Abstract
The Alcohol Tolerant and Alcohol Non-Tolerant rats (AT, ANT) were selectively bred for ethanol-induced ataxia as measured on the inclined plane. Here we report on a quantitative trait locus (QTL) study in an F2 intercross population derived from inbred AT and ANT (IAT, IANT) and a follow-up study of congenics that were bred to examine one of the mapped QTLs. Over 1200 F2 offspring were tested for inclined plane sensitivity, acute tolerance on the inclined plane, duration of the loss of righting reflex (LORR), and blood ethanol at regain of the righting reflex (BECRR). F2 rats that were in the upper and lower 20% for inclined plane sensitivity were genotyped with 78 SSLP markers. Significant QTLs for inclined plane sensitivity were mapped on chromosomes 8 and 20; suggestive QTLs were mapped on chromosomes 1, 2, and 3. Highly significant QTLs for LORR duration (LOD=12.4) and BECRR (LOD=5.7) were mapped to the same locus on chromosome 1. Breeding and testing of reciprocal congenic lines confirmed the chromosome 1 LORR/BECRR QTL. A series of recombinant congenic sub-lines were bred to fine-map this QTL. Current results have narrowed the QTL to an interval of between 5 and 20 Mb. We expect to be able to narrow the interval to less than 5 Mb with additional genotyping and continued breeding of recombinant sub-congenic lines.
Keywords: Alcohol tolerance, Genetics, Selected lines, Loss of righting reflex
Introduction
It has become evident that an individual’s acute sensitivity to alcohol (ethanol) early in their drinking career is a reliable predictor for the development of alcoholism later in life (Schuckit, 1998; Schuckit & Smith, 2006). Moreover, acute sensitivity is genetically determined, at least in part (Heath et al. 1999; Schuckit et al. 2005; Viken et al. 2003). Thus, elucidating the molecular and genetic substrates of acute sensitivity is an important goal towards a complete understanding of the factors that contribute to the initiation and maintenance of pathological drinking behavior.
Investigations of genetic effects on acute alcohol sensitivity have included the development of rodent lines that have been selected for a variety of acute responses to alcohol. One such selection is the Alcohol Tolerant (AT) and Alcohol Non-Tolerant (ANT) rat lines which were established by Dr. Kalervo Eriksson in 1973 (Eriksson & Rusi 1981). The heterogeneous stock from which these animals were selected was generated from a complex breeding scheme involving numerous rat lines including the Alko Alcohol and Alko Non-Alcohol selectively bred lines, Sprague-Dawley, Long-Evans hooded rats, and albino rats from the Helsinki Zoo (see Radcliffe et al. 2004a). Breeding pairs of these lines at generation 60 were brought to the University of Colorado in 1998. At that time, it was determined that the lines were approximately 90% inbred. The lines were subsequently bred through 6 additional generations using a brother-sister mating scheme to inbreed the lines before any testing was begun (now referred to as IAT and IANT) (Radcliffe et al. 2004a).
The AT and ANT lines were selectively bred using a measure of ataxic sensitivity to alcohol as determined by performance on the tilting plane test. In this test, the subject is given three trials in which it is placed on the far end of a mechanical tilting plane that is raised from a flat horizontal position to an 84-degree angle in 5 seconds. The average angle at which the animal slides to the base of the plane is recorded. An intraperitoneal injection of 2.0 g/kg alcohol is then administered. After a 30-minute interval, the average angle obtained on 3 additional trials is recorded. Ataxic sensitivity is measured as the difference between the baseline and post-injection average angle of sliding (Sellin & Laakso 1987). Using this test, rats demonstrating the lowest ataxic sensitivity at 30 minutes were selected for breeding as the AT line and those demonstrating the highest sensitivity were bred into the ANT line. After 7 generations of selection, the lines differed significantly in ataxic sensitivity, but not in ethanol metabolism or in blood acetaldehyde levels (Eriksson & Rusi 1981). The AT and ANT rats have been studied extensively, primarily by Korpi and colleagues (Hellevuo et al. 1989; Hellevuo & Korpi 1988; Kaheinen et al. 1988; Korpi & Uusi-Oukari 1989; Korpi et al. 1992a; Korpi et al. 1992b; Korpi et al. 1992c; Makela et al. 1995; Malminen & Korpi 1988; Malminen & Korpi 1989; Nakki et al. 1995; Sarviharju & Korpi 1993; Tuominen & Korpi 1991; Uusi-Oukari & Korpi 1989; Uusi-Oukari & Korpi 1990; Uusi-Oukari & Korpi 1991; Uusi-Oukari & Korpi 1992).
The finding of a mutation in the α6 subunit of the GABAA chloride channel in the AT and ANT lines is of particular interest (Korpi et al. 1993; Korpi & Seeburg 1993). Initial genotyping of a sample of rats from generations 45 and 46 showed that the mutant allele occurred with a frequency of 0.86 in the ANT while the frequency was only 0.09 in the AT (Makela et al. 1995). Among AT and ANT breeders that were transferred to the University of Colorado in 1998 (generation 60), all ANT rats were homozygous for the mutant allele, while both alleles were still present in the AT (Radcliffe et al. 2004a). The point mutation converts a critical arginine to glutamine which affects benzodiazepine binding and responses to benzodiazepines (Korpi et al. 1993; Uusi-Oukari & Korpi 1990). Because of the known relationship between alcohol and GABA signaling, the α6 mutation was thought to be an excellent candidate that might account for at least a portion of the selected acute sensitivity difference between the lines (Korpi et al. 1993). However, there is some disagreement about whether the mutation affects alcohol responses (Botta et al. 2007a; Botta et al. 2007b; Botta et al. 2008; Hanchar et al. 2005; Otis 2008). Our own studies lines do not support that the mutation contributes to genetic differences in the tilting plane test in the AT and ANT (Botta et al. 2007b).
A segregating population of over 1200 F2 rats was bred from the IAT and IANT several years ago to further investigate the genetic basis of variation in acute alcohol responses. The rats went through three weeks of phenotype testing during which several tilting plane-related tests were performed. In addition, the animals were tested for the duration of the loss of righting reflex (LORR) and blood ethanol concentration at regain of the righting reflex (BECRR) (Radcliffe et al., 2004a).
Here we describe a quantitative trait locus (QTL) mapping study that was conducted on the F2 animals described in Radcliffe et al. (2004a). Numerous QTLs were identified, although we did not conduct a full genome scan (see Methods, below). After the discovery of a major QTL on rat chromosome 1, genotyping for QTL mapping was suspended. The chromosome 1 QTL subsequently was investigated using a congenic strategy. Thus, the current report contains what can be considered a partial QTL mapping study with a focus on one particular alcohol sensitivity QTL on rat chromosome 1.
Methods
Animals
AT and ANT breeders (generation 60) were obtained in 1998. Fifteen families of AT and 13 families of ANT were established and inbred through brother-sister mating for a minimum of 6 generations. AT and ANT rats from this generation (now referred to as IAT and IANT) were tested as described below. One family from each line was selected for reciprocal mating to produce an F1 generation from which 1201 F2 progeny were bred (598 males, 603 females). The IAT and IANT progenitors of the F2 had undergone at least 9 generations of inbreeding. The 30-minute ataxic sensitivity scores (SENS30) and fertility were considered in selection of the two families. In addition, it was made certain that the selected IAT family carried the wild-type allele of the α-6 subunit of the GABAA receptor, while the IANT family carried the mutant allele.
For the congenic lines, a separate F1 and F2 intercross was bred as above using IAT and IANT rats that had undergone 15 to 16 generations of inbreeding. The progenitors were from the same families that were used to generate the QTL mapping F2. Male F2 offspring that carried the appropriate IANT interval as identified through genotyping were backcrossed to homozygous IAT females that were undergoing continued inbreeding (see Results for specific marker and interval information). The process was repeated for up to 6 generations as noted in the Results. All congenic rats that were tested were either homozygous for the recipient line throughout the genotyped interval (control) or heterozygous (congenic).
All rats were maintained on a 12 hour light/dark cycle in an environment of constant temperature and humidity (22°C., 40% humidity) and given access to normal rodent chow and water ad libitum. Phenotype testing began when the rats were 59 ± 0.12 days old (range: 51–70).
The procedures described in this report have been established to ensure that the animals received the absolute highest level of humane care. All procedures have been reviewed and approved by the University of Colorado Denver Anschutz Medical Campus IACUC.
Phenotyping
Rats were tested over a three week period. During week one, the tilting plane test was used in identical fashion as in selective breeding (Dr. Kalervo Kiianmaa graciously sent to us the tilting plane used in Helsinki for these studies). All animals received three trials while sober on the inclined plane to determine their baseline angle (BSLN1). This was immediately followed by a 2.0 g/kg dose of alcohol (15%w/v, IP). The animals were left undisturbed until 30-minutes post-injection at which time they were given three additional trials on the inclined plane (T30ANGL). The difference between the pre- and post-injection average angle of sliding was recorded as 30-minute ataxic sensitivity (SENS30). In all cases, all three values from the pre- and post-alcohol trials were used to calculate the mean angle of sliding.
In week two, the animals were tested on the inclined plane at the same dose and in similar fashion, but starting at 5 minutes, the angle at which they slid down the plane was recorded. They were then continuously tested at every 5 minutes until they regained their initial baseline angle (BSLN2). The time it took to regain the baseline angle (REGAIN) was recorded as a measure of acute tolerance. Note that acute tolerance was determined differently in the congenic study as described in the Results.
In week three, the animals were administered a 3.5 g/kg dose of alcohol (15%w/v, IP) to induce the loss of righting reflex, or the inability to turn itself over when placed on its back in a V-shaped trough. When the subject did manage to turn over three times in one minute, two 40-microliter blood samples were taken from the retro-orbital sinus. This testing produced two additional sensitivity scores: duration of loss of righting reflex (LORR) in minutes, and blood ethanol concentration at regain of the righting reflex (BECRR). If an animal failed to lose the righting reflex, this implied either that it was very resistant to the effects of alcohol or that there was a bad injection (Lewis et al. 1966). Such injections could have been subcutaneous, into the intestine or bladder, or into a fat pad. To decide between “bad injection” and resistance, we took a retro-orbital blood sample from all animals that had failed to lose the righting reflex by 30 minutes. By referring to data contained in a large metabolism study performed in our lab, we excluded LORR and BECRR data from those animals with a blood ethanol concentration below 390 mg/dl at 30 minutes post-injection (see Radcliffe et al. 2004b).
