Abstract
The greenhouse gas N2O is converted to N2 by a µ-sulfido-tetracopper active site in the enzyme nitrous oxide reductase (N2OR) via a process postulated to involve µ-1,3 coordination of N2O to two Cu(I) ions. In efforts to develop synthetic models of the site with which to test mechanistic hypotheses, we have prepared a localized mixed valent Cu(II)Cu(I)2 cluster bridged in µ-η2:η1:η1 fashion by disulfide, [L3Cu3(µ3-S2)]X2 (L = 1,4,7-trimethyl-triazacyclononane, X = O3SCF3 − or SbF6 −). This cluster exhibits spectroscopic features similar to those of the active site in N2OR and reacts with N2O to yield N2 in a reaction that models the function of the enzyme. Computations implicate a transition state structure that features µ-1,1-bridging of N2O via its O-atom to a [L2Cu2(µ-S2)]+ fragment and provide chemical precedence for an alternative pathway for N2O reduction by N2OR.
Nitrous oxide (N2O) is an important greenhouse gas and component of the global nitrogen cycle.1 Its reduction to dinitrogen (N2) is thermodynamically favorable (E° = 1.76 V), making it attractive as an environmentally benign oxidant, yet its utility in this regard is limited by high kinetic barriers that limit reaction rates. Transition metals facilitate the reduction of N2O, although in most heterogeneous catalytic systems high temperatures are required2 and homogeneous processes that operate under mild conditions generally use highly reducing low-valent metal complexes.3–5 In Nature, conversion of N2O to N2 and H2O is catalyzed under ambient conditions during microbial dentrification by the metalloenzyme nitrous oxide reductase, N2OR.6 X-ray crystallographic,7 spectroscopic, and theoretical studies8 have identified the active site of N2OR as a µ-sulfido-tetracopper cluster without precedent in biology or synthetic chemistry, which cycles through tetracopper(I) and mixed-valent states during catalysis.9 A provocative mechanism for N2O reduction has been suggested that involves µ-1,3-coordination and bending of N2O between two of the copper ions in the fully reduced (all copper(I)) cluster, with the µ-sulfide acting to facilitate electron delocalization during the redox process. 9 Inspired by a desire to test this mechanistic hypothesis and to better understand the properties of the intrinsically novel active site [(His)7Cu4S] cluster, multicopper-sulfur complexes supported by N-donor ligands have been targeted for synthesis and characterization.10 To date, however, none contain reduced copper or react with N2O. We have resolved these shortcomings, and report the characterization of a unique mixed-valent tricopper cluster bridged by disulfide that converts N2O to N2 at low temperature. A novel pathway for the reaction is suggested on the basis of theory that has potential implications for understanding the N2OR enzyme mechanism.
Reaction of Na2S2 with [LCu(CH3CN)]X (L = 1,4,7-trimethyltriazacyclononane, X = O3SCF3 − or SbF6 −)11 in THF at room temperature over ca. 90 min resulted in the formation of known complex 2a11b or new variant 2b (Figure 1). The structures and spectroscopic properties of these complexes are similar to each other and to those of others with antiferromagnetically coupled (µ-η2:η2-disulfido)dicopper(II) cores.11b,12 For example, they are EPR silent and exhibit an intense S2 2− → Cu(II) charge transfer transition at ~395 nm (ε ~15,000 M−1cm−1, Figure 1c, red line), excitation into which (λex 406.7 or 457.0 nm) results in resonance enhancement of a peak in the Raman spectrum at ~431 cm−1 (Δ34S = 19 cm−1) attributable to an S-S stretching mode.
Figure 1.
a) Synthesis of disulfido complexes. b) X-ray structure of cationic portion of 1b, with all nonhydrogen atoms shown as 50% thermal ellipsoids. Selected bond distances: Cu1-S1, 2.1416(8) Å; Cu2-S2, 2.1367(8) Å; Cu3-S1, 2.2548(8) Å; Cu3-S2 = 2.2751(8) Å; S1-S2, 2.1267(10) Å. c) UV/Vis spectra obtained during the reaction of [LCu(MeCN)]O3SCF3 (3.1 mM, black line) with Na2S2 in THF. The green line is the spectrum obtained after 90 min at −20 °C (1b), which transforms upon warming to rm temp to 2b (red line).
