Abstract
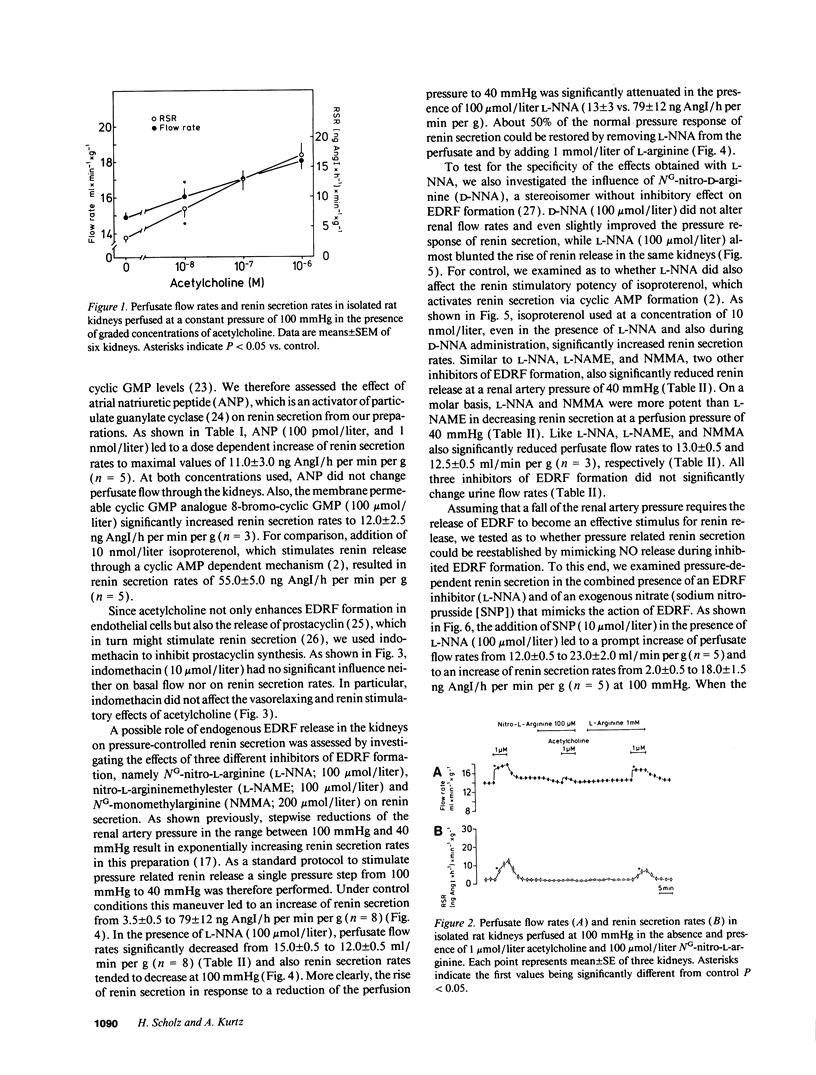
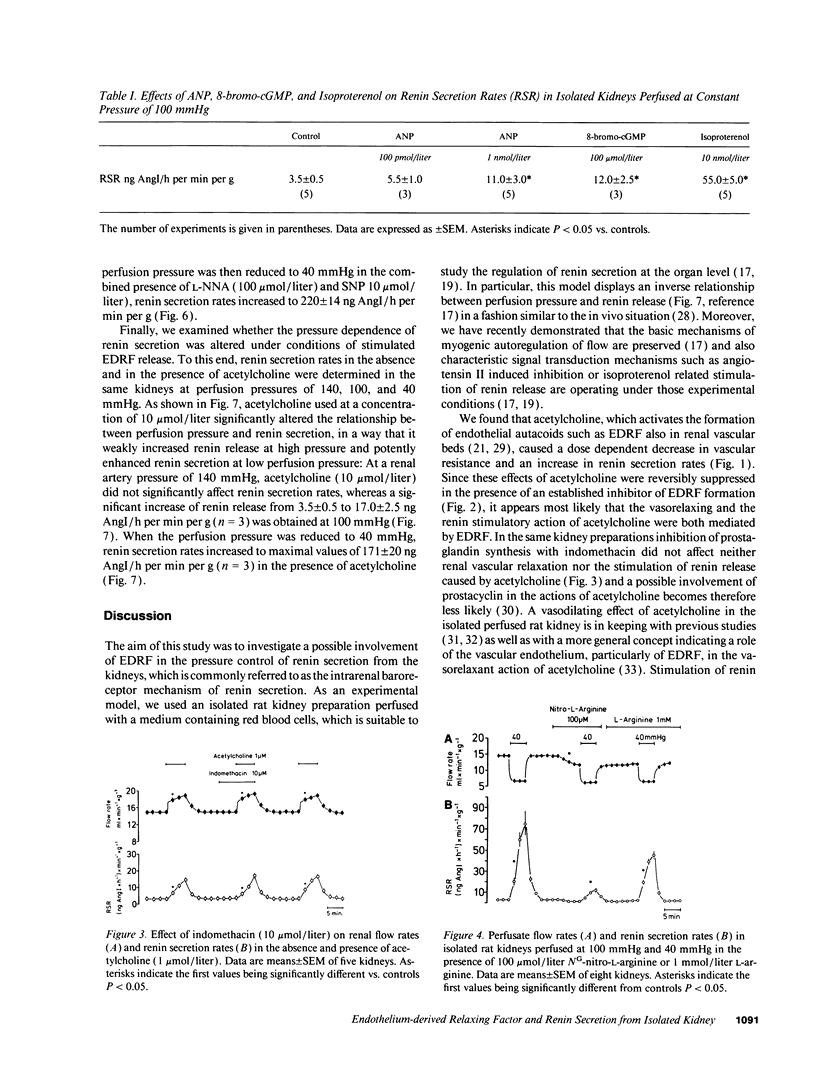
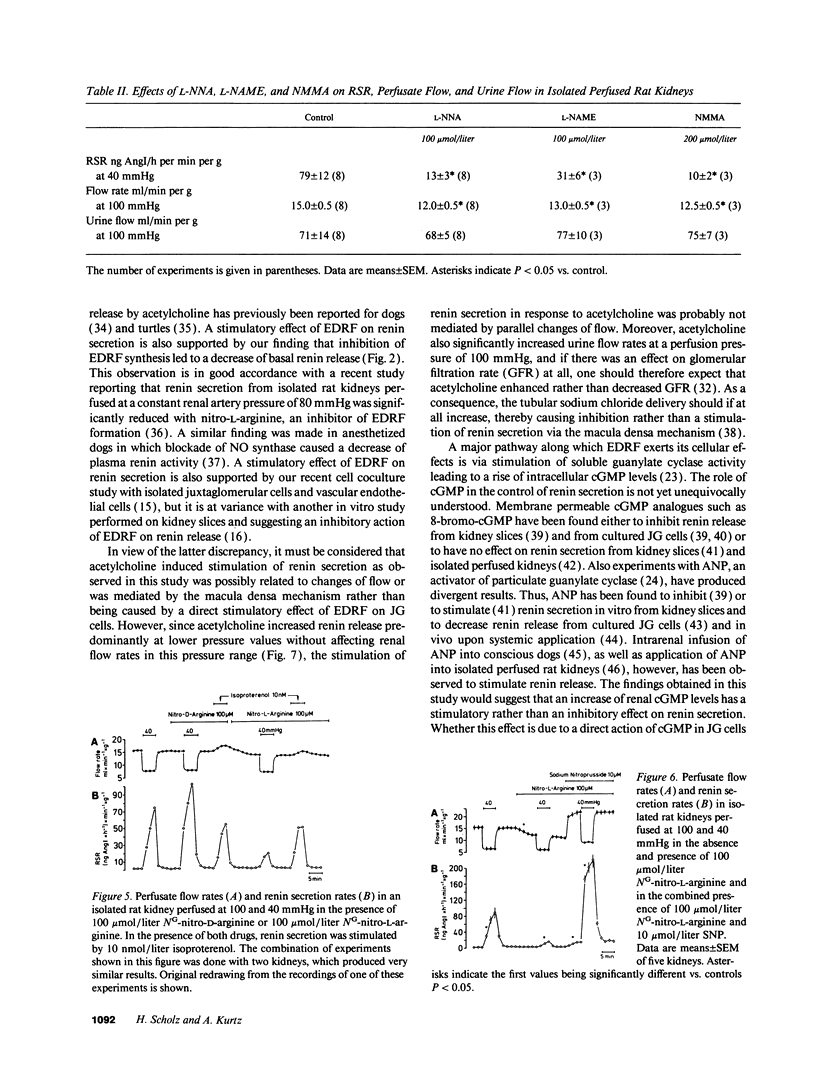
Using isolated rat kidneys perfused at controlled pressure, we examined a potential role of endothelium-derived relaxing factor (EDRF) in the pressure control of renin secretion. We found that stimulation of EDRF release by acetylcholine (1 mumol/liter) increased mean perfusate flow rates from 15.0 +/- 0.5 to 18.0 +/- 0.5 ml/min per g and average renin secretion rates from 3.5 +/- 0.5 to 16.0 +/- 2.0 ng angiotensin I/h per min per g at a perfusion pressure of 100 mmHg (mean +/- SEM, n = 6). Those effects of acetylcholine were significantly reduced during inhibition of EDRF formation with NG-nitro-L-arginine (100 mumol/liter), but they were not affected with the cyclooxygenase inhibitor indomethacin (10 mumol/liter). Lowering of the perfusion pressure from 100 mmHg to 40 mmHg resulted in an increase of average renin secretion rates from 3.5 +/- 0.5 to 79 +/- 12 ng AngI/h per min per g under control conditions (n = 8), and to 171 +/- 20 ng AngI/h per min per g in the presence of 10 mumol/liter acetylcholine (n = 3). The rise of renin secretion in response to a reduction of the renal artery pressure was markedly attenuated with inhibitors of EDRF formation such as NG-nitro-L-arginine (100 mumol/liter) and related compounds. During inhibition of EDRF formation, addition of sodium nitroprusside (10 mumol/liter) increased mean perfusate flow rates from 12.0 +/- 0.5 to 23.0 +/- 2.0 ml/min per g and average renin secretion rates from 2.0 +/- 0.5 to 18.0 +/- 1.5 ng AngI/h per min per g at 100 mmHg (n = 5). Lowering of the perfusion pressure from 100 mmHg to 40 mmHg under those conditions increased average renin secretion rates to 220 +/- 14 ng AngI/h per min per g (n = 5). Taken together, our findings suggest that EDRF and related activators of soluble guanylate cyclase stimulate renin secretion from isolated kidneys, predominantly at lower perfusion pressure. Moreover, pressure control of renin secretion appears to require the tonical stimulation by intrarenal EDRF.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayers C. R., Harris R. H., Jr, Lefer L. G. Control of renin release in experimental hypertension. Circ Res. 1969 May;24(5 Suppl):103–112. [PubMed] [Google Scholar]