The congenic and sub-congenic lines were treated in week 1 and 2 with a 2.0 g/kg dose of alcohol, but they were not tested on the inclined plane unless otherwise noted. This was because we wanted to duplicate the treatment schedule that was used for the F2 QTL mapping since we have noted effects of testing order (Radcliffe et al. 2004a), but had limited manpower to actually test them. In week 3, the congenic rats were tested for LORR duration and BECRR as described above using an alcohol dose of 3.2 g/kg. A subset of sub-congenic and control rats were tested on the inclined plane (2.0 g/kg) during week 1, but were not subsequently tested for any other phenotype.
Genotyping
QTL mapping is made more efficient by genotyping only the extreme responders in a segregating population, since it is these animals that capture the greatest proportion of the genetic variance (Darvasi & Soller 1992). Thus, 479 F2 animals that were in the upper and lower 20% of the distribution for 30 minute sensitivity – the trait for which the AT and ANT were originally selected – were genotyped (low responders, IAT-like: n=110 males, 129 females; high responders, IANT-like: n=140 males, 100 females).
This was not a full genome scan; all chromosomes (except X) were probed with from two to six informative simple sequence length polymorphism (SSLP) DNA markers, 78 in all. The average intermarker distance across all markers was 18.4 cM with 11 gaps of 30 cM or greater (see supplemental table S1). The intermarker distance was 14.1 cM if the gaps shown in table S1 are excluded. Under the assumption of an ideal marker spacing of 20 cM (10 cM from the end of the chromosome), the results shown in table S1 (along with the fact that chromosome X was not genotyped at all) suggest that the scan covered approximately 1206 cM out of a total length of 1562 cM (Rat genome Database, 2008; SHRSP X BN cross). The specific markers used and their map locations are provided in supplemental table S2. SSLP fragments were amplified using standard PCR methods and size differences were determined with the use of an ABI Prism 3100 Genetic Analyzer. IAT, IANT, and heterozygote controls were always included in each genotyping assay. Genotyping procedures were identical for the congenic lines.
Data analysis
Basic data analysis was conducted using SPSS (v. 16.0); specific procedures are indicated in the text and figure legends. QTL mapping was conducted using J/qtl which is a graphical user interface for the popular mapping software R/qtl (Broman et al. 2003; Sheppard et al. 2008). A genetic map was constructed using the Haldane mapping function with a genotype error rate of 10−4. There were cases when either the published or empirical distance between two adjacent markers was too far apart to reliably use interval mapping procedures; this distance was arbitrarily chosen as 35 cM. When the distance was less than 35 cM, QTLs were mapped using the extended Haley-Knott interval mapping method (Feenstra et al. 2006). For markers that were separated by 35 cM or greater from both of their adjacent markers, QTL mapping was conducted using the marker regression method (Soller et al. 1976). Significance thresholds were determined empirically using only the markers that were linked by 35 cM or less (58 markers, 1000 iterations; Churchill & Doerge 1994) and are reported according to the guidelines of Lander and Kruglyak (1995). Heritability of individual QTLs (h2QTL) was calculated as the proportion of variance that the QTL contributed to the overall phenotypic variance. It was calculated as the ratio of the residual variance to the total variance and was derived from the Haley-Knott regression method as implemented in Map Manager QTX (Manly et al. 2001). Epistatic interactions were examined using marker regression to test for all pair-wise interactions (Haley & Knott, 1992). As above, permutation testing was used to determine empirical significance thresholds (1000 iterations; Churchill & Doerge 1994).
Results
The results of phenotype testing of a sample of IAT and IANT rats are shown in table 1. A three-way ANOVA was conducted on BSLN1 and BSLN2 to determine if there were effects of line, sex, or week of testing. Indeed, there was a significant main effect of week on the baseline angle, as well as main effects of line and sex. As expected, T30ANGL showed a significant main effect of line and also sex. The results suggest that the lines were selected not only for a difference in acute alcohol sensitivity, but also for a difference in their tilting plane performance in the unintoxicated state. The combined effect of these two measures resulted in a significant difference in SENS30 which is the phenotype for which they were selected. There was also a significant main effect of line on the time it took to regain BSLN2 after 2.0 g/kg alcohol, a measurement made in the second week of testing (REGAIN) This is a measure of acute tolerance and is consistent with SENS30; i.e., the IAT showed much greater acute tolerance than the IANT.
Table 1.
Inclined plane and LORR-related measures in the IAT and IANT progenitors.
| Trait | Sex1 | Line2 | Mean ± SEM (n)3 |
|---|---|---|---|
| BSLN1 (degrees) | M** | IANT*** | 83.2 ± 0.2 (70) |
| IAT | 80.8 ± 0.6 (43) | ||
| F | IANT | 83.3 ± 0.2 (58) | |
| IAT | 81.6 ± 0.7 (44) | ||
|
| |||
| T30ANGL (degrees) | M** | IANT*** | 72.2 ± 1.1 (70) |
| IAT | 81.6 ± 0.4 (43) | ||
| F | IANT | 76.8 ± 1.0 (58) | |
| IAT | 82.9 ± 0.3 (44) | ||
|
| |||
| SENS30 (BSLN1 minus T30ANGL; degrees)4 | M* | IANT*** | 10.9 ± 1.0 (70)* |
| IAT | −0.8 ± 0.6 (43) | ||
| F | IANT | 6.4 ± 1.0 (58) | |
| IAT | −1.3 ± 0.8 (44) | ||
|
| |||
| BSLN2 (degrees) | M** | IANT*** | 83.4 ± 0.2 (70) |
| IAT | 81.0 ± 0.6 (43) | ||
| F | IANT | 83.7 ± 0.1 (58) | |
| IAT | 82.9 ± 0.3 (44) | ||
|
| |||
| REGAIN (seconds)4 | M | IANT*** | 1331 ± 138 (70) |
| IAT | 701 ± 52 (43) | ||
| F | IANT | 1277 ± 137 (58) | |
| IAT | 575 ± 50 (44) | ||
|
| |||
| BECRR (mg%)4 | M** | IANT*** | 401 ± 8 (62)* |
| IAT | 455 ± 9 (42) | ||
| F | IANT | 446 ± 9 (45) | |
| IAT | 463 ± 7 (42) | ||
|
| |||
| Duration of LORR (mins)4 | M*** | IANT | 100 ± 8 (67) |
| IAT | 77 ± 9 (42) | ||
| F | IANT | 45 ± 8 (52) | |
| IAT | 52 ± 6 (43) | ||
Asterisks indicate significant main effect of sex1, line2, or interaction3:
p<0.05;
p<0.01;
p<0.001
BSLN1, BSLN2: Main effects of sex (F1,211=8.5, p<0.01), line (F1,211=50.0, p<10−10), week (F1,211=4.9, p<0.05).
T30ANGL: Main effects of sex (F1,211=9.8, p<0.01), line (F1,211=68.3, p<10−13).
SENS30: Main effects of sex (F1,211=6.6, p<0.05), line (F1,211=101.8, p<10−19), interaction (F1,211=4.3, p<0.05).
REGAIN: Main effects of line (F1,211=29.5, p<10−6).
BECRR: Main effects of sex (F1,187=9.6, p<0.01), line (F1,187=17.2, p<10−4), sex-by-line interaction (F1,187=4.5, p<0.05).
Duration of LORR: Main effects of sex (F1,200=22.8, p<10−5).
Reanalysis of data originally presented in Radcliffe et al., 2004.
BECRR was found to be significantly different between the lines with the IANT being more sensitive than the IAT, consistent with their differences in tilting plane sensitivity (table 1). The line difference in BECRR was not as great for females compared to males resulting in a significant sex-byline interaction. There was not a significant line effect for duration of LORR, but there was a significant main effect of sex for duration as well as for BECRR with males being more sensitive than females for both measures (table 1).
Table 2 shows the phenotypic correlations among the traits measured in the IAT X IANT F2 rats. BSLN1 and T30ANGL both correlate well with SENS30. This was not unexpected since the latter is a composite of the two former measures. It is notable that T30ANGL is significantly positively correlated to BSLN1 and BSLN2 which indicates that these measures were not independent of one another.
Table 2.
| Inclined plane related measures3 | LORR-related measures | |||||
|---|---|---|---|---|---|---|
| T30ANGL | SENS30 | BSLN2 | REGAIN | Duration | BECRR | |
| BSLN1 | 0.43*(n=1170) | 0.36*(n=1170) | 0.37*(n=1185) | −;;0.13*(n=1176) | −;;0.19*(n=1184) | 0.08 (n=1000) |
| T30ANGL | −;;0.69*(n=1170) | 0.35*(n=1159) | −;;0.22*(n=1150) | −;;0.14*(n=1158) | 0.04 (n=974) | |
| SENS30 | −;0.05 (n=1159) | 0.11*(n=1150) | 0.00 (n=1158) | 0.03 (n=974) | ||
| BSLN2 | −;0.05 (n=1178) | −;;0.20*(n=1178) | 0.11*(n=995) | |||
| REGAIN | 0.07 (n=1169) | −;0.08 (n=988) | ||||
| LORR duration | −;;0.38*(n=1001) | |||||
Values represent Pearson’s product-moment correlation coefficients (r; missing values excluded in a pairwise manner);
asterisk indicates p<0.05, corrected by the Bonferroni procedure (nominal p<0.0014).
Some of these correlations were previously presented in Radcliffe et al. 2004a.
See methods for variable abbreviations.
LORR duration was significantly negatively correlated to BECRR, as expected (table 2). LORR duration also was significantly negatively correlated to BSLN1 and BSLN2, and to T30ANGL, but not to SENS30. BECRR was not significantly correlated to any of the inclined plane measures except for a positive correlation to BSLN2 which is consistent with the negative correlation between the BSLN2 and LORR duration.