Monitoring the reactions by UV-vis spectroscopy revealed the formation and subsequent decay (at room temperature, t1/2 ~ 45 min) of an intermediate with λmax = 634 (1a) or 631 (1b) nm, respectively, the lifetime of which can be extended significantly by lowering the temperature (Figure 1c). For the case of 1b, crystals suitable for characterization by X-ray diffraction were obtained, although larger scale solid samples for reactivity studies (see below) were more readily isolated for 1a. The structure of 1b (Figure 1b) features two O3SCF3 − anions associated with a [L3Cu3S2]2+ unit. This unit contains a disulfide (S2 2−) bridging the three copper ions in a manner unique in copper chemistry,10 albeit precedented for other metal ions13 and analogous to a proposed motif for a peroxide intermediate in dioxygen reduction by copper oxidases.14 A localized mixed valent Cu(II)Cu(I)2 electronic structure for the [Cu3S2]2+ core is suggested by metal-ligand bond distances15 and metal ion coordination geometries, with 4-coordinate C3v-distorted tetrahedral Cu1 and Cu2 in the +1 oxidation state and 5-coordinate Cu3 in the +2 state.
In the ESI mass spectrum of 1a in CH2Cl2 (Figure S5) a parent ion envelope with the appropriate isotope pattern for [L3Cu3S2](SbF6)+ confirms that the trinuclear cluster is retained in solution (calc. m/z 1003.1463; found 1003.1426). Nonetheless, loss of a [LCu(I)](SbF6) fragment to yield [L2Cu2S2]+ is facile, as indicated by an intense peak envelope for this species (calc. m/z = 532.1499; found 532.1521); this observation is relevant to reactivity studies described below. The EPR spectrum of a frozen solution of 1b (X-band, 3.5K, Figure S4) exhibits an essentially axial signal with slight rhombicity (gx = 2.079, gy = 2.024, gz = 2.023) and a four line hyperfine splitting in gx due to coupling to a single Cu nucleus (ACu = 138 × 10−4 cm−1). The 631 nm absorption band for 1b resembles that observed for a mixed-valent form of CuZ in N2OR (λmax = 640 nm) that has been attributed to a S → Cu ligand-to-metal charge transfer (LMCT) transition.8b,c Excitation into the 631 nm band for 1b leads to resonance enhancement of a peak in the Raman spectrum (Figure 2b) at 453 cm−1 (Δ34S = 19 cm−1); similar data were observed for 1a.16 These results are consistent with assignment of the 631 nm band as a Cu/S2 2− charge transfer transition and the 453 cm−1 peak as a predominantly ν(S-S) mode.
Figure 2.
(left) Experimental absorption spectrum of the crude reaction solution containing 1b obtained upon slow addition of Na2S2 (0.5 eq) to [LCu(MeCN)]O3SCF3 in THF at −20 °C (green line) and TD-B98 calculated spectrum (red line), with drawings of the acceptor orbitals for the respective features shown. (right) Resonance Raman spectrum obtained from the reaction of [LCu(MeCN)]O3SCF3 with Na2S2 in THF (−196 °C, λex = 647.1 nm; 32S, blue line; 34S, red line).