- Beierwaltes W. H., Schryver S., Sanders E., Strand J., Romero J. C. Renin release selectively stimulated by prostaglandin I2 in isolated rat glomeruli. Am J Physiol. 1982 Sep;243(3):F276–F283. doi: 10.1152/ajprenal.1982.243.3.F276. [DOI] [PubMed] [Google Scholar]
- Bhardwaj R., Moore P. K. The effect of arginine and nitric oxide on resistance blood vessels of the perfused rat kidney. Br J Pharmacol. 1989 Jul;97(3):739–744. doi: 10.1111/j.1476-5381.1989.tb12011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine E. H., Davis J. O., Prewitt R. L. Evidence for a renal vascular receptor in control of renin secretion. Am J Physiol. 1971 Jun;220(6):1593–1597. doi: 10.1152/ajplegacy.1971.220.6.1593. [DOI] [PubMed] [Google Scholar]
- Bock H. A., Hermle M., Fiallo A., Osgood R. W., Fried T. A. Measurement of renin secretion in single perfused rabbit glomeruli. Am J Physiol. 1990 May;258(5 Pt 2):F1460–F1465. doi: 10.1152/ajprenal.1990.258.5.F1460. [DOI] [PubMed] [Google Scholar]
- Brüne B., Lapetina E. G. Activation of a cytosolic ADP-ribosyltransferase by nitric oxide-generating agents. J Biol Chem. 1989 May 25;264(15):8455–8458. [PubMed] [Google Scholar]
- Cairns H. S., Rogerson M. E., Westwick J., Neild G. H. Regional heterogeneity of endothelium-dependent vasodilatation in the rabbit kidney. J Physiol. 1991 May;436:421–429. doi: 10.1113/jphysiol.1991.sp018558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. P., Rossitch E., Jr, Andon N. A., Loscalzo J., Dzau V. J. Flow activates an endothelial potassium channel to release an endogenous nitrovasodilator. J Clin Invest. 1991 Nov;88(5):1663–1671. doi: 10.1172/JCI115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmke H., Persson P. B., Just A., Nafz B., Seyfarth M., Hackenthal E., Kirchheim H. R. Physiological concentrations of ANP exert a dual regulatory influence on renin release in conscious dogs. Am J Physiol. 1992 Sep;263(3 Pt 2):R529–R536. doi: 10.1152/ajpregu.1992.263.3.R529. [DOI] [PubMed] [Google Scholar]
- Fray J. C. Stretch receptor model for renin release with evidence from perfused rat kidney. Am J Physiol. 1976 Sep;231(3):936–944. doi: 10.1152/ajplegacy.1976.231.3.936. [DOI] [PubMed] [Google Scholar]