Significant and suggestive QTLs that were mapped for the inclined plane measures are shown in table 3. There were a total of 15 QTLs, some of which were overlapping, with heritability values (h2QTL) that ranged from 1% to 9%. SENS30 had the largest number of QTLs (5) and all except one (chromosome 3) overlapped with QTLs for either BSLN1 or T30ANGL. The SENS30 QTL on chromosome 1 overlapped with a BSLN1 locus and the SENS30 QTLs on chromosomes 2 and 8 mapped to the same positions as those for T30ANGL; all three traits had a QTL that mapped to chromosome 20. The QTLs for acute tolerance (REGAIN) also mapped to the same loci as for SENS30on chromosomes 3 and 20. There were no significant pair-wise interaction effects for any of the inclined plane measures (p>0.6, genome-wide)
Table 3.
Summary of QTL mapping results for inclined plane measures in IAT X IANT F2 rats.
| Trait | Chromosome | Nearest marker | Peak location (95% confidence interval)1 | LOD 2 | h2QTL3 |
|---|---|---|---|---|---|
| BSLN1 | 1 | D1rat35 + 12.0 cM | 71.4 cM (65 to 79 cM) | 10.5*** | 9% |
| 6 | D6rat90 + 0 cM | 82.9 cM (55 to 83 cM) | 2.4* | 2% | |
| 19 | D19rat12 + 0 cM | 20.3 cM (20 to 40 cM) | 1.9* | 1% | |
| 20 | D20rat32† | 0 cM (NA) | 3.2** | 3% | |
| T30ANGL | 2 | D2rat339 + 0 cM | 43.3 cM (43 to 51 cM) | 2.4* | 2% |
| 8 | D8rat58† | 0.0 cM (NA) | 3.2** | 3% | |
| 20 | D20rat54 + 0 cM | 28.7 cM (28 cM to 37 cM) | 2.3* | 3% | |
| SENS30 | 1 | D1rat35 + 10 cM | 69.4 cM (59 to 106 cM) | 2.2* | 2% |
| 2 | D2rat339 + 0 cM | 43.3 cM (43 to 58 cM) | 2.1* | 2% | |
| 3 | D3rat1 + 0 cM | 91.5 cM (82 to 92 cM) | 1.8* | 2% | |
| 8 | D8rat58† | 0 cM (NA) | 4.6** | 5% | |
| 20 | D20rat32† | 0.0 cM (NA) | 3.4** | 4% | |
| BSLN2 | 1 | D1rat35 + 12 cM | 71.4 cM (67 to 75 cM) | 11.2*** | 8% |
| REGAIN | 3 | D3rat1 + 0 cM | 91.5 cM (82 cM to 92 cM) | 2.0* | 2% |
| 20 | D20rat54 +0 cM | 28.7 cM (28 cM to 37 cM) | 2.0* | 2% |
Location based on published location of marker (Rat Genome Database, 2008; SHRSP X BN cross) most proximal to the QTL peak plus distance to peak as calculated by J/qtl; NA=confidence interval not applicable.
LOD is for the free model; asterisks indicate level of significance (genome-wide):
(p < 0.63);
significant (p < 0.05);
highly significant (p < 0.001) (Lander & Kruglyak 1995).
h2QTL is the heritability of the QTL; i.e., the fraction of the phenotypic variance explained by the QTL.
Mapped using marker regression (published or observed interval > 35 cM).
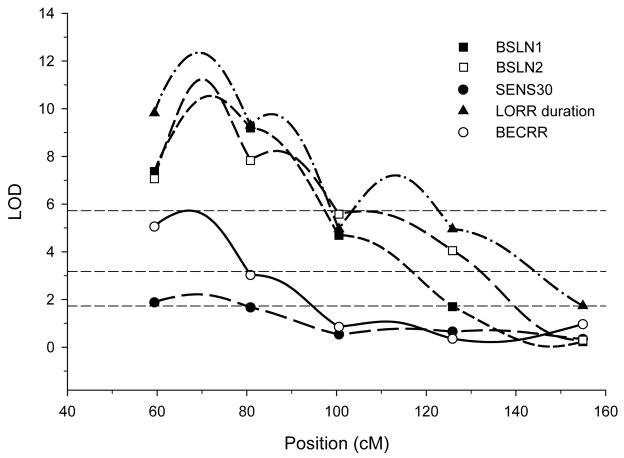
QTLs for LORR-related measures are shown in table 4. A large effect-size QTL was detected at the same locus on chromosome 1 for both LORR duration and BECRR. Notably, similarly large effect-size QTLs were mapped at this same locus for BSLN1 and BSLN2 as well as a suggestive QTL for SENS30 (see table 2). All five of these QTLs are shown in figure 1. It is not possible to determine from the mapping results if any or all of the QTLs are the same since their 95% confidence intervals all overlap (see tables 3 and 4). There were no significant pair-wise interaction effects for LORR duration or BECRR (p>0.1, genome-wide)
Table 4.
Summary of QTL mapping results for LORR and BECRR in IAT X IANT F2 rats.
| Trait | Chromosome | Nearest marker | Peak location (95% confidence interval, cM)1 | LOD2 | h2QTL3 |
|---|---|---|---|---|---|
| LORR duration | 1 | D1rat35 + 10 cM | 69.4 cM (65 to 75 cM) | 12.4*** | 11% |
| 14 | D14rat92 + 8 cM | 50.8 cM (29 to 61 cM) | 3.3* | 3% | |
| 20 | D20rat32† | 0 cM (NA) | 2.4* | 2% | |
| BECRR | 1 | D1rat35 + 8 cM | 67.4 cM (59 to 73 cM) | 5.7*** | 7% |
| 2 | D2rat68† | 103.3 cM (NA) | 2.1* | 2% |
Location based on published location of marker (Rat Genome Database, 2008; SHRSP X BN cross) most proximal to the QTL peak plus distance to peak as calculated by J/qtl; NA=confidence interval not applicable.
LOD is for the free model; asterisks indicate level of significance (genome-wide):
(p < 0.63);
significant (p < 0.05);
highly significant (p < 0.001) (Lander & Kruglyak 1995).
h2QTL is the heritability of the QTL; i.e., the fraction of the phenotypic variance explained by the QTL.
Mapped using marker regression (published or observed interval > 35 cM).
Figure 1.
QTLs were mapped to the approximate same location on chromosome 1 for five of the traits that were measured in the IAT X IANT F2 rats. The three horizontal lines indicate empirically derived genome-wide significance thresholds for BSLN2 which were the highest among all traits: bottom line, suggestive (p=0.63); middle line, significant (p=0.05); top line, highly significant (p=0.001) (Lander & Kruglyak 1995). The thresholds for the other traits were similar.
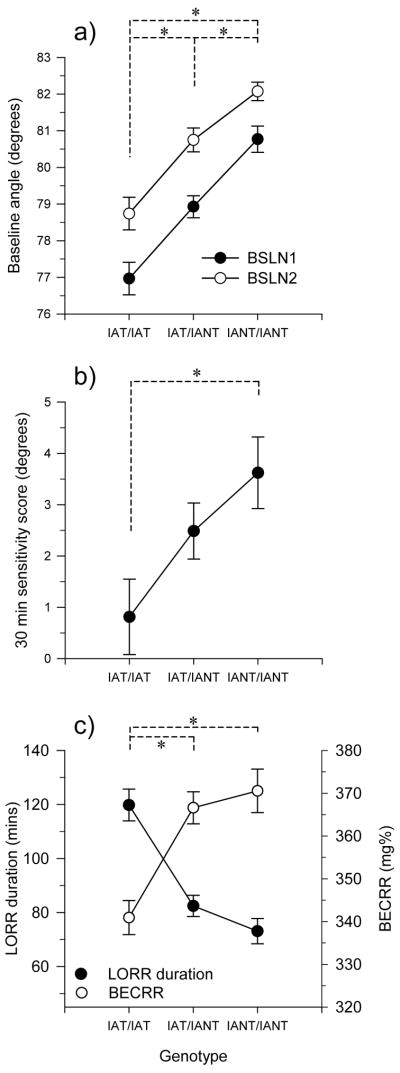
Figure 2 illustrates the chromosome 1 QTL allele effects for BSLN1, BSLN2, SENS30, LORR duration, and BECRR. Two-way ANOVA on the baseline angles indicated significant main effects of genotype and week (figure 2-a). Simple effects analysis indicated that all three genotypes were significantly different from one another in each week. There was an overall effect of genotype (one-way ANOVA, factor=genotype) on SENS30 (figure 2-b); only the two homozygote values were significantly different from one another. These results are consistent with an additive allele effect for the chromosome 1 QTLs for the baseline angles and SENS30.
Figure 2.
Allele effects for the chromosome 1 QTLs shown in figure 1. Values were derived using data from the marker nearest to the QTL peak (mean ± SEM). (a) BSLN1 and BSLN2, marker D1rat47; main effects of genotype (F2,464=28.5, p<10−11) and week (F1,464=38.2, p<10−8); (b) SENS30, marker D1rat35; main effect of genotype (F2,454=3.8, p<0.05); and (c) LORR duration and BECRR, marker D1rat35; main effect of genotype on LORR duration (F2, 454=22.9, p<10−9) and BECRR (F2, 383=12.5, p<10−5). Asterisk (*) indicates a significant difference (p<0.05) between genotypes. Number of subjects in each group: IAT/IAT, n=107–134; IAT/IANT, n=183–229; IANT/IANT, n=96–117. Marker distribution was not different from the expected 25:50:25 (χ2, P>0.10).
Allele effects for duration of LORR and BECRR are shown in figure 2-c. One-way ANOVA (factor=genotype) was conducted independently for each variable. In both cases, there was a significant effect of genotype on LORR duration and BECRR. Also for both traits, the IANT allele appears to be dominant since in both cases the heterozygotes were significantly different from the IAT homozygotes but not the IANT. IAT and ANT homozygotes also were significantly different from each other. Note that none of the marker distributions were significantly different from the expected Mendelian distribution of 25:50:25.