These assignments were investigated further using Density Functional Theory (DFT) calculations. Geometries of the [L3Cu3S2]2+ cluster were optimized in the gas phase and including self-consistent reaction-field THF solvation effects. The two optimized geometries were similar and differ little from the single-crystal X-ray structure, except that the S-S bond is longer (2.21 Å) in the computed structures than in the crystal structure (2.13 Å). UV-vis spectra calculated at the time-dependent B98 density functional level of theory were insensitive to choice of structure (computed or X-ray) and showed excellent agreement with the experimental spectrum when computed transitions were blue-shifted by 0.2 eV (Figure 2a; the blue shift is consistent with the tendency for most TD-DFT protocols to underestimate the energy of charge-transfer excitations17). Importantly, and in contrast to the assignments for CuZ,8b,c the absorption near 630 nm can be attributed to a metal-to-ligand charge transfer (MLCT) from the Cu(I) centers into a π* orbital of the S-S bond (SOMO, Figure 2a). Similarly, the peak at around 340 nm originates mainly from a MLCT from the Cu(I) centers into the σ* orbital of the S-S bond (LUMO). With respect to the resonance Raman spectrum, theory predicts an S-S stretch at 406 cm−1 and Cu(I)-S stretches at 366 cm−1 and 347 cm−1. The discrepancy of the S-S stretch with the experimental value is probably due to the elongated S-S bond in the gas-phase optimized structure, which was used for these computations (a frequency of 504 cm−1 is predicted when the non-stationary X-ray crystal structure is used). Finally, the calculations show that 71% of the spin density is located on the sulfur atoms and the Cu center coordinated η2 to the disulfide unit (therefore denoted Cu(II) with all other atoms having less than 5%).
In addition to converting to 2a upon warming to room temperature, 1a in CH2Cl2 (or generated in situ in THF) under a He atmosphere at −80 °C slowly (t1/2 ~ 6 h) reacts with excess N2O to quantitatively (based on 1a) yield N2 as determined by GC/MS. In control experiments, identical procedures were followed but with either no complex present, or with the complex present but without injecting N2O; in both cases, N2 production was not observed. UV-vis spectroscopic changes during the reaction of 1a with N2O show bleaching of the 631 nm band and concomitant formation of the features due to 2a (~60% yield18), the identity of which was confirmed by resonance Raman spectroscopy.11b In addition, an ESI mass spectrum of the final reaction solution revealed the presence of {[L2Cu2(OH)2]SbF6}+ (calc. m/z 739.1048, found 739.0960).19,20 Peaks corresponding to this product are not present in the spectrum of the solution resulting from decay of 1a in the absence of N2O. We have not been able to isolate [L2Cu2(OH)2](SbF6)2 from the product mixture, and suspect that it may not be the primary oxygen-containing copper byproduct of the reduction of N2O; further experimental mechanistic evaluation is needed to address this issue. Importantly from a mechanistic perspective, the presence of a ~7-fold excess of [LCu(CH3CN)](SbF6) in solutions of 1a in CH2Cl2 inhibited both the decomposition of 1a in the absence of N2O (t1/2 ~ several days at −20 °C) and its reaction with N2O to form N2 (40 ±10% yield after 10 h). These results, in conjunction with the ESI-MS data indicating the feasibility of [L2Cu2S2]+, are consistent with a pre-equilibrium step (eq. 1) that generates [L2Cu2S2]+ as the active species in the N2O activation process, with added LCu(I) shifting the equilibrium to the presumably less reactive trinuclear cluster.
| (1) |
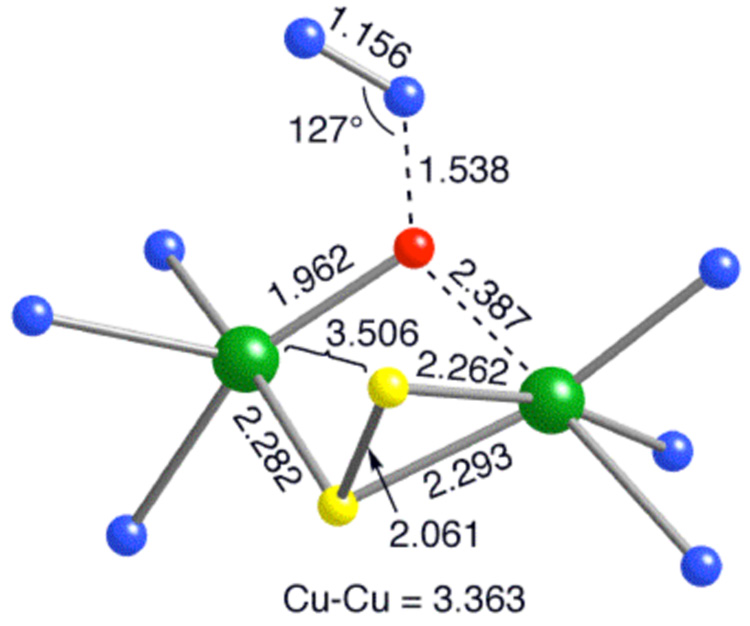