- Freeman R. H., Davis J. O., Villarreal D. Role of renal prostaglandins in the control of renin release. Circ Res. 1984 Jan;54(1):1–9. doi: 10.1161/01.res.54.1.1. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Gardes J., Poux J. M., Gonzalez M. F., Alhenc-Gelas F., Menard J. Decreased renin release and constant kallikrein secretion after injection of L-NAME in isolated perfused rat kidney. Life Sci. 1992;50(14):987–993. doi: 10.1016/0024-3205(92)90092-4. [DOI] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Nitric oxide decreases cytosolic free calcium in Balb/c 3T3 fibroblasts by a cyclic GMP-independent mechanism. J Biol Chem. 1991 Jan 5;266(1):9–12. [PubMed] [Google Scholar]
- Hackenthal E., Lang R. E., Bührle C. P. Atrial natriuretic factor stimulates renin release from the isolated rat kidney. J Hypertens Suppl. 1985 Dec;3(3):S323–S325. [PubMed] [Google Scholar]
- Hackenthal E., Paul M., Ganten D., Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev. 1990 Oct;70(4):1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- Henrich W. L., McAllister E. A., Smith P. B., Campbell W. B. Guanosine 3',5'-cyclic monophosphate as a mediator of inhibition of renin release. Am J Physiol. 1988 Sep;255(3 Pt 2):F474–F478. doi: 10.1152/ajprenal.1988.255.3.F474. [DOI] [PubMed] [Google Scholar]
- Hiruma M., Ikemoto F., Yamamoto K. Rat atrial natriuretic factor stimulates renin release from renal cortical slices. Eur J Pharmacol. 1986 Jun 5;125(1):151–153. doi: 10.1016/0014-2999(86)90095-6. [DOI] [PubMed] [Google Scholar]
- Katsuki S., Arnold W., Mittal C., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977 Feb;3(1):23–35. [PubMed] [Google Scholar]
- Kirchheim H. R., Ehmke H., Hackenthal E., Löwe W., Persson P. Autoregulation of renal blood flow, glomerular filtration rate and renin release in conscious dogs. Pflugers Arch. 1987 Nov;410(4-5):441–449. doi: 10.1007/BF00586523. [DOI] [PubMed] [Google Scholar]
- Kon V., Harris R. C., Ichikawa I. A regulatory role for large vessels in organ circulation. Endothelial cells of the main renal artery modulate intrarenal hemodynamics in the rat. J Clin Invest. 1990 Jun;85(6):1728–1733. doi: 10.1172/JCI114628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz A., Della Bruna R., Pfeilschifter J., Bauer C. Role of cGMP as second messenger of adenosine in the inhibition of renin release. Kidney Int. 1988 Apr;33(4):798–803. doi: 10.1038/ki.1988.70. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Della Bruna R., Pfeilschifter J., Taugner R., Bauer C. Atrial natriuretic peptide inhibits renin release from juxtaglomerular cells by a cGMP-mediated process. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4769–4773. doi: 10.1073/pnas.83.13.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz A., Kaissling B., Busse R., Baier W. Endothelial cells modulate renin secretion from isolated mouse juxtaglomerular cells. J Clin Invest. 1991 Oct;88(4):1147–1154. doi: 10.1172/JCI115415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz A., Pfeilschifter J., Hutter A., Bührle C., Nobiling R., Taugner R., Hackenthal E., Bauer C. Role of protein kinase C in inhibition of renin release caused by vasoconstrictors. Am J Physiol. 1986 Apr;250(4 Pt 1):C563–C571. doi: 10.1152/ajpcell.1986.250.4.C563. [DOI] [PubMed] [Google Scholar]
- Lamontagne D., Pohl U., Busse R. NG-nitro-L-arginine antagonizes endothelium-dependent dilator responses by inhibiting endothelium-derived relaxing factor release in the isolated rabbit heart. Pflugers Arch. 1991 Apr;418(3):266–270. doi: 10.1007/BF00370525. [DOI] [PubMed] [Google Scholar]
- Lansman J. B., Hallam T. J., Rink T. J. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers? 1987 Feb 26-Mar 4Nature. 325(6107):811–813. doi: 10.1038/325811a0. [DOI] [PubMed] [Google Scholar]
- Majid D. S., Navar L. G. Suppression of blood flow autoregulation plateau during nitric oxide blockade in canine kidney. Am J Physiol. 1992 Jan;262(1 Pt 2):F40–F46. doi: 10.1152/ajprenal.1992.262.1.F40. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989 Jun 1;38(11):1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest. 1986 Jul;78(1):1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen S. P., Clapham D. E., Davies P. F. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988 Jan 14;331(6152):168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Peart W. S., Quesada T., Tenyi I. The effects of cyclic adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate and theophylline on renin secretion in the isolated perfused kidney of the rat. Br J Pharmacol. 1975 May;54(1):55–60. doi: 10.1111/j.1476-5381.1975.tb07409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radermacher J., Förstermann U., Frölich J. C. Endothelium-derived relaxing factor influences renal vascular resistance. Am J Physiol. 1990 Jul;259(1 Pt 2):F9–17. doi: 10.1152/ajprenal.1990.259.1.F9. [DOI] [PubMed] [Google Scholar]
