induced changes were different from 0 (Aickin & Gensler 1996). The neurochemical data were analyzed using univariate anova followed by post hoc Tukey HSD tests when appropriate.
Results
Alcohol concentration in the holding tanks during chronic treatment
For chronic alcohol treatment we replaced the appropriate alcohol water in the holding tanks once a day. However, because of evaporation of alcohol, or may be even to its metabolism by the experimental subjects, alcohol concentration may have diminished between water changes and the actual alcohol exposure may have fluctuated or may have differed from the experimentally set level. Figure 1 shows the actual alcohol concentration measured in the water samples taken from the holding tanks. The results show that alcohol concentration remained stable with negligible variability across the different time points of testing. Variation among the multiple holding tanks sampled was also small (error bars are often not visible on the figure as they are smaller than the symbols,). Furthermore, the measured alcohol concentration was very close to the desired dose and showed a slight (but consistent) reduction only at the highest (0.50%) concentration, which may have been because of evaporation of alcohol during sample processing. Briefly, the chronic alcohol exposure paradigm provided a temporally stable alcohol dose.
Figure 1. The concentration of alcohol (EtOH) in the holding tank of experimental fish during the first 16 days of chronic alcohol exposure period.
The mean ± SEM is shown. The first data point was obtained before alcohol was introduced to the water and subsequently each tank was maintained at a given alcohol concentration for 4 days. Notice the stepwise increase of alcohol concentration every 4 days (4 days × 3 data points per day = 12 data points per concentration) that followed the 0.125% alcohol dose increases of the chronic dose-escalation schedule employed.
Minute-to-minute changes of the distance between zebrafish and the stimulus computer screen
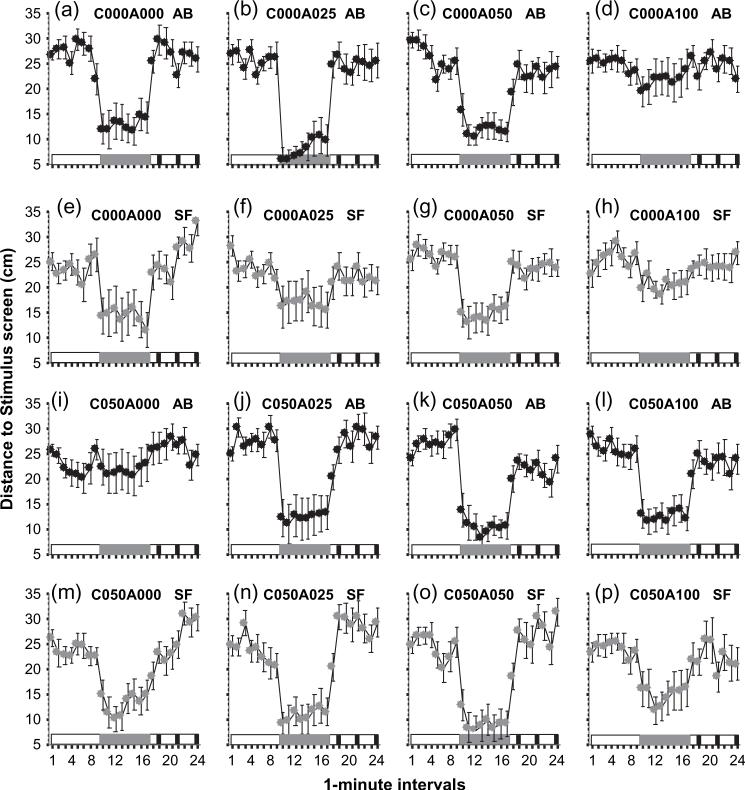
Figure 2 depicts the average distance the experimental zebrafish were from the stimulus screen during each of the 1-min intervals of the 24-min session. The effects of gender (both males and females were measured), the side of stimulus presentation (images were shown either on the right or the left side), and the experimental tank (two tanks were used) were found non-significant. The interaction terms of these factors with the other independent factors (population, acute alcohol dose, chronic alcohol dose) and/or with Interval were also found non-significant (statistical results not shown). Therefore, data were pooled for gender, stimulus side and experimental tank. The most apparent feature of the responses is the robust reduction of distance from the stimulus screen during the presentation of the zebrafish shoal. It is also apparent that not all zebrafish responded the same way: the trajectories of the curves are not identical across different treatment groups. Repeated measures anovas confirmed these observations and found significant acute alcohol dose × chronic alcohol dose interactions and also interactions between these treatment factors with population (statistical results not shown). Notably, confirmatory post hoc tests could not be performed to identify which group differed from which because such tests are inappropriate for repeated measures designs. To circumvent this problem, we calculated a composite measure that reflected the decrease of distance in response to the shoal image and another composite measure that reflected the increase of distance in response to the predator image.
Figure 2. The effect of chronic and subsequent acute alcohol (or freshwater) exposure on the distance zebrafish swam from the stimulus computer screen.
Mean ± SEM are shown for 1-min intervals of the 24-min recording session. Timing of stimulus delivery is shown above the abscissa (below each graph): white represents the periods during which no stimulus was presented, gray represents the period during which an image of a moving zebrafish shoal was presented on the computer screen, and black represents the periods during which a moving image of a predator was shown. The group designations above the graphs represent the chronic and subsequent acute alcohol exposure employed (e.g. C000A000 was chronic 0.00% and acute 0.00% alcohol and C050A100 was chronic 0.50% and acute 1.00% alcohol). The population of fish (AB or SF) is also indicated above the graphs. Sample sizes were as follows: nC000A000_AB = 17, nC000A025_AB = 13, nC000A050_AB = 18, nC000A100_AB = 16, nC000A000_SF = 15, nC000A025_SF = 15, nC000A050_SF = 17, nC000A100_SF = 14, nC050A000_AB = 15, nC050A025_AB = 17, nC050A050_AB = 17, nC050A100_AB = 18, nC050A000_SF = 14, nC050A025_SF = 16, nC050A050_SF = 15, nC050A100_SF = 15.
