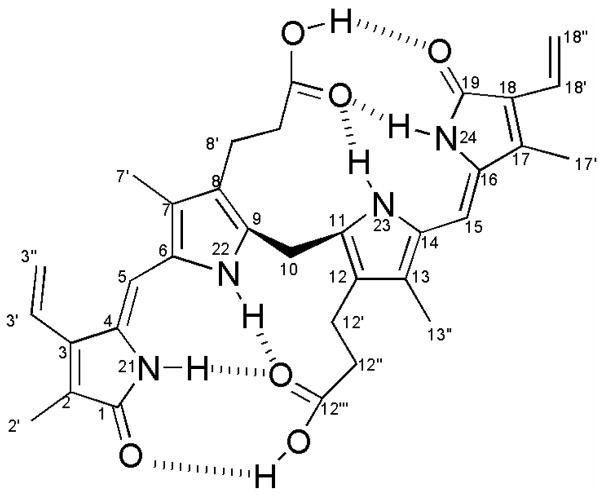
Cyclic tetrapyrroles occur throughout the plant kingdom and include vital biosynthetic products such as chlorophyll and heme. In plants, oxidative degradation of heme forms first biliverdin-IXα and subsequently phytochromobilin, the precursor of the phytochrome chromophore, an essential light sensing molecule1. In animals, oxidative degradation of heme also leads to the formation of biliverdin-IXα, but it is transformed into the yellow-orange pigment bilirubin-IX α. Here, we present spectroscopic and chromatographic evidence that bilirubin (Figure 1) is the major pigment of the orange aril of Strelitzia nicolai Regel & Koern. (Strelitziaceae, order Zingiberales), the white bird of paradise tree.
Figure 1.
Bilirubin-IXα demonstrating intramolecular H-bonding.
This is the first example of bilirubin in a plant2, a finding which likely necessitates the revision of the plant tetrapyrrole pathway since there is currently no known mechanism of bilirubin production in the plant kingdom.
S. nicolai is native to South Africa and widely cultivated in the tropics. It produces woody capsular fruits which contain orange arillate seeds. Analytical high-performance liquid chromatography (HPLC) of the aril extract3 revealed one major peak, which had a UV-visible spectrum with a maximum absorbance at 444 nm. After purification using preparative scale HPLC, the isolated pigment was analyzed by 1H NMR, 13C NMR (Brucker, 400 MHz, (CD3)2S=O), and liquid chromatography-positive ion electrospray mass spectrometry (LC-ESI), (Thermo-Finnigan LCQ).
The 1H NMR and 13C NMR spectra of the isolated pigment matched published values of authentic bilirubin4,5,10 (Tables S1 and S2). Identification was further supported by 1H NMR analysis of bilirubin standard (Aldrich). This yielded a spectrum which matched that of the S. nicolai pigment (Figure S1). Both the positive ion ES mass spectrum and the product ion spectrum matched those of authentic bilirubin standard and previous published data6 (molecular ion, m/z 585 (M + H)+, product ion m/z 299).
Given the unexpected discovery of bilirubin in plants, it was essential to confirm the identity of the pigment as bilirubin-IX α, and not other isomers. Previous chromatographic studies have demonstrated that the ability of bilirubin-IX α to undergo intramolecular hydrogen bonding makes it significantly less polar than bilirubin-IX β,γ or δ7,8 (Figure 1).
In our HPLC analyses, a single peak was observed when bilirubin-IX α standard was co-injected with the isolated pigment, thereby eliminating the possibility that the pigment was bilirubin-IX β,γ or δ. Furthermore, the visible spectrum of bilirubin-IX α has an intense peak at 458 nm in dimethylsulfoxide (DMSO), which is approximately 50 nm longer than bilirubin-IX β,γ or δ7. Other bilirubin isomers, including bilirubin-IIIα and bilirubin-XIIIα, were eliminated because their 1H NMR spectra are substantially different9.
The occurrence of bilirubin is not restricted to S. nicolai. Two other species in the Strelitziaceae, Phenakospermum guyanense Endl., and S. reginae Aiton, the bird of paradise, contain aril pigments which co-eluted with authentic bilirubin in HPLC and had similar UV-visible spectra. We are currently examining species in related families. This information, in combination with studies on the synthesis of bilirubin-IXα in S. nicolai, will provide the basis for a more thorough understanding of the evolutionary origin of this pigment in plants.
Supplementary Material
Acknowledgments
This research has been funded in part by the United States Environmental Protection Agency (EPA) under the Greater Research Opportunities (GRO) Graduate Program, the McBryde Science Program at the National Tropical Botanical Garden, and the RISE Biomedical Research Initiative (NIH). We thank the Center for Ethnobiology and Natural Products (CeNAP) at FIU for lab facilities.
Footnotes
Supporting Information Available: Methods, UV-VIS spectrum, and 1H and 13C NMR data. This material is available free of charge on the internet at http://pubs.acs.org.
References
- 1.Tanaka R, Tanaka A. Ann Rev Plant Biol. 2007;58:321–346. doi: 10.1146/annurev.arplant.57.032905.105448. [DOI] [PubMed] [Google Scholar]
- 2.Willows RD, Cuttriss A, Pogson B, Schwinn KE, Davies KM, Zryd JP, Christinet L. In: Plant Pigments and Their Manipulation. Davies KM, editor. Chapters 2–7 Blackwell; Oxford, UK: 2004. [Google Scholar]
- 3.Spivak W, Yuey W. Biochem J. 1986;234:101–109. doi: 10.1042/bj2340101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuenzle C. Biochem J. 1970;119:395–409. doi: 10.1042/bj1190395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan D, Navon G. Org Mag Res. 1980;13:59–62. [Google Scholar]
- 6.Lightner DA. In: Bilirubin. Heirwegh KPM, Brown SB, editors. Chapter 1 CRC Press; Boca Raton, FL: 1982. p. 1. [Google Scholar]
- 7.Blanckeart N, Heirwegh KPM, Compernolle F. Biochem J. 1976;155:405–417. doi: 10.1042/bj1550405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnett R, Davies JE, Hursthouse MB, Sheldrich GM. Proc R Soc Lond B. 1978;202:249–268. doi: 10.1098/rspb.1978.0066. [DOI] [PubMed] [Google Scholar]
- 9.McDonagh AF, Palma LA, Lightner DA. J Am Chem Soc. 1982;104:6865–6867. [Google Scholar]
- 10.Muller N. Magn Reson Chem. 1985;23:688–689. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.