In computational efforts to evaluate this hypothesis, we calculated that the disproportionation shown in eq. 1 in THF is endergonic by only 2.7 kcal/mol (with THF bound to [LCu(I)]+). As a result, exhaustive searches at the M06L DFT level for bound complexes and transition-state (TS) structures with N2O were performed for both [L3Cu3S2]2+ and [L2Cu2S2]+. In addition to a number of TS structures found for both clusters with free energies of activation too high to be experimentally relevant (see supporting information), we discovered a low-energy (ΔG‡ = 26.6 kcal mol−1) TS structure for [L2Cu2S2]+ that evolves to an oxo-bridged intermediate (not observed experimentally) in an exergonic process (ΔG = −12.5 kcal mol−1, Figure 3 and Figure S13). The nature of the bridging in the TS structure, involving only the substrate O atom, differs from the µ-1,3 binding of N2O proposed for N2OR by Solomon and coworkers,9 but the ΔG‡ we compute is similar to their calculated value for the enzyme model (after entropic effects are added to their reported activation potential energies). We were unable to find similar µ-1,3-bridgedspecies for either binuclear or trinuclear clusters; plausible starting structures inevitably dissociated N2O or relaxed to monodentate coordination.
Figure 3.
Transition state structure for the N-O bond cleavage computed at the M06L DFT level with selected interatomic distances (Å). C and H atoms of the Me3tacn ligands are not shown for clarity. Key: green = Cu, blue = N, yellow = S, and red = O.
The ground state of our computed product is a quartet and this, together with an analysis of its charge and spin density, indicates oxidation of the original Cu(I)/Cu(II) binuclear reactant to a Cu(II)/-O•−/Cu(II) product. As has been noted previously,21 high-spin (triplet) Cu(II)-O•− is preferred over a closed-shell Cu(III)/oxo formalism, and coupling of this fragment with the remaining Cu(II) is ferromagnetic leading to an S = 3/2 ground state. In the TS structure, oxidation of the original Cu(I) atom is moderately advanced, but the doublet S = 1/2 state remains the ground state. Thus, spin crossing occurs subsequent to commitment to reaction.
We conclude by noting that although the disulfido (S2 2−) unit in complex 1 differs from the sulfido (S2−) ligand in the active site of N2OR, key properties of CuZ are modeled, such as S-bridging between Cu(I) and Cu(II) sites supported by N-donor ligands and similar UV-vis absorption features. Importantly, the complex exhibits reactivity relevant to that of the enzyme, insofar as it converts N2O to N2. The finding of N2O reduction by a discrete copper-sulfur complex under mild conditions is significant, as such reactions have only been observed for Cu oxide surfaces22 and Cu-doped zeolites23 at elevated temperatures and for excited state Cu(I) ions in the gas phase.24 We propose a mechanism involving pre-equilibrium formation of a dicopper complex, which subsequently reduces N2O via a transition state that features bridging of substrate between the two copper ions through a single O atom. Although previously discounted,9 the evidence we have obtained suggests that such a pathway may represent a feasible alternative to the mechanism involving µ-1,3-coordination of N2O proposed previously for the enzyme.
Supplementary Material
Materials and Methods, details of X-ray crystallography and calculations, and full citation for ref 1b. This information is available free of charge via the Internet at http://pubs.acs.org/
Acknowledgments
We thank the NIH, DAAD, and NSF for support of this research through grants GM47365 to W.B.T., a postdoctoral fellowship to S.M.H., and CHE-0610183 to C.J.C, respectively. We also thank Dr. Dana Reed, Sean Murray, and Dr. Lei Yang for assistance with the GC/MS measurements, Joe Kumka for help with the EPR simulation, John T. York for useful discussions, and Benjamin Kucera for help with the X-ray crystallography.