The effect of alcohol treatment on the shoaling response: reduction of distance from stimulus screen on presentation of the shoal image
The decrease of distance in response to the shoal image was calculated as follows: ΔD = (∑Sj)/8 – (∑Nj)/8 where N is the distance value obtained for no-stimulus interval ‘i’ (i running from interval 1−8) and S is the distance value obtained for the stimulus interval ‘j’ (j running from interval 9−16). Larger negative ΔD values represent bigger distance reduction, i.e. a stronger shoaling response (Fig. 3). Three-factorial non-repeated measures anova of ΔD showed that acute alcohol dose had a significant effect (F3,236 = 4.167, P < 0.001) and the interaction acute alcohol dose × Chronic alcohol dose was also significant (F3,236 = 3.957, P < 0.01). The triple-interaction term acute alcohol dose × chronic alcohol dose × population did not reach significance (F3,236 = 2.025, P > 0.10). The other main effects (chronic alcohol dose or population) and the interaction term chronic alcohol dose × population were also non-significant. As a result of the identified significant main effect and interaction term, and the known insensitivity of anova to detect interactions (Wahlsten 1990), we conducted post hoc multiple comparison tests (Tukey HSD) without pooling the data across any main factor. This analysis showed that the AB fish exposed to chronic freshwater and subsequently to the 1.00% acute alcohol dose reduced their distance from the shoal image significantly (P < 0.05) less than those treated with acute alcohol doses, 0.25% or 0.50% or with acute freshwater [the latter three groups did not significantly (P > 0.05) differ from each other, Fig. 3, panel A]. Briefly, 1.00% acute alcohol treatment abolished the shoaling response in AB (Fig. 3 panel A, and also see Fig. 2 panels A, B, C vs. D), a conclusion supported by comparing the size of reduction to zero (no reduction). This comparison showed that all AB groups (t-tests with Holm correction: acute dose groups 0.00% t = 7.346, df = 16, P < 0.0001; 0.25% t = 8.013, df = 12, P < 0.001; and 0.50% t = 7.697, df = 17, P < 0.0001) but the one treated with the 1.00% acute alcohol dose (t = 0.874, df = 15, P > 0.35) significantly differed from zero. Such abolishment of the shoaling response, however, was less apparent in SF (Fig. 3 panel B, also see Fig. 2 panels E, F, G, vs. H), and indeed the Tukey HSD test found no significant (P > 0.05) differences among the four acute dose groups in this population. The t-tests comparing the size of reduction to zero also showed that all acute alcohol dose groups but the lowest one significantly differed from zero (t-tests with Holm correction: acute treatment group 0.00% t = 3.392, df = 14, P = 0.01; 0.25% t = 2.001, df = 14, P > 0.05; 0.50% t = 3.821, df = 16, P < 0.01; 1.00% t = 3.105, df = 13, P < 0.01).
Figure 3. The effect of acute alcohol administered after chronic freshwater (C000, panels A, B) or after chronic alcohol (C050, panels C, D) on the shoaling response (reduction of distance in response to the shoal image) of zebrafish of the AB and SF populations.
The mean ± SEM is shown. Sample sizes are as indicated on Fig. 2. The significance of the reduction of distance from the computer screen as compared with 0 cm is also shown: **P < 0.01, *P < 0.05, ns, not significant P > 0.05. For the method of calculation of this measure and details of results of the statistical analysis see Results section.
Panels C and D on Fig. 3 depict the shoaling responses after chronic alcohol exposure. Tukey HSD analysis confirmed that among these groups the 0.00% acute alcohol dose-exposed AB fish differed significantly (P < 0.05) from all other groups (0.25%, 0.50% and 1.00% acute alcohol dose groups) and that these latter three groups did not differ from each other (P > 0.05). It appears that withdrawal from alcohol abolished the shoaling response in AB and this effect was absent when the fish received any alcohol acutely after chronic exposure (Fig. 3, panel C first bar vs. the other bars, also see Fig. 2 panel I vs. panels J, K and L). Comparison of the group means with zero (no shoaling response) confirmed this observation and showed that indeed the 0.00% acute alcohol dose group was not significantly different from zero (t-test with Holm correction, t = 0.347, df = 14, P > 0.70), but the other three groups were (0.25% acute alcohol dose t = 3.477, df = 16, P < 0.01; 0.50% acute alcohol dose t = 6.210, df = 16, P < 0.0001; 1.00% acute alcohol dose t = 5.133, df = 17, P < 0.0001). Notably, abolishment of shoaling as a result of withdrawal from alcohol was not seen in SF fish (Fig. 3, panel D first bar vs. other bars, also see Fig. 2 panel M vs. panels N, O and P) as Tukey HSD test showed no differences among the treatment groups (P > 0.05) and one-sample t-tests with Holm correction also confirmed all groups to differ significantly from zero (acute alcohol doses 0.00% t = 4.213, df = 13, P < 0.01; 0.25% t = 3.900, df = 15, P < 0.01; 0.50% t = 5.869, df = 14, P < 0.01; 1.00% t = 2.965, df = 14, P < 0.05).
In summary, first, acute alcohol exposure after chronic freshwater abolished shoaling at the highest dose in AB fish but this acute effect was blunted in SF; and second, withdrawal from alcohol abolished shoaling in AB but not in SF fish.