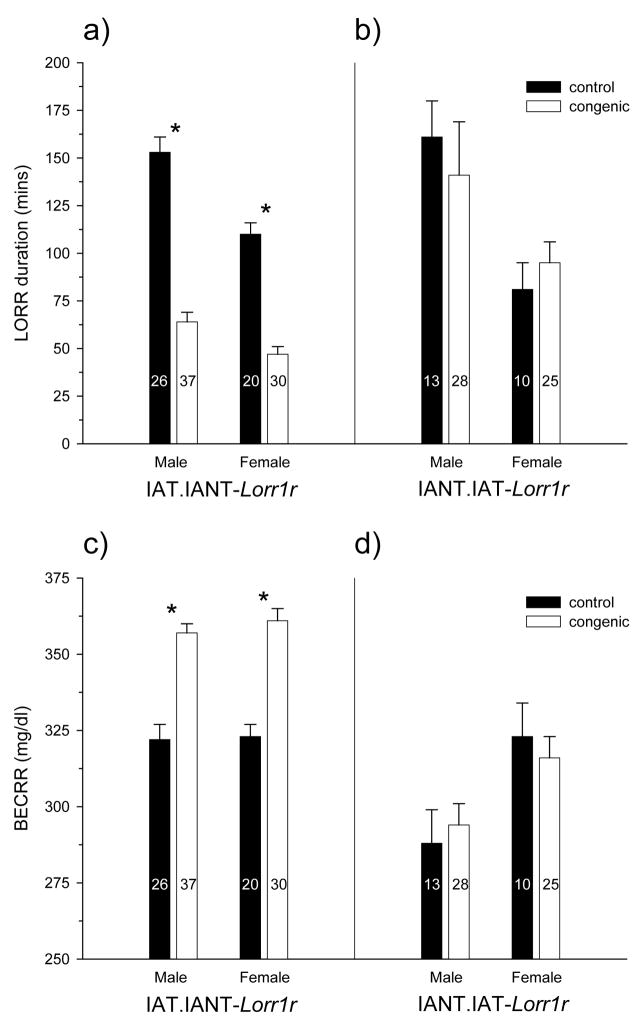
Following the discovery of the major QTL on chromosome 1, marker-assisted congenics were constructed as described under Methods. Offspring that carried the appropriate chromosome 1 interval were identified and bred through repeated backcrossing. Shown in figure 3 are the results of testing the congenic lines for LORR duration and BECRR. Rats were tested after 4 generations of backcrossing at which time each line was in theory 93.75% recipient strain and 6.25% donor strain; the actual values undoubtedly varied somewhat from these hypothetical values. Four chromosome 1 markers that covered an interval of approximately 151 Mb (72 cM) were used to genotype the offspring (D1rat234: 22.7 cM, 41.0 Mb; D1rat35: 59.4 cM, 124.7 Mb; D1rat47: 77.9 cM, 158.6 Mb; and D1rat288: 94.8 cM, 192 Mb). Testing backcross offspring is an excellent design because each litter contains an approximately equal number of offspring that are homozygous for the recipient strain (controls) and heterozygotes (congenics), meaning that maternal, litter, and background effects are controlled. A disadvantage is that since the congenic animals are heterozygous, the phenotypic effect of the QTL may be reduced if its alleles are additive in nature. The accepted nomenclature for a congenic is to first list the recipient strain followed by the donor strain and then the name of the QTL which, in this case, has been named Lorr1r (Loss of righting reflex 1 for the rat). Figures 3-a and 3-c show results of the IANT interval introgressed onto an IAT background while figures 3-b and 3-d show the results from animals that had the IAT interval introgressed onto an IANT background.
Figure 3.
Duration of LORR and BECRR for congenic lines at the fourth generation of backcrossing. For the IAT.IANT-Lorr1r (a, c), there were significant effects of genotype (F1,109=163.0, p<10−20), sex (F1,109=26.2, p<10−5), and interaction (F1,109=4.8, p<0.05) on duration of LORR (a), and a significant genotype effect for BECRR (c; F1,109=74.9, p<10−13). There was only a significant effect of sex for LORR duration (F1,72=16.9, p<10−3) and BECRR (F1,72=9.4, p<0.01) in the IANT.IAT-Lorr1r rats (b, d). All congenic subjects were heterozygous for this experiment. Asterisks (*) indicate the significant genotype effects. The number in each group is shown in each bar.
Two-way ANOVA (genotype-by-sex) was conducted for LORR duration and BECRR independently for each backcross grouping (i.e., IAT.IANT and IANT.IAT). There were significant main effects of genotype (control vs. congenic), sex, and interaction (figure 3-a) for duration of LORR in the IAT.IANT animals. This was not the case when the IAT allele was introgressed onto an IANT background (figure 3-b); there was only a significant main effect of sex. Similarly, there was a significant genotype effect for BECRR in the IAT.IANT and not the IANT.IAT, but there were no sex or interaction effects in the IAT.IANT animals (figure 3-c); the sex effect was still present in the IANT.IAT (figure 3-d).
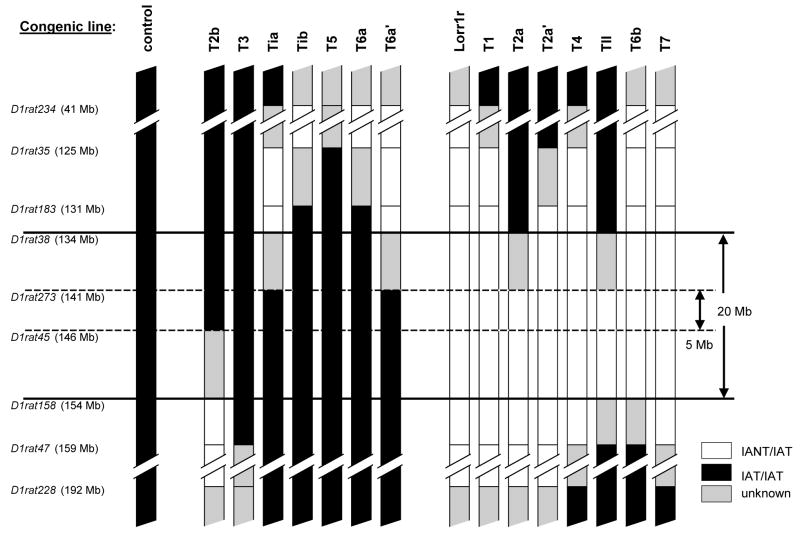
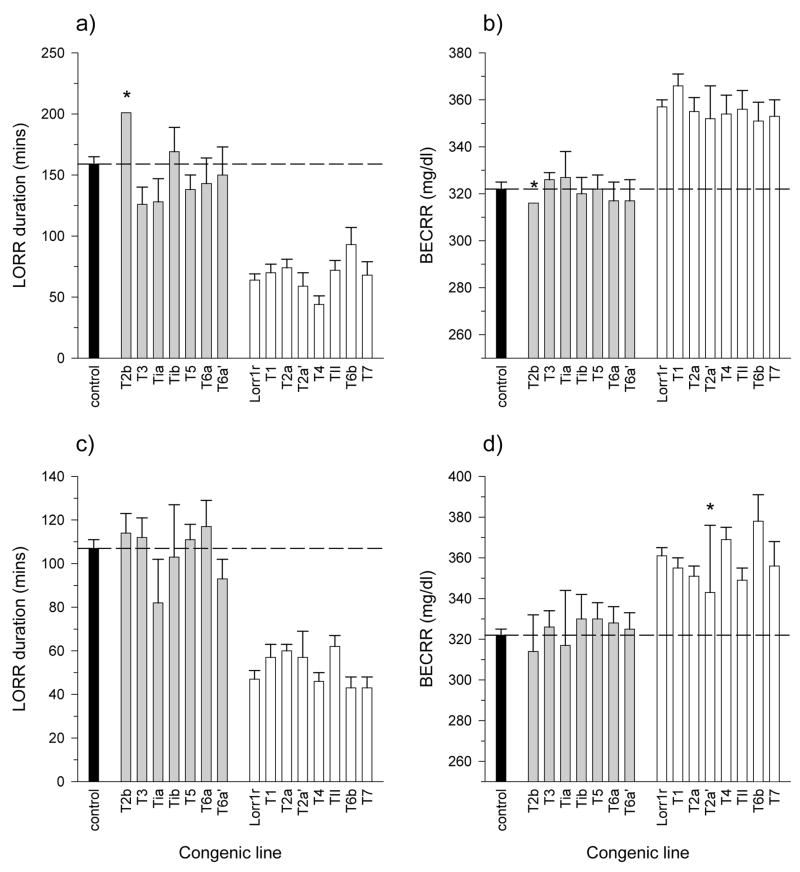
During the breeding of marker-assisted congenics, there will be some proportion of offspring that will be recombinant within the interval being introgressed. These animals can be identified and bred into separate sub-congenic lines and then tested as a way to reduce the size of the QTL-containing interval (Darvasi 1997). This strategy was used in the current study. Figure 4 illustrates the introgressed intervals of the sub-congenic lines that have been bred and tested to date. Only backcrosses of the IANT onto the IAT background were pursued because of the apparent dominance of the IANT allele (figures 2 and 3). The white areas show the IANT interval that was backcrossed onto an IAT background which is depicted in black. Grey indicates an interval that was flanked on one side by a heterozygous genotype (IAT/IANT) and on the other side by a homozygous genotype (IAT/IAT). These intervals contain a recombination whose location is unknown at this time. Lines T1, T3, T4, T5, T7, and TII were independently established directly from offspring of a cross between the IAT and the IAT.IANT-Lorr1r lines. All other sub-congenics were derived from another sub-congenic line. For example, lines T2a, T2a′, and T2b were bred from the offspring of a cross between the IAT and a sub-congenic line called T2 which initially was established similar to lines T1, T3, etc. Very few of the progenitors of these “sub-sub-congenics” were ever bred and/or phenotyped, and are therefore not shown in the figures (i.e., lines Ti, T2, and T6).
Figure 4.
Location of crossovers in the IAT.IANT recombinant sub-congenic lines. Lines on the right side of the figure (lines Lorr1r through T7) showed a significant effect of line on LORR duration and BECRR compared to the control line (far left); lines on the left side (lines T2b through T6a′) did not (see figure 5). The red areas indicate the IANT intervals that were introgressed onto an IAT background which is shown by black. Grey areas indicate the region in which there is a crossover between markers whose exact position is unknown. Markers used to characterize the lines are shown on the left. The solid and dashed horizontal lines show the maximum and minimum predicted QTL-containing intervals, respectively.