References
- 1.(a) Trogler WC. Coord. Chem. Rev. 1999;187:303–327. [Google Scholar]; (b) Duce R, et al. Science. 2008;320:893–897. doi: 10.1126/science.1150369. [DOI] [PubMed] [Google Scholar]
- 2.Smeets PJ, Groothaert MH, van Teeffelen RM, Leeman H, Hensen EJM, Schoonheydt RA. J. Catal. 2007;245:358–368. [Google Scholar]
- 3.Lee JH, Pink M, Tomaszewski J, Fan H, Caulton KG. J. Am. Chem. Soc. 2007;129:8706–8707. doi: 10.1021/ja071452f. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 4.Harman WH, Chang CJ. J. Am. Chem. Soc. 2007;129:15128–15129. doi: 10.1021/ja076842g. [DOI] [PubMed] [Google Scholar]
- 5.McNeill K, Bergman RG. J. Am. Chem. Soc. 1999;121:8260–8269. [Google Scholar]
- 6.Zumft WG, Kroneck PMH. Adv. Microb. Phys. 2007;52:107–227. doi: 10.1016/S0065-2911(06)52003-X. [DOI] [PubMed] [Google Scholar]
- 7.(a) Brown K, Tegon M, Prudencio M, Pereira AS, Besson S, Moura JJ, Moura I, Cambillau C. Nat. Struct. Biol. 2000;7:191–195. doi: 10.1038/73288. [DOI] [PubMed] [Google Scholar]; (b) Brown K, Djinovic-Carugo K, Haltia T, Cabrito I, Saraste M, G.Moura JJ, Moura I, Tegoni M, Cambillau C. J. Biol. Chem. 2000;275:41133–41136. doi: 10.1074/jbc.M008617200. [DOI] [PubMed] [Google Scholar]; (c) Paraskevopoulos K, Antonyuk SV, Sawers RG, Eady RR, Hasnain SS. J. Mol. Biol. 2006;362:55–65. doi: 10.1016/j.jmb.2006.06.064. [DOI] [PubMed] [Google Scholar]
- 8.(a) Rasmussen T, Berks BC, Sanders-Loehr J, Dooley DM, Zumft WG, Thomson AJ. Biochemistry. 2000;39:12753–12756. doi: 10.1021/bi001811i. [DOI] [PubMed] [Google Scholar]; (b) Chen P, Cabrito I, Moura JJG, Moura I, Solomon EI. J. Am. Chem. Soc. 2002;124:10497–10507. doi: 10.1021/ja0205028. [DOI] [PubMed] [Google Scholar]; (c) Alvarez ML, Ai J, Zumft W, Sanders-Loehr J, Dooley DM. J. Am. Chem. Soc. 2001;123:576–587. doi: 10.1021/ja994322i. [DOI] [PubMed] [Google Scholar]; (d) Ghosh S, Gorelsky SI, DeBeer George S, Chan JM, Cabrito I, Dooley DM, Moura JJG, Moura I, Solomon EI. J. Am. Chem. Soc. 2007;129:3955–3965. doi: 10.1021/ja068059e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Oganesyan VS, Rasmussen T, Fairhurst S, Thomson AJ. Dalton Trans. 2004:996–1002. doi: 10.1039/b313913a. [DOI] [PubMed] [Google Scholar]
- 9.(a) Chen P, Gorelsky SI, Ghosh S, Solomon EI. Angew. Chem. Int. Ed. 2004;43:4132–4140. doi: 10.1002/anie.200301734. [DOI] [PubMed] [Google Scholar]; (b) Solomon E, Sarangi R, Woertink J, Augustine A, Yoon J, Ghosh S. Acc. Chem. Res. 2007;40:581–591. doi: 10.1021/ar600060t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) York JT, Bar-Nahum I, Tolman WB. Inorg. Chim. Acta. 2008;361:885–893. doi: 10.1016/j.ica.2007.06.047. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sarangi R, York JT, Helton ME, Fujisawa K, Karlin KD, Tolman WB, Hodgson KO, Hedman B, Solomon EI. J. Am. Chem. Soc. 2008;130:676–686. doi: 10.1021/ja0762745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Cole AP, Mahadevan V, Mirica LM, Ottenwaelder X, Stack TDP. Inorg. Chem. 2005;44:7345–7364. doi: 10.1021/ic050331i. [DOI] [PubMed] [Google Scholar]; (b) Bar-Nahum I, York JT, Young VG, Jr, Tolman WB. Angew. Chem. Int. Ed. 2008;47:533–536. doi: 10.1002/anie.200704690. [DOI] [PubMed] [Google Scholar]