The effect of alcohol treatment on predator image induced behavioral responses
Analysis of the behavioral responses to the predator image also showed notable results. The statistical analysis followed the same logic as explained for the analysis of shoaling responses. Briefly, repeated measures anova conducted for 1-min intervals following the cessation of the shoaling stimulus, i.e. the last 8 min during which the predator image was presented intermittently (Fig. 2) showed significant acute alcohol dose and chronic alcohol dose interactions as well as interactions of these factors with population (results not shown). Next, we conducted a non-repeated measure three-factorial anova on the composite measure (ΔP), the predator image induced increase of distance from the computer screen. ΔP was calculated as follows: ∑(Si+1 – Ni)/3, where N was the distance value obtained for no-stimulus interval ‘i’ preceding the predator presentation and S was the distance value obtained for the stimulus interval i + 1. The results are shown on Fig. 4 (panels A and B, freshwater during the chronic treatment period and panels C and D alcohol during the chronic treatment period). anova showed a significant acute alcohol dose effect (F3,232 = 3.274, P < 0.05) but found the other factors (population and chronic alcohol dose) non-significant. All double interaction terms were non-significant, but the triple-interaction acute alcohol dose × chronic alcohol dose × population was significant F3,232 = 3.813, P < 0.05), and thus we conducted the follow-up post hoc multiple comparison analysis without pooling the data across any of the main factors. Briefly, Tukey HSD tests showed that SF fish treated with 1.00% alcohol acutely after chronic alcohol exposure differed significantly (P < 0.05) from those that were exposed to lower acute concentrations of alcohol or freshwater (Fig. 4 panel D first and fourth bars vs. second and third bars). Although the dose–response trajectories and thus the interaction between acute alcohol dose and chronic alcohol dose appeared to be different for AB and SF (Fig. 4, panels A, B and C), Tukey HSD tests found no significant (P > 0.05) differences among groups within each of these panels or among groups between these panels. One-sample t-tests comparing the means of the groups to zero (no increase of distance in response to predator image) largely confirmed these findings and showed a significant difference only for the chronic alcohol treated SF fish exposed to the subsequent 0.25% (t = 3.475, df = 15, P < 0.01) or 0.50% (t = 2.671, df = 14, P < 0.05) acute alcohol doses. These analyses also showed three other groups to significantly differ from zero. First, the chronic freshwater plus acute 1.00% alcohol dose-exposed AB fish showed a significant (t = 2.234, df = 15, P < 0.05) reduction of distance (as opposed to increase), a result probably reflecting anxiolytic effects of this highest acute alcohol dose. Second, the chronic freshwater-treated and acute freshwater-treated SF fish showed a robust avoidance (significant increase of distance from predator image, t = 2.673, df = 14, P < 0.05), probably reflecting a species-typical avoidance reaction that in this population could be abolished by acute alcohol treatment. Finally, the chronic alcohol-treated AB fish that received a subsequent 0.25% acute alcohol dose showed a significant (t = 2.673, df = 14, P < 0.05) increase of predator stimulus-induced distance from the stimulus screen.
Figure 4. The effect of acute alcohol administered after chronic freshwater (C000, panels A, B) or after chronic alcohol (C050, panels C, D) on predator image avoidance (increase of distance in response to the predator image) of zebrafish of the AB and SF populations.
The mean ± SEM is shown. Sample sizes are as indicated on Fig. 2. Positive values represent increase of distance in response to the predator image (avoidance), whereas negative values a decrease (approach). The significance of the change of distance from the computer screen as compared with 0 cm is also indicated: **P < 0.01, *P < 0.05, ns, not significant P > 0.05. For the method of calculation of this measure and details of results of the statistical analysis see Results section.
The effect of acute alcohol doses on concentration of neurochemicals on the zebrafish brain
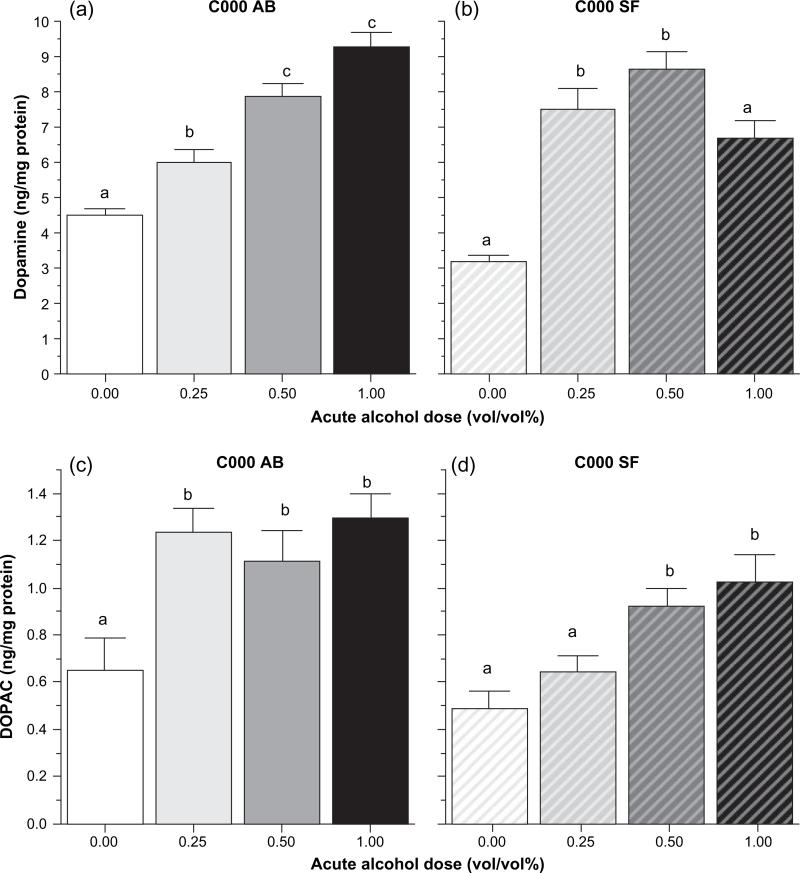
Analysis of neurochemical responses showed numerous significant alcohol treatment effects that were dependent on the population origin of the fish. First, we discuss the effects of different acute alcohol doses on fish that were not exposed to alcohol chronically. Figure 5 shows the results obtained for dopamine and its metabolite, DOPAC. The dose–response curves appear to be different for AB and SF fish. anova confirmed this observation for dopamine and showed a significant acute alcohol dose effect (F3,40 = 45.501, P < 0.0001) and acute alcohol dose × population interaction (F3,40 = 9.942, P < 0.0001) but found no significant population main effect. In case of AB, Tukey HSD post hoc tests showed that all acute treatment groups significantly (P < 0.05) differed from each other except the two highest dose groups, 0.50% and 1.00%. For SF fish, Tukey HSD test found the 0.25% and 0.50% dose groups not to significantly differ (P > 0.05). The 0.25% and 1.00% dose groups also did not significantly (P > 0.05) differ, but the differences between other pairs of groups were found significant (P < 0.05). For DOPAC, anova found a significant acute alcohol dose effect (F3,40 = 12.269, P < 0.0001) and a significant population effect (F1,40 = 18.056, P < 0.0001) but no significant acute alcohol dose × population interaction was detected. Post hoc Tukey HSD tests showed that the 0.00% alcohol dose group differed significantly (P < 0.05) from the other groups, but the 0.25%, 0.50% and 1.00% alcohol groups did not differ from each other.