The sub-congenic lines shown in figure 4 were tested for LORR duration and BECRR; the results are shown in figure 5. These rats had been backcrossed for a total of 5 or 6 generations. Two-way ANOVA was used to determine if there was an overall effect of line or sex. There was a significant effect of line and sex on duration of LORR as well as a significant interaction (males: figure 5-a; females: figure 5-c). There was a significant effect of only line for BECRR (males: figure 5-b; females: figure 5-d). The ANOVA was followed by a Dunnett’s t post hoc analysis in which each congenic line was compared to the control line. The results are depicted graphically in figure 5. The lines that were significantly different from the control line are shown with white bars on the right side of each panel while those that were not different are shown with grey bars on the left side of the panel; the control line is indicated by a black bar on the far left. The separation of the lines was remarkably consistent across the two variables and sexes. There were two lines for which one of the sex groups had only one or two subjects, indicated in the figure by an asterisk (T2b males and T2a′ females, BECRR only). These were not included in the statistical analysis, but were included in the figure because the other sex group of these lines was found to be significantly different (T2a′ males) or not (T2b females) from their controls. In addition, the single or mean value of these two lines was very similar to other lines in their respective overall grouping. Note that LORR duration in the control lines is considerably longer for males compared to females, but the BECRR is nearly identical suggesting similar sensitivity, but different pharmacokinetic properties between the sexes.
Figure 5.
Duration of LORR and BECRR the IAT.IANT recombinant sub-congenic lines. Panels a and b show duration of LORR and BECRR, respectively, for males and panels c and d show duration of LORR and BECRR, respectively, for females. All subjects for the congenic lines were heterozygous. The white bars on the right side of each figure are significantly different than the black bar (control) with the exception of line T2a′ for BECRR which had an n of 2 and was therefore not included in the statistical analysis (females only, identified with an asterisk *). Note also that line T2b only had an n of 1 and therefore also was not included in the statistical analysis (males only, identified with an asterisk *). There were significant effects of line (F15,457=31.3, p<10−30), sex (F1,457=43.3, p<10−9), and interaction (F15,457=12.8, p<0.01) for LORR duration, and for line only for BECRR (F15,443=14.1, p<10−28). The number of subjects in each group were as follows (in some cases, BECRR samples were lost due to a failure of the BEC assay; the difference in n is shown in parentheses): males: control=66 (BECRR=64), T2b=1, T3=4, Tia=7, Tib=6 (BECRR=4), T5=12, T6a=10 (BECRR=9), T6a′=9, Lorr1r=37, T1=24, T2a=29, T2a′=4 (BECRR=3), T4=12, TII=20, T6b=8, T7=10; females: control=58 (BECRR=57), T2b=4 (BECRR=3), T3=9, Tia=3, Tib=4 (BECRR=3), T5=12, T6a=6 (BECRR=2), T6a′=8, Lorr1r=30, T1=19, T2a=26, T2a′=3 (BECRR=2), T4=16, TII=15, T6b=8, T7=13 (BECRR=9).
The results shown in figure 5 are recapitulated in figure 4: lines that were not different from control (solid black) are shown on the left and lines that were significantly different than control are shown on the right. The regions that were introgressed into lines whose phenotypes were not different than the control line can be eliminated from consideration for containing the QTL. Similarly, those areas that retain the IAT background in the lines that were different than the controls can also be eliminated. Using this sort of logic, the QTL can be localized between 134 and 154 Mb. The interval is probably substantially smaller since it is unlikely that the recombination event occurred right at the marker and also because there is a probability that the grey region (i.e., the area in which the recombination is known to be located) was reduced with each backcross. The smallest interval to which the QTL could be definitively located is between 141 and 146 Mb. The regions are defined by horizontal lines in the figure. The larger and smaller intervals contain 175 and 56 known and hypothesized genes, respectively (Twigger et al. 2007).
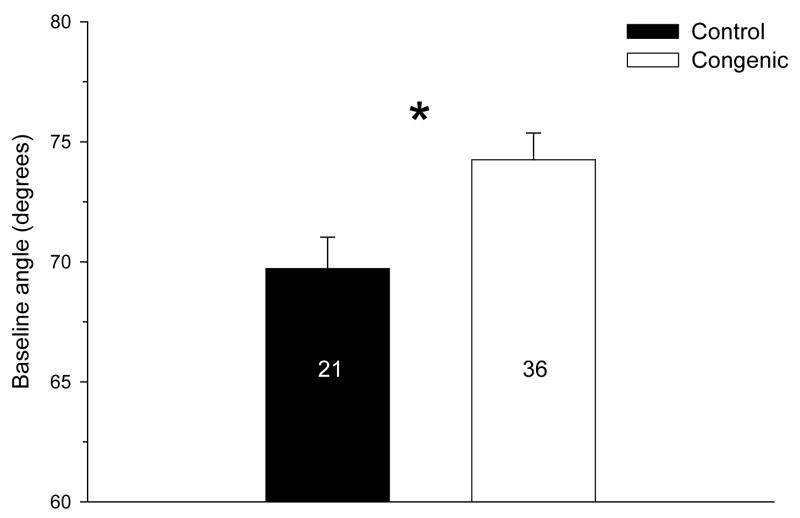
A small number of control and congenic animals were tested on the inclined plane. All rats used in this experiment were naive prior to being tested and not tested for any other phenotype. The animals were randomly selected from among the offspring of 5 of the sub-congenic lines that were significantly different than the control for LORR-related responses (T1, T2a, T4, T7, and TII). The control animals were the homozygous IAT littermates. Figure 6 shows the baseline angle for these animals, collapsed across sex. Two-way ANOVA (line-by-sex) indicated only a significant main effect of line.
Figure 6.
Inclined plane baseline angle in IAT.IANT congenics (lines T1, T2a, T4, T7, and TII). Asterisk (*) indicates significant effect of line (F1,53=8.4, p<0.01). The number in each group is shown in each bar.
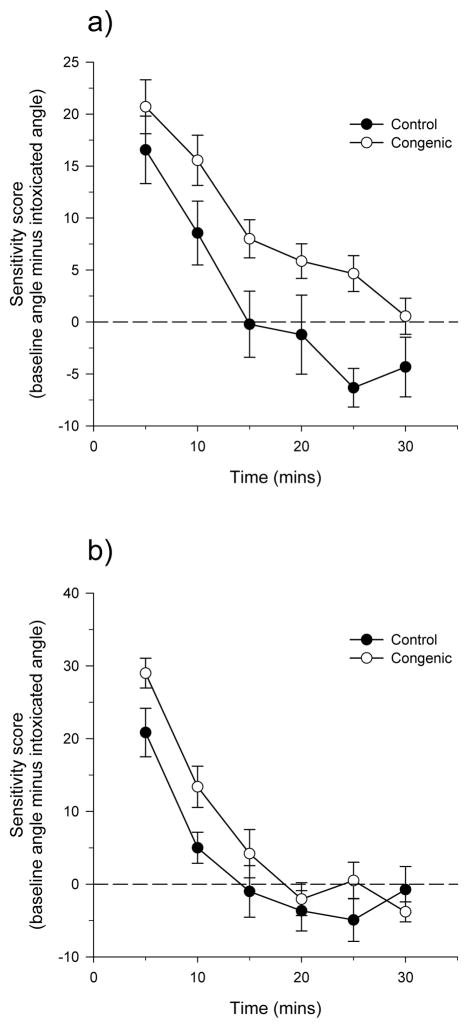
Following their baseline test, the rats were administered 2.0 g/kg alcohol and tested on the inclined plane every 5 minutes over a 30 minute period. Sensitivity scores – baseline angle minus intoxicated angle at each time point – are illustrated in figure 7. Three-way ANOVA (line-by-sex-by-time) indicated significant effects of genotype, time, and a time-by-sex interaction.
Figure 7.
Inclined plane sensitivity scores across time in male (a) and female (b) IAT.IANT congenics (same rats as in figure 6). Rats were administered 2.0 g/kg ethanol and tested at the times indicated for the angle at which they would slide to the base of the plane. This angle was subtracted from the baseline angle to derive the sensitivity score. There was a significant effect of time in both sexes and a significant effect of line in only males. The horizontal dashed line indicates the time at which the baseline angle is equivalent to the angle at 30 minutes after alcohol administration. There were significant effects of genotype (F5,265=3.0, p<0.05), time (F5,265=3.0, p<0.05), and a time-by-sex interaction (F5,265=3.0, p<0.05). The number of subjects in each group was: male controls, n=9; female controls, n=12; male congenics, n=20, female congenics, n=16.
Acute tolerance (REGAIN) was not specifically tested in the control and congenic rats shown in figure 7. This value was estimated, however, by fitting an exponential curve to the data from each individual animal and from that calculating the X-intercept (i.e., the time at which SENS30 is estimated to be equal to zero). The results are shown in table 5; values are collapsed across sex. Consistent with the difference in REGAIN in the IAT and IANT (table 1), the control animals had a shorter estimated REGAIN time than the congenics. This was significant as tested by a genotype-by-sex two-way ANOVA; there were no significant sex or interaction effects.
Table 5.
Estimated values of the time it took control and congenic rats to reach their original baseline angle after 2.0 g/kg (same subjects as shown in figures 6 and 7).
| Sex | Genotype | REGAIN ± SEM (seconds)1 |
|---|---|---|
| Males | Control | 1026 ± 57 |
| Congenic | 1574 ± 148 | |
| Females | Control | 980 ± 79 |
| Congenic | 1405 ± 229 |
REGAIN is the estimated time it took to regain the baseline angle after 2.0 g/kg alcohol. Significant main effect of line, p< 0.01F1,53=7.4, p<0.01. Values were determined as described in text.
Discussion
The hypothesis that an inherent low sensitivity to acute alcohol is an important risk factor for the development of alcoholism is well supported (Schuckit & Smith 2006). How this risk is actually manifested, however, is unclear. It has been postulated that low sensitivity to alcohol may permit an individual to drink more heavily early in their drinking career thereby accelerating the development of tolerance and/or the neuronal changes that trigger the progression from casual to pathologic drinking behavior (Schuckit et al., 2004). Low sensitivity also could promote social interactions with others who are already heavy drinkers thus reinforcing that this behavior is acceptable if not desirable (Schuckit et al., 2004). Alternatively, alcohol sensitivity may be pleiotropic with something else that is the true basis of its association to alcoholism risk; for example, some component of learning and memory (Chandler et al. 1998; Kelley 2004; Quadros et al. 2003; Radcliffe et al. 1998). Here, we have begun to dissect the genetic factors that contribute to two measures of acute sensitivity – the inclined plane and the widely used loss of righting reflex assay – with the goal of furthering our understanding of its genetic and molecular basis, and ultimately clarifying its role in alcoholism.