- 12.(a) Fujisawa K, Moro-oka Y, Kitajima N. J. Chem. Soc., Chem. Commun. 1994:623–624. [Google Scholar]; (b) Brown EC, Bar-Nahum I, York JT, Aboelella NW, Tolman WB. Inorg. Chem. 2007;46:486–496. doi: 10.1021/ic061589r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Helton ME, Maiti D, Zakharov LN, Rheingold AL, John A. Porco J, Karlin KD. Angew. Chem. Int. Ed. 2006;45:1138–1141. doi: 10.1002/anie.200503216. [DOI] [PubMed] [Google Scholar]; (d) Inosako M, Shimokawa C, Sugimoto H, Kihara N, Takata T, Itoh S. Chem. Lett. 2007;36:1306–1307. [Google Scholar]
- 13.Brunner H, Gehart G, Leblanc J-C, Moise C, Nuber B, Stubenhofer B, Volpato F, Wachter J. J. Organomet. Chem. 1996;517:47–51. [Google Scholar]
- 14.Solomon EI, Augustine AJ, Yoon J. Dalton Trans. 2008:3921–3932. doi: 10.1039/b800799c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.A bond valence sum analysis gave values of 1.19, 1.21 and 2.25 for the oxidation states of Cu1, Cu2 and Cu3, respectively, using the reported empirical values (ro) from: Thorp HH. Inorg. Chem. 1998;37:5690–5692. doi: 10.1021/ic9804420. Thorp HH. Inorg. Chem. 1992;31:1585–1588.
- 16.Other features in the Raman spectrum between 345–410 cm−1 shift by smaller amounts upon 34S substitution and are assigned as Cu-S modes.
- 17.Cramer CJ. Essentials of Computational Chemistry: Theories and Models. 2nd ed. Chichester: John Wiley & Sons; 2004. [Google Scholar]
- 18.The yield is based on the absorption intensity, but is a lower limit due to observable precipitation of the product(s).
- 19.Chaudhuri P, Ventur D, Wieghardt K, Peters E-M, Peters K, Simon A. Angew. Chem. Int. Ed. 1985;24:57–59. [Google Scholar]
- 20.Complex 1a also converts to 2a upon reaction with O2, but at a rate (t1/2 ~ 30 s at −80 °C) much faster than the reaction with N2O. In addition, the ESI mass spectrum of the product solution differs from that obtained from the reaction with N2O (see supporting information for details).
- 21.Hong S, Huber SM, Gagliardi L, Cramer CJ, Tolman WB. J. Am. Chem. Soc. 2007;129:14190–14192. doi: 10.1021/ja0760426. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(a) Dell RM, Stone FS, Tiley PF. Trans. Faraday Soc. 1953;49:201–209. [Google Scholar]; (b) Scholten JJF, Konvalinka JA. Trans. Faraday Soc. 1969;65:2465–2473. [Google Scholar]; (c) Sankar G, Thomas JM, Waller D, Couves JW, Catlow CRA, Greaves GN. J. Phys. Chem. 1992;96:7485–7489. [Google Scholar]
- 23.Schneider WF, Hass KC, Ramprasad R, Adams JB. J. Phys. Chem. B. 1998;102:3692–3705. [Google Scholar]
- 24.(a) Rodgers MT, Walker B, Armentrout PB. Int, J. Mass Spectrom. 1999;182:99–120. [Google Scholar]; (b) Delabie A, Pierloot K. J. Phys. Chem. A. 2002;106:5679–5685. [Google Scholar]
- 25.In pulse radiolysis studies, rate constants for reactions of Cu(I) complexes with N2O were reported, but no proof of N2 generation was provided: Tait AM, Hoffman MZ, Hayon E. Inorg. Chem. 1976;15:934–939.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and Methods, details of X-ray crystallography and calculations, and full citation for ref 1b. This information is available free of charge via the Internet at http://pubs.acs.org/