Figure 5. The effect of acute alcohol dose on dopamine (A and B) and DOPAC (panels C and D) levels in the brain, an HPLC analysis in AB and SF strain zebrafish.
The mean ± SEM is shown. Sample sizes n = 6 for each group. C000 above the graph means that these fish received freshwater as chronic treatment. The strain of fish (AB or SF) is also indicated above the graphs. The small letter above each bar shows the results of Tukey HSD tests. Bars that have the same letter do not significantly differ from each other (P > 0.05).
Figure 6 shows the acute alcohol dose-induced changes in serotonin and its metabolite 5-HIAA. The dose–response curves for serotonin in AB and SF (Fig. 6 panels A and B) appear similar to those obtained for dopamine. anova found a significant acute alcohol dose effect (F3,40 = 6.747, P = 0.001) and significant acute alcohol dose × population interaction (F3,40 = 9.993, P < 0.0001) but no significant population effect. Post hoc Tukey HSD tests showed that in AB the highest alcohol dose group (1.00% EtOH) differed significantly (P < 0.05) from all other groups but other differences were non-significant. In case of SF, the 0.50% dose group differed significantly (P < 0.05) from the 1.00% dose group and also from the 0.25% and 0.00% dose groups, but other differences were non-significant. Acute alcohol treatment appeared to have a more robust effect on 5-HIAA levels (Fig. 8 panels C and D). anova found a significant acute alcohol dose effect (F3,40 = 49.343, P < 0.0001) and a significant acute alcohol dose population interaction term (F3,4015 = 15.871, P < 0.0001) but no significant population effect. Similar to what was found for serotonin in AB fish, post hoc Tukey HSD test showed that the highest dose group (1.00% alcohol) differed from all other groups and that these other groups did not significantly differ from each other. In case of SF, post hoc Tukey HSD test showed that the 0.50% alcohol group, having the highest value, significantly differed from all other groups and that the 1.00% alcohol group (second highest value) also differed from the 0.00% and the 0.25% alcohol groups.
Figure 6. The effect of acute alcohol administration on serotonin (panels A and B) and 5-HIAA (panels C and D) levels in the brain, an HPLC analysis in AB and SF strain zebrafish.
The mean ± SEM is shown. Sample sizes n = 6 for each group. C000 above the graph means that these fish received freshwater as chronic treatment. The strain of fish (AB or SF) is also indicated above the graphs. The small letter above each bar shows the results of Tukey HSD tests. Bars that have the same letter do not significantly differ from each other (P > 0.05).
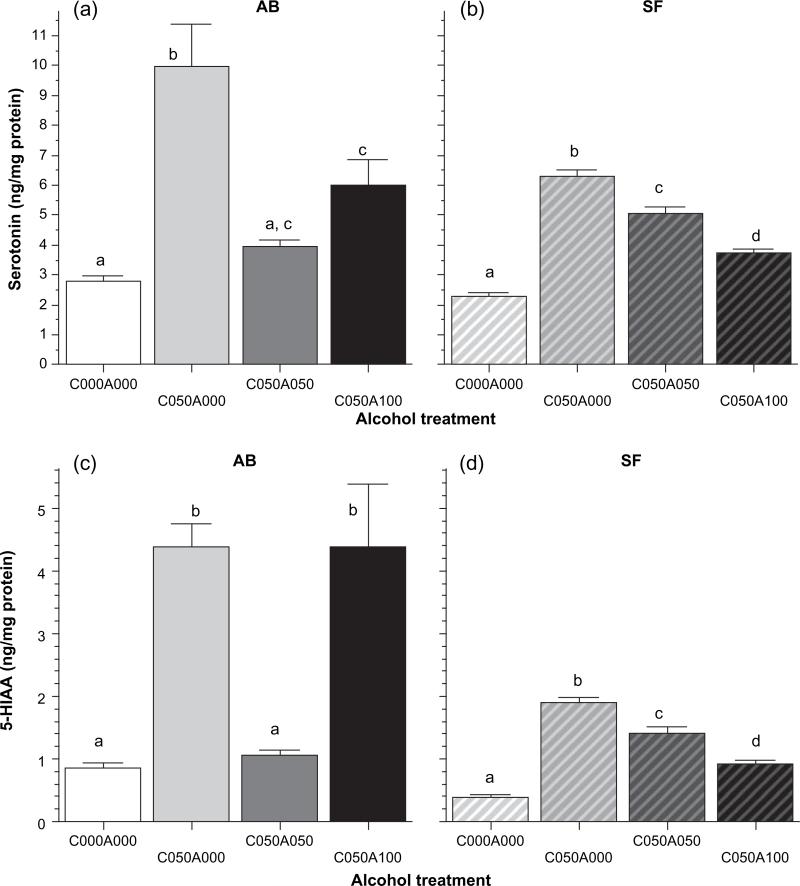
Figure 8. The effect of chronic alcohol administration on serotonin (panels A and B) and 5-HIAA (panels C and D) levels in the brain, an HPLC analysis in AB and SF strain zebrafish.
The mean ± SEM is shown. Sample sizes n = 6 for each group. The strain of fish (AB or SF) is indicated above the graphs. The group designation is indicated on the abscissa and represents the chronic and subsequent acute alcohol exposure employed (e.g. C000A000 is chronic 0.00% alcohol and acute 0.00% alcohol, C050A100 is chronic 0.50% alcohol and acute 1.00% alcohol). The small letter above each bar shows the results of Tukey HSD tests. Bars that have the same letter do not significantly differ from each other (P > 0.05).