The AT and ANT lines were selected for their acute response on the inclined plane at a single time point which was 30 minutes after alcohol administration (Eriksson & Rusi 1981). Thus, they were not specifically selected for tolerance per se despite being called Tolerant and Non-Tolerant. However, in any acute paradigm such as this, tolerance can develop between the time of alcohol administration and the behavioral measurement, perhaps in more acute alcohol-response tests than is generally appreciated. The development of acute tolerance (REGAIN) was significantly greater in the IAT than in the IANT, an observation that was also made by Le and Kiianmaa (1989). It is thus possible if not likely that acute tolerance accounts for at least some proportion of the SENS30 difference between the lines, although differences in initial sensitivity cannot be ruled out. This is further supported by the positive phenotypic correlation between REGAIN and SENS30 among the F2 rats; i.e., the longer it takes to regain the baseline angle (decreased acute tolerance), the greater the 30 minute sensitivity. The development of acute tolerance has been noted for a variety of low- and high-dose measures in both rodents and humans, and may be an important component of most if not all genetic differences in acute alcohol responses (LeBlanc et al. 1975; O’Connor et al. 1999; Ponomarev & Crabbe 2004; Radcliffe et al. 2006; Radlow & Hurst 1985; Ramchandani et al. 2002; Varlinskaya & Spear 2006). Indeed, acute tolerance, as opposed to true initial sensitivity, may be the more relevant phenotype in the context of the relationship between acute sensitivity and alcoholism risk (Newlin & Thomson 1990).
T30ANGL was substantially less than BSLN1 in the IANT rats; thus, obviously, SENS30 was much greater than zero. However, it took less than 30 minutes (approximately 20 to 22 minutes) for the IANT to regain BSLN2, the baseline angle measured in week 2 as part of the acute tolerance phenotype. Learning-related tolerance may have contributed to this apparent discrepancy. In week 1, the animals were tested at only two time points – just prior to and then 30 minutes after alcohol – whereas in week 2 they were tested repeatedly until they regained the baseline angle. The repeated within-session testing may have contributed to learning-related tolerance, a phenomenon that has been well described (Le & Kalant 1992). In addition, the rats had been tested a week prior to the tolerance experiment which gave them yet another opportunity to develop some learning-related tolerance as well as the possibility of developing longer term functional tolerance (Le & Kalant 1990). These possibilities are supported by results from a previous test-order experiment that had been conducted on groups of IAT X IANT F2 rats (Radcliffe et al. 2004a). Rats that were tested for acute tolerance one week after the 30 minute sensitivity test in that experiment, exactly as has been done in the current experiment, showed greater acute tolerance than naïve rats.
A second indication that some learning may have taken place between week 1 and 2 was the slight but significant increase in the baseline angle in the IAT and IANT rats. A similarly small but significant increase in BSLN2 compared to BSLN1 (approximately 0.9 degrees) was observed in the F2 rats (data not shown). An even greater effect was seen at the chromosome 1 QTL (approximately 1.5 degrees; figure 2). Taken together, the results suggest that learning may have contributed to acute tolerance on the inclined plane when tested in week 2. The increase in baseline angle tested during week 2 (BSLN2) was similar in the IAT and IANT and also across all three chromosome 1 genotypes in the F2 rats (figure 2); however, the extent to which the development of learning-related tolerance differs in the IAT and IANT is not known since this was not specifically examined.
We previously mapped QTLs for LORR duration and BECRR in an F2 derived from the selectively bred High and Low Alcohol Sensitivity rats (Radcliffe et al. 2004b; Radcliffe et al. 2006). It was subsequently found that the basis of many these acute sensitivity QTLs was probably acute tolerance rather than initial sensitivity (Radcliffe et al. 2007). It may be that some or all of the SENS30 QTLs in the current study are also related to acute tolerance. This is consistent with the mapping of two suggestive QTLs for acute tolerance (REGAIN) to chromosomes 3 and 20 near to the SENS30 QTLs on those same chromosomes. In addition, the congenic results shown in table 5 are consistent with an acute tolerance QTL on chromosome 1 which was mapped at the suggestive level at this locus for SENS30. Note that REGAIN did not map to chromosome 1 in the F2 study which may have been the result of insufficient power to map this particular locus. Heritability in a congenic as compared to an F2 population is as much as three times greater which may have been why it was “mapped” in the congenic, but not in the F2 (J.K. Belknap, personal communication; Belknap 2003).
In addition to acute tolerance, another trait for which the IAT and IANT were probably inadvertently selected was the baseline angle. SENS30 is a composite of BSLN1 and T30ANGL, and therefore either or both variables theoretically could have responded to selection pressure. This seems to be the case for BSLN1 which is higher in the IANT than the IAT. This is what would have been predicted since an increase in BSLN1 would effectively increase the 30 minute sensitivity score in the IANT and vice versa for the IAT. The effect is even more pronounced at the chromosome 1 QTL in which both BSLN1 and BSLN2 were almost 4 degrees higher in the IANT genotypes than in the IAT genotypes.
In fact, if BSLN1 was under selective pressure as argued above, then it is curious as to why the IAT and IANT progenitors did not show at least a 4 degree difference in baseline angle. Bidirectional selection should have maximized the baseline difference between the lines, and a difference of 4 degrees apparently was possible from the chromosome 1 QTL alone. A clue may come from the positive correlation between BSLN1 and T30ANGL among the F2 (r = 0.43) which indicates that F2 rats with a high baseline tended to also have a relatively high angle of sliding after alcohol. It may be that some proportion of the genetic control over T30ANGL is not dissociable from the genes that control the baseline angle. This could be a result of pleiotropy, linkage disequilibrium, a direct functional relationship, or simply a consequence of starting from a high baseline angle. Whatever the underlying mechanism, the responsible factor(s) would then tend to negate the effects of genes that control the baseline angle independent of any alcohol-related effects; e.g., the chromosome 1 QTL.
The genome scan for the QTL analysis was only partial meaning that some QTLs or interactions may not have been detected. Nonetheless, many QTLs were identified for the various traits tested in the F2 population, although the majority of them were mapped at only the suggestive level; i.e., they should be viewed as preliminary until shown otherwise. The QTLs mapped on chromosome 1 for duration of LORR, BECRR, and baseline angle stand apart from the others because their effects were so large. Efforts were focused on this region following its discovery with an emphasis on the LORR-related responses. A variation of a breeding scheme originally proposed by Darvasi (1997) was used to confirm and fine-map this QTL. The results of this experiment confirm the QTL for LORR duration, BECRR, and baseline angle. Further, the QTL-containing interval has been reduced from approximately 65 Mb to less than 20 Mb.
An interesting question regarding the chromosome 1 QTLs is the extent to which they are functionally related. It is likely that the chromosome 1 QTLs for LORR duration and BECRR are mediated by the same gene or genes. Similarly, it is almost certain that the BSLN1 and BSLN2 QTLs have the same molecular underpinning. It is not so clear, however, if the genes that are responsible for the baseline QTLs are one and the same as those for the LORR-related responses. BSLN1 was confirmed in a subset of the congenics, although still within a fairly large interval. In addition, the observed correlations between LORR-related measures and inclined plane measures suggests some kind of genetic relationship between these variables; this has been discussed previously (Radcliffe et al. 2004a). Yet the measurement of the baseline angle is completely independent of any alcohol-related effects meaning that its QTL impacts normal balance or postural control and is not (necessarily) part of an alcohol-response pathway. It is thus possible that animals with the IANT genotype at the chromosome 1 QTL on the IAT background recover the righting reflex more quickly not because they were less sensitive to the higher dose of alcohol, but because their apparent greater postural capabilities allow them to regain the righting reflex sooner than the IAT genotypes. However, it seems unlikely that this could account completely for the very large effect on LORR duration and BECRR that is mediated by this QTL.
A second possibility is that the LORR-related QTL and the baseline angle QTL are not actually the same. The congenic results and the significant correlation between the measures could have been the result of linkage disequilibrium. It is not possible to assess this interpretation with the current results, but one finding that does support it is that the nature of the QTL allele effects was different among the traits. The QTL mapping results as well as the congenic results suggest a model in which the IANT allele is dominant for both duration of LORR and BECRR. For baseline angle, however, the IAT and IANT alleles are additive. This result is consistent with distinct genes for the QTLs, although pleiotropy at some level cannot be ruled out.
As discussed above, the chromosome 1 QTL for baseline angle was not necessarily related to alcohol responses. This is consistent with the absence of a QTL for T30ANGL in this region of chromosome 1. In addition, interval mapping for SENS30 with BSLN1 as a covariate completely eliminates the chromosome 1 SENS30 QTL which also suggests that this was not an alcohol responsive QTL (data not shown). However, there was a main effect of line on alcohol sensitivity in the inclined plane experiment that was conducted in the congenics (figure 7). It is interesting that there was no difference between the control and congenic lines for the sensitivity score at 30 minutes, although the experiment was conducted differently than for the SENS30 phenotype. Together, these results cannot conclusively determine the extent to which the chromosome 1 QTL influenced specifically alcohol effects on the inclined plane.
It is notable that no QTLs mapped near 27 Mb or anywhere else on chromosome 10 which is where the gene for the α6 subunit of the GABAA chloride channel is located (Gabra6; Rat Genome Database 2008). There also were no significant interactions between the α6 locus and any other locus. This was true for the inclined plane tests as well as the LORR-related measures. It was not because the two α6 alleles were not present since the grandparents of the F2 were deliberately selected to ensure that the F2 included these alleles. Indeed, the three genotypes were found to be present at a ratio of approximately 25%:50%:25% in a random sample of 72 of the F2 rats suggesting that the alleles were distributed throughout the population at their expected Mendelian frequency (Botta et al. 2007b). The absence of QTLs or interactions on chromosome 10 suggests that the aforementioned α6 mutation does not contribute to genetic differences in inclined plane sensitivity in the IAT and IANT or in the acute alcohol responses that were tested in the F2 animals, although the possibility that the mutation contributes to an undetectably small portion of the genetic variance cannot be ruled out. It is also possible that interactions could have occurred with loci that were not included in the genome scan. These results are more thoroughly discussed in Botta et al. (2007b; 2008).