- SKINNER S. L., MCCUBBIN J. W., PAGE I. H. CONTROL OF RENIN SECRETION. Circ Res. 1964 Jul;15:64–76. doi: 10.1161/01.res.15.1.64. [DOI] [PubMed] [Google Scholar]
- Salomonsson M., Skøtt O., Persson A. E. Influence of intraluminal arterial pressure on renin release. Acta Physiol Scand. 1991 Feb;141(2):285–286. doi: 10.1111/j.1748-1716.1991.tb09081.x. [DOI] [PubMed] [Google Scholar]
- Scholz H., Kaissling B., Inagami T., Kurtz A. Differential response of renin secretion to vasoconstrictors in the isolated perfused rat kidney. J Physiol. 1991 Sep;441:453–468. doi: 10.1113/jphysiol.1991.sp018761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz H., Kurtz A. Disparate effects of calcium channel blockers on pressure dependence of renin secretion and flow in the isolated perfused rat kidney. Pflugers Arch. 1992 Jun;421(2-3):155–162. doi: 10.1007/BF00374822. [DOI] [PubMed] [Google Scholar]
- Schurek H. J., Alt J. M. Effect of albumin on the function of perfused rat kidney. Am J Physiol. 1981 Jun;240(6):F569–F576. doi: 10.1152/ajprenal.1981.240.6.F569. [DOI] [PubMed] [Google Scholar]
- Skøtt O., Briggs J. P. Direct demonstration of macula densa-mediated renin secretion. Science. 1987 Sep 25;237(4822):1618–1620. doi: 10.1126/science.3306925. [DOI] [PubMed] [Google Scholar]
- Struthers A. D., Anderson J. V., Payne N., Causon R. C., Slater J. D., Bloom S. R. The effect of atrial natriuretic peptide on plasma renin activity, plasma aldosterone, and urinary dopamine in man. Eur J Clin Pharmacol. 1986;31(2):223–226. doi: 10.1007/BF00606663. [DOI] [PubMed] [Google Scholar]
- TOBIAN L. Relationship of juxtaglomerular apparatus to renin and angiotensin. Circulation. 1962 Jan;25:189–192. doi: 10.1161/01.cir.25.1.189. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Rubanyi G. M., Miller V. M., Houston D. S. Modulation of vascular smooth muscle contraction by the endothelium. Annu Rev Physiol. 1986;48:307–320. doi: 10.1146/annurev.ph.48.030186.001515. [DOI] [PubMed] [Google Scholar]
- Vidal M. J., Romero J. C., Vanhoutte P. M. Endothelium-derived relaxing factor inhibits renin release. Eur J Pharmacol. 1988 May 10;149(3):401–402. doi: 10.1016/0014-2999(88)90679-6. [DOI] [PubMed] [Google Scholar]