The effect of chronic alcohol treatment on concentration of neurochemicals on the zebrafish brain
The changes induced by different alcohol treatments are shown in Fig. 7 for dopamine and DOPAC and in Fig. 8 for serotonin and 5-HIAA. The alcohol treatment-dependent differences between the AB and SF fish appear to be pronounced for all neurochemicals tested. For example, for dopamine (Fig. 7 panels A and B) anova showed that both the effects of alcohol treatment (F3,56 = 114.182, P < 0.0001) and population (F1,56 = 302.390, P < 0.0001) as well as their interaction (F3,56 = 56.108, P < 0.0001) were significant. Post hoc Tukey HSD test showed that in case of AB fish only the C050A100 and C050A050 groups did not differ from each other significantly (P > 0.05). In the SF, the Tukey HSD test also found only two groups not to differ significantly (P > 0.05), the C050A100 and C050A000 groups. The differences in chronic treatment-induced changes between the two populations were also robust in DOPAC (Fig. 7 panels C and D). anova showed a significant alcohol treatment (F3,56 = 15.899, P < 0.0001), Population (F1,56 = 53.686, P < 0.0001) as well as alcohol treatment × population interaction effect (F3,56 = 14.876, P < 0.0001). Post hoc Tukey HSD test showed that for AB fish the C000A000 vs. C050A050 groups did not significantly (P > 0.05) differ from each other and that the C050A000 vs. C050A100 groups also did not significantly (P > 0.05) differ from each other. The other pairs of groups were found significantly (P < 0.05) different. The pattern of pairwise differences in case of SF was unlike in AB. For this population, Tukey HSD test showed no significant difference (P > 0.50) between C000A000 vs. C050A000 and also between C050A050 and C050A100, while all other pairs of groups were found to differ significantly (P < 0.05).
Figure 7. The effect of chronic alcohol administration on dopamine (panels A and B) and DOPAC (panels C and D) levels in the brain, an HPLC analysis in AB and SF strain zebrafish.
The mean ± SEM is shown. Sample sizes n = 6 for each group. The strain of fish (AB or SF) is indicated above the graphs. The group designation is indicated on the abscissa and represents the chronic and subsequent acute alcohol dose employed (e.g. C000A000 is chronic 0.00% alcohol and acute 0.00% alcohol, C050A100 is chronic 0.50% alcohol and acute 1.00% alcohol). The small letter above each bar shows the results of Tukey HSD tests. Bars that have the same letter do not significantly differ from each other (P > 0.05).
Serotonin and 5-HIAA also showed alcohol treatment induced changes and the differences between AB and SF fish appear robust in these neurochemicals too (Fig. 8). Statistical analysis for serotonin (Fig. 8 panels A and B) showed a significant alcohol treatment effect (anova F3,56 = 30.004, P < 0.0001), population effect (F1,56 = 9.862, P < 0.01) and alcohol treatment × population interaction (F3,56 = 6.019, P = 0.001). Tukey HSD test showed that in case of AB fish the C050A000 group significantly (P < 0.05) differed from all other groups, C000A000 significantly (P < 0.05) differed from all but C050A050, and C050A100 significantly (P < 0.05) differed from all but C050A050. The pattern of pairwise differences for SF fish was unlike the above. For SF fish Tukey HSD showed that all groups significantly (P < 0.05) differed from one another. Analysis of 5-HIAA levels (Fig. 8 panels C and D) also showed that both main effects and their interaction were significant (anova alcohol treatment F3, 56 = 18.571, P < 0.0001; population F1,56 = 30.735, P < 0.0001; Alcohol treatment × Population F3, 56 = 10.430, P < 0.0001). For AB, Tukey HSD test found no significant (P > 0.05) differences between two pairs of groups C000A000 vs C050A050 and C050A000 vs. C050A100. All other pairwise comparisons turned out to show significant (P < 0.05) differences. For SF, again the results were different from the above. Tukey HSD test confirmed that all groups differed from each other significantly (P < 0.05).
In summary, acute alcohol and also the combination of chronic and acute alcohol exposure led to zebrafish population-dependent changes in the concentrations of neurochemicals tested.
Discussion
Alcohol engages several molecular mechanisms in a complex dose and administration regimen-dependent manner (e.g. Vengeliene et al. 2008). To tackle this complexity one could employ broad-spectrum screening for mutation or drug induced modification of alcohol's effects. Zebrafish screens have proven cost effective because of the small size and prolific nature of this species (Grunwald & Eisen 2002). Although a vertebrate with high similarity to mammals at the nucleotide and amino-acid sequence levels (Renier et al. 2007) and in syntenic relationships (Woods et al. 2005), zebrafish suffers from a drawback: unlike in rodents (Gerlai 2002), behavioral phenotyping is only starting for zebrafish (Sison et al. 2006). The current paper is an attempt to contribute to this start.
Here, we report acute alcohol, chronic alcohol and alcohol withdrawal-induced behavioral and neurochemical changes in zebrafish, some of which were population dependent. For example, we found the highest acute alcohol dose (1.00%) to abolish shoaling in AB zebrafish. Previously, alcohol was shown to disrupt group preference, essentially the same phenomenon (Gerlai et al. 2000). But now we also report that the abolishment of shoaling was not seen in SF fish, indicating genetic variability in this response. In the past we have compared AB and SF fish in their responses to acute alcohol treatment (Gerlai et al. 2008), and although we found trends in shoaling responses similar to current results, the differences were not significant. Notably, stimulus delivery was not computerized previously (Gerlai et al. 2008) as live stimulus fish were employed. The current method with its computerized stimulus presentation reduced error variation and was more sensitive to detect the acute alcohol-induced and population-dependent changes in shoaling.
Chronic alcohol treatment significantly blunted the effect of subsequent acute alcohol exposure in zebrafish, showing adaptation to alcohol in both populations tested. It is in line with previous results that showed adaptation to alcohol in activity levels of zebrafish (Gerlai et al. 2006). However, we now also discovered significant alcohol withdrawal effects leading to abolishment of shoaling in AB but not in SF zebrafish, showing genetic variability in alcohol withdrawal.
The pattern of responses to the predator image was different from those shown towards the shoal image, suggesting that the chronic and acute alcohol treatment-induced changes in the distance from the stimulus screen are unlikely to be the result of alterations in performance characteristics, e.g. perception or motor function, a conclusion also supported by previous findings (Gerlai et al. 2006). Confirming previous results, predator avoidance was found blunted by acute alcohol treatment in SF zebrafish possibly as a result of the anxiolytic effect of alcohol (Gerlai et al. 2000). However, the acute alcohol dose–response curve was different in AB fish, showing significant genetic variation. Chronic alcohol exposure reversed the U-shaped acute dose–response curve in SF but not in AB, suggesting the involvement of genetic factors again. In summary, our results showed genetic variability in acute and chronic alcohol-induced behavioral responses.