We had previously mapped a LORR-related QTL to the same region of chromosome 1 in an F2 intercross of the Inbred High and Low Alcohol Sensitivity rat lines (IHAS, ILAS) which were selectively bred for a differential duration of LORR phenotype (Draski et al. 1992; Radcliffe et al. 2004b). However, this QTL failed to replicate in a follow-up study of an independent group of IHAS X ILAS F2 rats (Radcliffe et al. 2006). Whether the putative IHAS/ILAS QTL and the current chromosome 1 QTL have anything in common remains to be determined. Beyond that, 60 rat QTLs that overlap with the 20 Mb chromosome 1 interval have been mapped (Rat Genome Database 2008). None, however, show an obvious functional relationship to LORR or to the baseline angle measurement. Similarly, there are no obvious QTLs from the mouse (chromosome 7, 86.1–104.4 Mb) or human (chromosome 1, 245.9246.0 Mb; chromosome 11, 77.7–88.9 Mb; chromosome 15, 78.1–80.4 Mb, 81.1–83.5 Mb, 86.1–89.4 Mb) that map to the syntenic region of the rat interval (Entrez Gene 2008).
The absence of any alcohol-response or related QTLs that overlap with or are syntenic with the current rat chromosome 1 QTL was not surprising. The specific alleles that are driving this QTL may be unique to the IAT and IANT or alternatively they may not have been present in other mapping populations in which relevant traits were examined. The underlying mechanisms that control mammalian behavioral or cognitive traits undoubtedly engage dozens if not hundreds of gene products, any one of which could potentially have more than one allele controlling its expression or function. Thus, for a given trait, even one as seemingly simple as LORR, many QTLs will be mapped among differing populations. The underlying genes, however, are postulated to be components of a larger common pathway(s). Our goals in the project described here are to identify QTLs for acute alcohol responses, identify the underlying genes for these QTLs, and, perhaps most importantly, elucidate the pathways in which these genes are found. This report describes a degree of success for the first goal. The second goal is certainly within the realm of possibility given the availability of powerful genomic tools such as high-throughput sequencing and high-density gene expression microarrays (DiPetrillo et al. 2005). With knowledge of these genes, it will then be possible to decipher the pathways controlling acute alcohol sensitivity which ultimately will help in gaining a more complete understanding and appreciation of the relationship between acute alcohol sensitivity and alcoholism in humans.
Supplementary Material
Acknowledgments
Supported by NIAAA grants RO1 AA12650, R01 AA13177, R37 AA10556, and K05 AA00093.
Reference List
- Belknap JK. Chromosome substitution strains: some quantitative considerations for genome scans and fine mapping. Mamm Genome. 2003;14:723–732. doi: 10.1007/s00335-003-2264-1. [DOI] [PubMed] [Google Scholar]
- Botta P, Mameli M, Floyd KL, Radcliffe RA, Valenzuela CF. Ethanol sensitivity of GABAergic currents in cerebellar granule neurons is not affected by a single amino acid change (R100Q) in the α6 GABAA receptor subunit. J Pharmacol Exp Ther. 2007a;323:684–691. doi: 10.1124/jpet.107.127894. [DOI] [PubMed] [Google Scholar]
- Botta P, Mameli M, Valenzuela CF, Radcliffe RA. Reply to Comment on “Ethanol Sensitivity of GABAergic Currents in Cerebellar Granule Neurons is not Increased by a Single Amino Acid Change (R100Q) in the α6 GABAA Receptor Subunit”. J Pharmacol Exp Ther. 2008;324:401–403. doi: 10.1124/jpet.107.127894. [DOI] [PubMed] [Google Scholar]
- Botta P, Radcliffe RA, Carta M, Mameli M, Daly E, Floyd KL, Deitrich RA, Valenzuela CF. Modulation of GABAA receptors in cerebellar granule neurons by ethanol: Genetic and electrophysiological studies. Alcohol. 2007b;41:187–199. doi: 10.1016/j.alcohol.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman KW, Wu H, Sen Œ, Churchill GA. R/qtl: QTL mapping in experimental crosses. Bioinformatics. 2003;19:889–890. doi: 10.1093/bioinformatics/btg112. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Harris RA, Crews FT. Ethanol tolerance and synaptic plasticity. Trends Pharmacol Sci. 1998;19:491–495. doi: 10.1016/s0165-6147(98)01268-1. [DOI] [PubMed] [Google Scholar]
- Churchill GA, Doerge RW. Empirical threshold values for quantitative trait mapping. Genetics. 1994;138:963–971. doi: 10.1093/genetics/138.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvasi A. Interval-specific congenic strains (ISCS): an experimental design for mapping a QTL into a 1-centimorgan interval. Mamm Genome. 1997;8:163–7. doi: 10.1007/s003359900382. [DOI] [PubMed] [Google Scholar]
- Darvasi A, Soller M. Selective genotyping for determination of linkage between a marker locus and a quantitative trait locus. Theor Appl Genet. 1992;85:353–359. doi: 10.1007/BF00222881. [DOI] [PubMed] [Google Scholar]
- DiPetrillo K, Wang X, Stylianou IM, Paigen B. Bioinformatics toolbox for narrowing rodent quantitative trait loci. Trends Genet. 2005;21:683–692. doi: 10.1016/j.tig.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Draski LJ, Spuhler KP, Erwin VG, Baker RC, Deitrich RA. Selective breeding of rats differing in sensitivity to the effects of acute ethanol administration. Alcohol Clin Exp Res. 1992;16:48–54. doi: 10.1111/j.1530-0277.1992.tb00634.x. [DOI] [PubMed] [Google Scholar]
- Ensembl (2008) [WWW document]. URL http://archive.ensembl.org/ (Date of last access : October 2008).
- Entrez Gene (2008) [WWW document]. URL http://www.ncbi.nlm.nih.gov/sites/entrez?db=gene (Date of last access : October 2008).
- Eriksson K, Rusi M. Finnish selection studies on alcohol-related behaviors: General outline. In: McClearn GE, Deitrich RA, Erwin VG, editors. Development of Animal Models as Pharmacogenetic Tools. NIAAA Research Monograph; Rockville, Maryland: 1981. pp. 87–117. [Google Scholar]
- Feenstra B, Skovgaard IM, Broman KW. Mapping quantitative trait loci by an extension of the Haley-Knott regression method using estimating equations. Genetics. 2006;173:2111–2119. doi: 10.1534/genetics.106.058537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley CS, Knott SA. A simple regression method for mapping quantitative trait loci in line crosses using flanking markers. Heredity. 1992;69:315–324. doi: 10.1038/hdy.1992.131. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity. Nature Neurosci. 2005;8:339–345. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Madden PAF, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, Rohrbaugh JW, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol Med. 1999;29:1069–1081. doi: 10.1017/s0033291799008909. [DOI] [PubMed] [Google Scholar]
- Hellevuo K, Kiianmaa K, Korpi ER. Effect of GABAergic drugs on motor impairment from ethanol, barbital and lorazepam in rat lines selected for differential sensitivity to ethanol. Pharmacol Biochem Behav. 1989;34:399–404. doi: 10.1016/0091-3057(89)90333-x. [DOI] [PubMed] [Google Scholar]
- Hellevuo K, Korpi ER. Failure of Ro 15–4513 to antagonize ethanol in rat lines selected for differential sensitivity to ethanol and in Wistar rats. Pharmacol Biochem Behav. 1988;30:183–188. doi: 10.1016/0091-3057(88)90441-8. [DOI] [PubMed] [Google Scholar]
- Kaheinen P, Korpi ER, Pyykko I, Mantysalo S, Ignatius J. Hippocampal rhythmic slow activity in rat lines selected for differences in ethanol-induced motor impairment. Pharmacol Biochem Behav. 1988;30:177–181. doi: 10.1016/0091-3057(88)90440-6. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–79. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Keingoor C, Kettenmann H, Seeburg PH. Benzodiazepine-induced motor impairment linked to point mutation in cerebellar GABAA receptor. Nature. 1993;361:356–359. doi: 10.1038/361356a0. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Seeburg PH. Natural mutation of GABAA receptor α6 subunit alters benzodiazepine affinity but not allosteric GABA effects. Eur J Pharmacol Mol Pharmacol. 1993;247:23–27. doi: 10.1016/0922-4106(93)90133-t. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Uusi-Oukari K, Wegelius K, Casanova M, Zito M, Kleinman JE. Cerebellar and frontal cortical benzodiazepine receptors in human alcoholics and chronically alcohol-drinking rats. Biol Psychiatry. 1992a;31:774–786. doi: 10.1016/0006-3223(92)90309-n. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Uusi-Oukari K, Castren E, Suzdak PD, Seppala T, Sarviharju M, Tuominen K. Cerebellar GABAA receptors in two rat lines selected for high and low sensitivity to moderate alcohol doses: Pharmacological and genetic studies. Alcohol. 1992b;9:225–231. doi: 10.1016/0741-8329(92)90058-i. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Uusi-Oukari M, Wegelius K. Substrate specificity of diazepam-insensitive cerebellar [3H] Ro 15–4513 binding sites. Eur J Pharmacol. 1992c;213:323–329. doi: 10.1016/0014-2999(92)90620-j. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Uusi-Oukari M. GABAA receptor-mediated chloride flux in brain homogenates from rat lines with differing innate alcohol sensitivities. Neurosci. 1989;32:387–392. doi: 10.1016/0306-4522(89)90087-0. [DOI] [PubMed] [Google Scholar]
- Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nature Genetics. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- Le AD, Kalant H. Learning as a factor in ethanol tolerance. NIDA Res Monograph. 1990;97:193–207. [PubMed] [Google Scholar]
- Le AD, Kalant H. Influence of intoxicated practice on the development of acute tolerance to the motor impairment effect of ethanol. Psychopharmacologia. 1992;106:57257–6. doi: 10.1007/BF02244833. [DOI] [PubMed] [Google Scholar]
- Le AD, Kiianmaa K. Initial sensitivity and the development of acute and rapid tolerance to ethanol in the AT and ANT rats. In: Kiianmaa K, Tabakoff B, Saito T, editors. Genetic Aspects of Alcoholism. Vol. 37. The Finnish Foundation for Alcohol Studies; Helsinki, Finland: 1989. pp. 147–155. [Google Scholar]
- LeBlanc AE, Kalant H, Gibbins RJ. Acute tolerance to ethanol in the rat. Psychopharmacologia. 1975;41:43–46. doi: 10.1007/BF00421304. [DOI] [PubMed] [Google Scholar]
- Lewis RR, Kunz AL, Bell RE. Error of intraperitoneal injections in rats. Lab Anim Care. 1966;16:505–509. [PubMed] [Google Scholar]
- Mäkelä R, Wong G, Luddens H, Korpi ER. Phenotypic and genotypic analysis of rats with cerebellar GABAA receptors composed from mutant and wild-type α6 subunits. J Neurochem. 1995;65:2401–2408. doi: 10.1046/j.1471-4159.1995.65062401.x. [DOI] [PubMed] [Google Scholar]
- Malminen O, Korpi ER. GABA/benzodiazepine receptor/chloride ionophore complex in brains of rat lines selectively bred for differences in ethanol-induced motor impairment. Alcohol. 1988;5:239–249. doi: 10.1016/0741-8329(88)90059-6. [DOI] [PubMed] [Google Scholar]
- Malminen O, Korpi ER. Diazepam-insensitive [3H] Ro 15–4513 binding in intact cultured cerebellar granule cells. Eur J Pharmacol. 1989;169:53–60. doi: 10.1016/0014-2999(89)90816-9. [DOI] [PubMed] [Google Scholar]
- Manly KF, Cudmore RH, Meer JM. Map Manager QTX, cross-platform software for genetic mapping. Mamm Genome. 2001;12:930–932. doi: 10.1007/s00335-001-1016-3. [DOI] [PubMed] [Google Scholar]
- Mouse Genome Database (MGD) The Jackson Laboratory, Bar Harbor, Maine (2008) [WWW document]. URL http://www.informatics.jax.org (Date of last access: October 2008).
- Näkki R, Wong G, Korpi ER. [3H] MK-801 binding in various brain regions of rat lines selected for differential alcohol sensitivity. Alcohol. 1995;12:335–340. doi: 10.1016/0741-8329(95)00013-h. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- O’Connor S, Sorbel J, Morzorati S, Li TK, Christian JC. A twin study of genetic influences on the acute adaptation of the EEG to alcohol. Alcohol Clin Exp Res. 1999;23:494–501. [PubMed] [Google Scholar]
- Otis TS. Comments on “Ethanol Sensitivity of GABAergic Currents in Cerebellar Granule Neurons Is Not Increased by a Single Amino Acid Change (R100Q) in the α6 GABAA Receptor Subunit”. J Pharmacol Exp Ther. 2008;324:399–400. doi: 10.1124/jpet.107.131557. [DOI] [PubMed] [Google Scholar]
- Ponomarev I, Crabbe JC. Characterization of acute functional tolerance to the hypnotic effects of ethanol in mice. Alcohol Clin Exp Res. 2004;28:991–997. doi: 10.1097/01.alc.0000131978.79857.5e. [DOI] [PubMed] [Google Scholar]
- Quadros IMH, Souza-Formigoni MLO, Fornari RV, Nobrega JN, Oliveira MGM. Is behavioral sensitization to ethanol associated with contextual conditioning in mice? Behav Pharmacol. 2003;14:129–136. doi: 10.1097/00008877-200303000-00004. [DOI] [PubMed] [Google Scholar]
- Radcliffe RA, Bludeau P, Asperi W, Fay T, Deng XS, Erwin VG, Deitrich RA. Confirmation of quantitative trait loci for ethanol sensitivity and neurotensin receptor density in crosses derived from the Inbred High and Low Alcohol Sensitive selectively bred rat lines. Psychopharmacology. 2006;188:343–354. doi: 10.1007/s00213-006-0512-2. [DOI] [PubMed] [Google Scholar]
- Radcliffe RA, Bludeau P, Deng XS, Erwin VG, Deitrich RA. Short-term selection for acute ethanol tolerance and sensitization from an F2 population derived from the High and Low Alcohol Sensitive selectively bred rat lines. Alcohol. 2007;41:557–566. doi: 10.1016/j.alcohol.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliffe RA, Floyd KL, Lee MJ. Rapid ethanol tolerance mediated by adaptations in acute tolerance in inbred mouse strains. Pharmacol Biochem Behav. 2006;84:524–534. doi: 10.1016/j.pbb.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Radcliffe RA, Erwin VG, Draski L, Hoffmann S, Edwards J, Deng XS, Bludeau P, Fay T, Lundahl KR, Asperi W, Deitrich RA. QTL mapping for ethanol sensitivity and neurotensin receptor density in an F2 intercross derived from Inbred High and Low Alcohol Sensitivity selectively bred rat lines. Alcohol Clin Exp Res. 2004b;28:1796–1804. doi: 10.1097/01.alc.0000148106.71801.d7. [DOI] [PubMed] [Google Scholar]
- Radcliffe RA, Hoffmann SE, Deng XS, Asperi W, Fay T, Bludeau P, Erwin VG, Deitrich RA. Behavioral characterization of Ethanol-tolerant and Ethanol-nontolerant rat lines and an F2 generation. Behav Genet. 2004a;34:453–463. doi: 10.1023/B:BEGE.0000023650.32243.39. [DOI] [PubMed] [Google Scholar]
- Radlow R, Hurst PM. Temporal relations between blood alcohol concentration and alcohol effect: an experiment with human subjects. Psychopharmacologia. 1985;85:260–266. doi: 10.1007/BF00428184. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Flury L, Morzorati SL, Kareken D, Blekher T, Foroud T, Li TK, O’Connor S. Recent drinking history: association with family history of alcoholism and the acute response to alcohol during a 60 mg% clamp. J Stud Alcohol. 2002;63:734–744. doi: 10.15288/jsa.2002.63.734. [DOI] [PubMed] [Google Scholar]
- Rat Genome Database, Medical College of Wisconsin, Milwaukee, Wisconsin (2008) Rat QTLs [WWW document] URL http://rgd.mcw.edu/ (Date of last access: October 2008).
- Schuckit MA. Biological, psychological, and environmental predictors of the alcoholism risk: A longitudinal study. J Stud Alcohol. 1998;59:485–494. doi: 10.15288/jsa.1998.59.485. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An evaluation of the level of response to alcohol, externalizing symptoms, and depressive symptoms as predictors of alcoholism. J Stud Alcohol. 2006;67:215–227. doi: 10.15288/jsa.2006.67.215. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Anderson KG, Brown SA. Testing the level of response to alcohol: social information processing model of alcoholism risk – a 20-year prospective study. Alcohol Clin Exp Res. 2004;28:1881–1889. doi: 10.1097/01.alc.0000148111.43332.a5. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko G, Kuperman S, Bierut LJ, Hesselbrock V. Correlations among first-degree relatives for responses on the Self-Rating of the Effects of Alcohol (SRE) Questionnaire in teenagers. J Stud Alcohol. 2005;66:62–65. doi: 10.15288/jsa.2005.66.62. [DOI] [PubMed] [Google Scholar]
- Sellin LC, Laakso PS. Effect of ethanol on motor performance and hippocampal population spikes in some standard and selectively outbred rat strains. Alcohol Clin Exp Res. 1987;11:502–505. doi: 10.1111/j.1530-0277.1987.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Sheppard, K., Wu, H., Wu, L., Smith, R. & Churchill, G.A. (2008) J/qtl, version 1.2.0 [WWW document]. URL http://research.jax.org/faculty/churchill/software/Jqtl (Date of last access: July 2008).
- Soller M, Brody T, Genizi A. On the power of experimental designs for the detection of linkage between marker loci and quantitative loci in crosses between inbred lines. Theor Appl Genet. 1976;47:35–39. doi: 10.1007/BF00277402. [DOI] [PubMed] [Google Scholar]
- Twigger SN, Shimoyama M, Bromberg S, Kwitek AE, Jacob HJ RGD Team. The Rat Genome Database, update 2007 – easing the path from disease to data and back again. Nucleic Acids Res. 2007;35:D658–62. doi: 10.1093/nar/gkl988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusi-Oukari M, Korpi ER. Cerebellar GABAA receptor binding and function in vitro in two rat lines developed for high and low alcohol sensitivity. Neurochem Res. 1989;14:733–739. doi: 10.1007/BF00964950. [DOI] [PubMed] [Google Scholar]
- Uusi-Oukari M, Korpi ER. Functional properties of GABAA receptors in two rat lines selected for high and low alcohol sensitivity. Alcohol. 1992;9:261–269. doi: 10.1016/0741-8329(92)90063-g. [DOI] [PubMed] [Google Scholar]
- Uusi-Oukari M, Korpi ER. Diazepam sensitivity of the binding of an imidazobenzodiazepine, [3H]R0 15–4513, in cerebellar membranes from two rat lines developed for high and low alcohol sensitivity. J Neurochem. 1990;54:1980–1987. doi: 10.1111/j.1471-4159.1990.tb04901.x. [DOI] [PubMed] [Google Scholar]
- Uusi-Oukari M, Korpi ER. Specific alterations in the cerebellar GABAA receptors of an alcohol-sensitive ANT rat line. Alcohol Clin Exp Res. 1991;15:241–248. doi: 10.1111/j.1530-0277.1991.tb01864.x. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Ontogeny of acute tolerance to ethanol-induced social inhibition in Sprague-Dawley rats. Alcohol Clin Exp Res. 2006;30:1833–1844. doi: 10.1111/j.1530-0277.2006.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viken RJ, Rose RJ, Morzorati SL, Christian JC, Li T-K. Subjective intoxication in response to alcohol challenge: Heritability and covariation with personality, breath alcohol level, and drinking history. Alcohol Clin Exp Res. 2003;27:795–803. doi: 10.1097/01.ALC.0000067974.41160.95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.