Another point to make concerns our methods: Both stimulus delivery and behavioral response quantification were computerized: the paradigm was automated. Automation not only increases precision, and thus improves sensitivity, but also facilitates running parallel sessions thereby increasing throughput, a requirement for screening mutants or compounds. Briefly, alcohol-induced changes in social behavior now can be tested in a high-throughput manner in a vertebrate, the zebrafish.
Similarly to the behavioral findings, our neurochemical analyses also showed significant chronic and acute alcohol effects that were often population dependent. In mammals, dopamine and serotonin mediate acute alcohol (e.g. Di Chiara & Imperato 1988; Lovinger & Zhou 1994; Lovinger 1999; Rodd-Henricks et al. 2000; Spanagel & Weiss 1999), chronic alcohol (e.g. Crabbe et al. 1996, 2006) and/or alcohol withdrawal (Rodd-Henricks et al. 2000; Sari et al. 2006; Thielen et al. 2004) effects. We have now showed significant changes in the levels of these neurotransmitters and their metabolites in zebrafish. Acute alcohol treatment increased dopamine in AB fish in a linear concentration-dependent manner. In SF fish, intermediate alcohol concentrations induced the largest dopamine increase, and the highest alcohol concentration was ineffective. This is noteworthy because presynaptic release of dopamine may underlie reinforcing effects of alcohol (Gonzales et al. 2004). If correct, this implies that alcohol may lose its reinforcing property at the highest dose (1.00%) in SF but not in AB fish, a prediction that will be empirically tested.
Analysis of DOPAC also confirmed an acute alcohol dose × population interaction: While increased DOPAC levels reached a plateau after 0.25% alcohol treatment in AB fish, in SF fish the response was more gradual. The acute alcohol dose-dependent and population-specific differences in dopa-mine metabolism did not parallel the dopamine level changes. Thus, the population differences in dopaminergic function may arise from at least two separate sets of genes.
Acute alcohol-induced changes in serotonin levels were similar to those we found in dopamine: the highest alcohol dose (1.00%) increased serotonin in AB, but in SF it was an intermediate dose of alcohol (0.50%) that induced the most robust increase. Acute alcohol enhances the release of serotonin in both the rat (Langen et al. 2002; Thielen et al. 2002; Yan 1999) and the human brain (Lovinger 1997). The behavioral function of the serotonin level increase is debated but an association between alcohol abuse and serotonin deficiency has been suggested, and serotonin's role in alcohol craving has been hypothesized (Ciccocioppo 1999). The pattern of changes in the amount of 5-HIAA induced by acute alcohol in the two populations paralleled those seen in serotonin itself implying a common underlying genetic difference.
Chronic alcohol treatment and subsequent acute administration of different doses of alcohol also resulted in population-dependent changes in neurotransmitter levels. Chronic alcohol consumption may induce neuroadaptations, blunting the acute effects of alcohol (Tambour & Quertemont 2007). In both humans and animals, chronic alcohol administration impairs dopamine synthesis, reduces concentrations of extracellular dopamine and reduces sensitivity of dopamine receptors (Diana et al. 1996; Rossetti et al. 1992). Similarly, blunted serotonin function because of chronic alcohol consumption has been reported (Ciccocioppo 1999; McBride et al. 1995), a serotoninergic hypofunction that may be counteracted by increased alcohol consumption induced serotonin release (Lovinger 1997). Our current results partially contradict these findings. In both AB and SF fish, chronic alcohol exposure resulted in increased, and not decreased, dopamine levels as compared with those seen in freshwater-treated fish. In case of serotonin, the increase was significant in SF fish, and a similar, but non-significant, trend was seen in AB. The pattern of results was similar in the metabolites of these neurotransmitters. Comparisons across experiments (C000A050 of Figs 5 and 6 vs. C050A050 of Figs 7 and 8) suggest no reduction of dopamine levels as a result of chronic alcohol exposure, and if anything, a potential increase of DOPAC. But serotonin and its metabolite 5-HIAA appeared reduced by chronic alcohol, findings to be confirmed in a properly randomized analysis.
Notably, the most robust change (increase) in the levels of the studied neurotransmitters and their metabolites was seen in the withdrawal group (C050A000, Figs 7 and 8) in AB fish, but this change was much less pronounced in SF fish. This parallels our behavioral results that showed withdrawal from alcohol to affect shoaling in AB but not in SF fish.
Do these neurotransmitter changes explain the behavioral findings? Can we make conclusions about causality? Addressing these questions will require further studies. Several neurotransmitter systems may change in response to alcohol (Vengeliene et al. 2008). We only studied two. Other molecular mechanisms may also mediate alcohol's actions. Pharmacological or genetic manipulation of known targets and/or comprehensive analysis of alcohol-induced responses (gene expression studies or drug and mutation screening) will be needed. Our current results represent only the first step.
Zebrafish is new in behavioral neuroscience research. The questions of face, predictive and construct validity remain to be answered. Alcohol reduces affiliative social interaction in non-human and human primates (Shively et al. 2002). Alcohol induced reduction of shoaling in zebrafish may thus have face validity. Acute alcohol may reduce and chronic alcohol or withdrawal from alcohol may elevate anxiety (Kushner et al. 2000). Thus alcohol-induced alterations in fear responses in zebrafish may also represent face validity. Pharmacological predictive validity is hard to forecast: drug studies with zebrafish are scarce. Construct validity can only be determined once we understand mechanistic details underlying alcohol's effects in zebrafish. Therefore, for now, we do not think zebrafish is a ‘model’ for human alcohol abuse. It is simply a promising tool.
Nevertheless, the showed genetic variability in acute and chronic alcohol effects and in alcohol withdrawal in zebrafish has important implications. First, it shows that our methods are sensitive to detect genetic differences. Second, it opens the door to research harnessing genetic variability, including quantitative trait locus analysis, in zebrafish. Third, it signifies the importance of a systematic comparison of zebrafish strains for alcohol-related traits for future mutagenesis studies in which appropriate mutation host and mapping strains must be chosen (Gerlai et al. 2000).
Supplementary Material
Appendix
Appendix S1: Additional information on methodological details.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author.
Footnotes
Note
During the publication process of the current study, a paper appeared in which dopamine levels from the brain of zebrafish of the AB strain were analyzed (López Patiño et al. 2008). Although the analysis did not include dopamine metabolites or other neurotransmitters, this study is significant and supports our current findings as follows. First, the basal dopamine level in the untreated control fish (2.2 ng/mg) was around the same order of magnitude as in our study (4.18 ng/mg). The slight difference may be as a result of the more sensitive recording technique we employed. Second, and more importantly, withdrawal from cocaine induced an increase, and not a decrease, of dopamine levels, a finding that is similar to what we observed after withdrawal from chronic alcohol.
Supporting Information
Additional supporting information may be found in the online version of this article.
References
- Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: The Bonferroni vs. Holm methods. Am J Public Health. 1996;86:726–728. doi: 10.2105/ajph.86.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass SLS, Gerlai R. Zebrafish (Danio rerio) responds differentially to stimulus fish: The effects of sympatric and allopatric predators and harmless fish. Behav Brain Res. 2008;186:107–117. doi: 10.1016/j.bbr.2007.07.037. [DOI] [PubMed] [Google Scholar]
- Blaser R, Gerlai R. Behavioral phenotyping in Zebrafish: Comparison of three behavioral quantification methods. Behav Res Methods. 2006;38:456–469. doi: 10.3758/bf03192800. [DOI] [PubMed] [Google Scholar]
- Browman KE, Crabbe JC. Alcohol and genetics: new animal models. Mol Med Today. 1999;5:310–318. doi: 10.1016/s1357-4310(99)01480-x. [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Gerlai R. High Precision Liquid Chromatography Analysis of Dopaminergic and Serotoninergic Responses to Acute Alcohol Exposure in Zebrafish. Behav Brain Res. 2009 doi: 10.1016/j.bbr.2009.01.016. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R. The role of serotonin in craving: from basic research to human studies. Alcohol Alcohol. 1999;34:244–253. doi: 10.1093/alcalc/34.2.244. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Feller DJ, Hen R, Wenger CD, Lessov CN, Schafer GL. Elevated alcohol consumption in null mutant mice lacking 5-HT1B serotonin receptors. Nat Genet. 1996;14:98–101. doi: 10.1038/ng0996-98. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF. Alcohol-related genes: contributions from studies with genetically engineered mice. Addict Biol. 2006;11:195–269. doi: 10.1111/j.1369-1600.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- Diana M, Pistis M, Muntoni A, Gessa G. Mesolimbic dopaminergic reduction outlasts ethanol withdrawal syndrome: evidence of protracted abstinence. Neuroscience. 1996;71:411–415. doi: 10.1016/0306-4522(95)00482-3. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugos CA, Rabin RA. Ethanol effects on three strains of zebrafish: model system for genetic investigations. Pharm Biochem Behav. 2003;74:471–480. doi: 10.1016/s0091-3057(02)01026-2. [DOI] [PubMed] [Google Scholar]
- Fernandes Y, Gerlai R. Long-term behavioral changes in response to early developmental exposure to ethanol in zebrafish. Alcohol Clin Exp Res. 2009;33:601–609. doi: 10.1111/j.1530-0277.2008.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RK, Hiller-Sturmhöfel S. Alcoholism treatment in the United States. An overview. Alcohol Res Health. 1999;23:69–77. [PMC free article] [PubMed] [Google Scholar]
- Gerlai R, Lahav M, Guo S, Rosenthal A. Drinks like a fish: Zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav. 2000;67:773–782. doi: 10.1016/s0091-3057(00)00422-6. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Phenomics: Fiction or the Future? Trends Neurosci. 2002;25:506–509. doi: 10.1016/s0166-2236(02)02250-6. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Zebra fish: An uncharted behavior genetic model. Behav Genet. 2003;33:461–468. doi: 10.1023/a:1025762314250. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Lee V, Blaser R. Effects of acute and chronic ethanol exposure on the behavior of adult zebrafish (Danio rerio). Pharmacol Biochem Behav. 2006;85:752–761. doi: 10.1016/j.pbb.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R, Ahmad F, Prajapati S. Differences in acute alcohol-induced behavioral responses among zebrafish populations. Alcohol Clin Exp Res. 2008;32:1763–1773. doi: 10.1111/j.1530-0277.2008.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessa GL, Serra S, Vacca G, Carai MA, Colombo G. Suppressing effect of the cannabinoid CB1 receptor antagonist, SR147778, on alcohol intake and motivational properties of alcohol in alcohol-preferring sP rats. Alcohol. 2004;40:46–53. doi: 10.1093/alcalc/agh114. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Grunwald DJ, Eisen JS. Timeline: Headwaters of the zebrafish—emergence of a new model vertebrate. Nat Rev Genet. 2002;3:717–724. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- Guarnieri DJ, Heberlein U. Drosophila melanogaster, a genetic model system for alcohol research. Int Rev Neurobiol. 2003;54:199–228. doi: 10.1016/s0074-7742(03)54006-5. [DOI] [PubMed] [Google Scholar]
- Guryev V, Koudijs MJ, Berezikov E, Johnson SL, Plasterk RH, van Eeden FJ, Cuppen E. Genetic variation in the zebrafish. Genome Res. 2006;16:491–497. doi: 10.1101/gr.4791006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood HJ, Fountain D, Livermore G. Economic costs of alcohol abuse and alcoholism. Recent Dev Alcohol. 1998;14:307–330. doi: 10.1007/0-306-47148-5_14. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149–171. doi: 10.1016/s0272-7358(99)00027-6. [DOI] [PubMed] [Google Scholar]
- Langen B, Dietze S, Fink H. Acute effect of ethanol on anxiety and 5-HT in the prefrontal cortex of rats. Alcohol. 2002;27:135–141. doi: 10.1016/s0741-8329(02)00219-7. [DOI] [PubMed] [Google Scholar]
- Lockwood B, Bjerke S, Kobayashi K, Guo S. Acute effects of alcohol on larval zebrafish: a genetic system for large-scale screening. Pharmacol Biochem Behav. 2004;77:647–654. doi: 10.1016/j.pbb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- López Patiño MA, Yu L, Yamamoto BK, Zhdanova IV. Gender differences in zebrafish responses to cocaine withdrawal. Physiol Behav. 2008;95:36–47. doi: 10.1016/j.physbeh.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM. Serotonin's role in alcohol's effects on the brain. Alcohol Health Res World. 1997;21:114–120. [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM. 5-HT3 receptors and the neural actions of alcohols: an increasingly exciting topic. Neurochem Int. 1999;35:125–130. doi: 10.1016/s0197-0186(99)00054-6. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Zhou Q. Alcohols potentiate ion current mediated by recombinant 5-HT3RA receptors expressed in a mammalian cell line. Neuropharmacology. 1994;33:1567–1572. doi: 10.1016/0028-3908(94)90131-7. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Bodart B, Lumeng L, Li TK. Association between low contents of dopamine and serotonin in the nucleus accumbens and high alcohol preference. Alcohol Clin Exp Res. 1995;19:1420–1422. doi: 10.1111/j.1530-0277.1995.tb01001.x. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Eckardt MJ, Linnoila MI. Pharmacotherapy of alcoholism. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology. The Fourth Generation of Progress. Raven Press Ltd; New York: 1995. pp. 1745–1755. [Google Scholar]
- Renier C, Faraco JH, Bourgin P, Motley T, Bonaventure P, Rosa F, Mignot E. Genomic and functional conservation of sedative-hypnotic targets in the zebrafish. Pharmacogenet Genomics. 2007;17:237–253. doi: 10.1097/FPC.0b013e3280119d62. [DOI] [PubMed] [Google Scholar]
- Rice DP. Economic costs of substance abuse. Proc Assoc Am Physicians. 1995;111:119–125. doi: 10.1046/j.1525-1381.1999.09254.x. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Weissman MM, Orvaschel H, Gruenberg E, Burke JD, Regier DA. Lifetime prevalence of specific psychiatric disorders in three sites. Arch Gen Psychiatry. 1984;41:949–958. doi: 10.1001/archpsyc.1984.01790210031005. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Edmundson VE, Dagon CL, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of 5-HT(3) receptor antagonists on daily alcohol intake under acquisition, maintenance, and relapse conditions in alcohol-preferring (P) rats. Alcohol. 2000;21:73–85. doi: 10.1016/s0741-8329(00)00083-5. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Melis F, Carboni S, Diana M, Gessa GL. Alcohol withdrawal in rats is associated with a marked fall in extraneuronal dopamine. Alcohol Clin Exp Res. 1992;16:529–532. doi: 10.1111/j.1530-0277.1992.tb01411.x. [DOI] [PubMed] [Google Scholar]
- Sari Y, Bell RL, Zhou FC. Effects of chronic alcohol and repeated deprivations on dopamine D1 and D2 receptor levels in the extended amygdala of inbred alcohol-preferring rats. Alcohol Clin Exp Res. 2006;30:46–56. doi: 10.1111/j.1530-0277.2006.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saverino C, Gerlai R. The social zebrafish: Behavioral responses to conspecific, heterospecific, and computer animated fish. Behav Brain Res. 2008;191:77–87. doi: 10.1016/j.bbr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Grant KA, Register TC. Effects of long-term moderate alcohol consumption on agonistic and affiliative behavior of socially housed female cynomolgus monkeys (Macaca fascicularis). Psychopharmacology. 2002;165:1–8. doi: 10.1007/s00213-002-1223-y. [DOI] [PubMed] [Google Scholar]
- Sison M, Cawker J, Buske C, Gerlai R. Fishing for genes of vertebrate behavior: zebra fish as an upcoming model system. Lab Animal. 2006;35:33–39. doi: 10.1038/laban0506-33. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Sullivan EJ, Handley SM. Alcohol and drug abuse. Annu Rev Nurs Res. 1993;11:281–297. [PubMed] [Google Scholar]
- Tambour S, Quertemont E. Preclinical and clinical pharmacology of alcohol dependence. Fundam Clin Pharmacol. 2007;21:9–28. doi: 10.1111/j.1472-8206.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Romeo HE, Beylin AV, Tio DL, Rahman SU, Hovda DA. Alcohol consumption in traumatic brain injury: attenuation of TBI-induced hyperthermia and neurocognitive deficits. J Neurotrauma. 2002;19:1597–1608. doi: 10.1089/089771502762300256. [DOI] [PubMed] [Google Scholar]
- Thielen RJ, Bare DJ, McBride WJ, Lumeng L, Li TK. Ethanol-stimulated serotonin release in the ventral hippocampus: an absence of rapid tolerance for the alcoholpreferring P rat and insensitivity in the alcohol-nonpreferring NP rat. Pharmacol Biochem Behav. 2002;71:111–117. doi: 10.1016/s0091-3057(01)00633-5. [DOI] [PubMed] [Google Scholar]
- Thielen RJ, Engleman EA, Rodd ZA, Murphy JM, Lumeng L, Li TK, McBride WJ. Ethanol drinking and deprivation alter dopaminergic and serotonergic function in the nucleus accumbens of alcohol preferring rats. J Pharmacol Exp Ther. 2004;309:216–225. doi: 10.1124/jpet.103.059790. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlsten D. Insensitivity of the analysis of variance to heredity- environment interaction. Behav Brain Sci. 1990;13:109–161. [Google Scholar]
- Woods IG, Wilson C, Friedlander B, Chang P, Reyes DK, Nix R, Kelly PD, Chu F, Postlethwait JH, Talbot WS. The zebrafish gene map defines ancestral vertebrate chromosomes. Genome Res. 2005;15:1307–1314. doi: 10.1101/gr.4134305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan QS. Extracellular dopamine and serotonin after ethanol monitored with 5-minute microdialysis. Alcohol. 1999;19:1–7. doi: 10.1016/s0741-8329(99)00006-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.