Abstract
Recently two serologically and biochemically distinct subtypes, designated 11Aα and 11Aβ, were discovered among serotype 11A isolates of Streptococcus pneumoniae. Sequence comparison of the capsular polysaccharide synthesis (cps) loci of the two subtypes identified disruption of the wcjE gene, a putative O-acetyltransferase, as the genetic hallmark of the 11Aβ phenotype. Directed disruption of wcjE in vitro in an 11Aα strain switched the strain to the 11Aβ phenotype, confirming the role of the gene in the divergence between the subtypes. Furthermore, sequences from seven 11Aβ clinical strains each contained unrelated disruptive mutations in the wcjE gene, displaying an unprecedented degree of genetic heterogeneity in a pneumococcal serotype. We propose to name the 11Aα subtype as serotype 11A and the 11Aβ subtype as 11E, a new serotype. Our findings also suggest that the diversity of pneumococcal capsule is much greater than it was previously recognized.
Keywords: Streptococcus pneumoniae, capsule, infections, serotype emergence, serotype 11A
Introduction
Streptococcus pneumoniae is a leading cause of pneumonia, bacteremia, otitis media, and bacterial meningitis. Almost all pathogenic strains of pneumococci express a polysaccharide (PS) capsule, which shields pneumococci from the host's natural immune defense and increases pneumococcal virulence [1]. As antibodies to the capsule made in response to either natural infection or vaccination [2, 3] can abrogate the protective effect of the capsule, pneumococci, as a species, produce antigenically diverse capsule types (commonly known as serotypes) and evade host's adaptive immunity. Currently, 91 pneumococcal serotypes are recognized according to their unique serological profiles and chemical structures [4, 5].
For almost all serotypes, all the genes involved in capsule synthesis are located in a region between the genes dexB and aliA labeled the capsule synthesis (cps) locus [6]. The DNA sequences of all pneumococcal cps loci have been determined [7-10]. All sequences contain a highly conserved region at the 5′ end of the locus, which includes genes associated with regulation of capsule production levels [11] (Figure 1A). The region downstream to the conserved region is serotype specific and includes “core” and “accessory” genes. The core genes include glycosyl-transferases, flippases, and polymerases, which are essential for capsule production. The accessory genes, although not essential for capsule production, can modulate the structure of capsular PSs and increase serologic diversity of the capsule [9, 12]. O-acetyltransferases (OAcT) are important accessory genes, as reflected by the presence of 14 different putative OAcT genes in the cps loci of 47 pneumococcal serotypes [8, 9]. However, the functions of most OAcT are unknown since there is a lack of obvious correlation between OAcT gene presence in the cps locus and capsule structures [9], and since the acetylation sites in PS structures are often not determined [13].
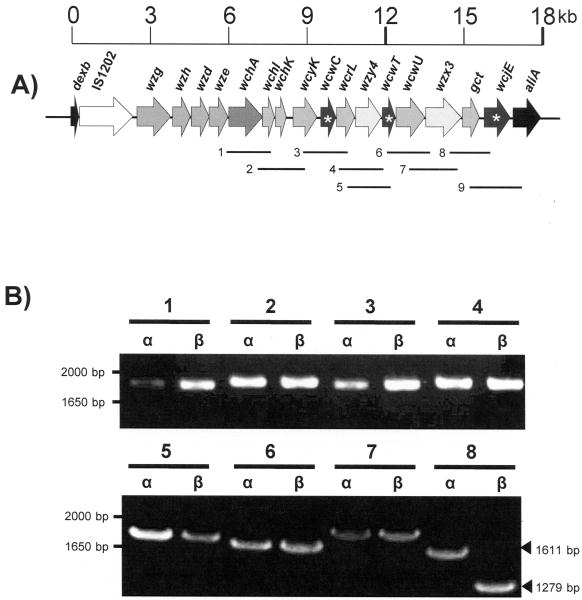
Figure 1. Comparison of cps PCR products identify discrepancy in 3′ region of 11A subtypes.
A) The 11A cps locus (GenBank accession # CR931653) [8]. The nine regions amplified by PCR and their assigned numbers are shown. Putative O-acetyltransferase genes are marked with a white asterisk. B) PCR products of regions 1-8 obtained from MNZ272 (α) and MNZ264 (β) were run on 1% agarose gel for size comparison.
Recent epidemiological studies show that serotype 11A has become one of the top five most prevalent serotypes isolated from diseased and colonized individuals in North America [15-17]. We recently identified antigenic subtypes among strains originally typed as 11A according to classical Quellung methods [18, 19]. The more common subtype is now labeled 11Aα and the rarer subtype, 11Aβ. Recent NMR analysis identified O-acetylation of a 1-phosphoglycerol (1-p-Gro) residue as the major biochemical distinction between 11Aα and 11Aβ capsular PS (Figure 2) [13]. To investigate the genetic basis for these subtypes, we examined the cps loci of the two subtypes and showed wcjE (cps11aP) to be the basis for the distinction between the subtypes.
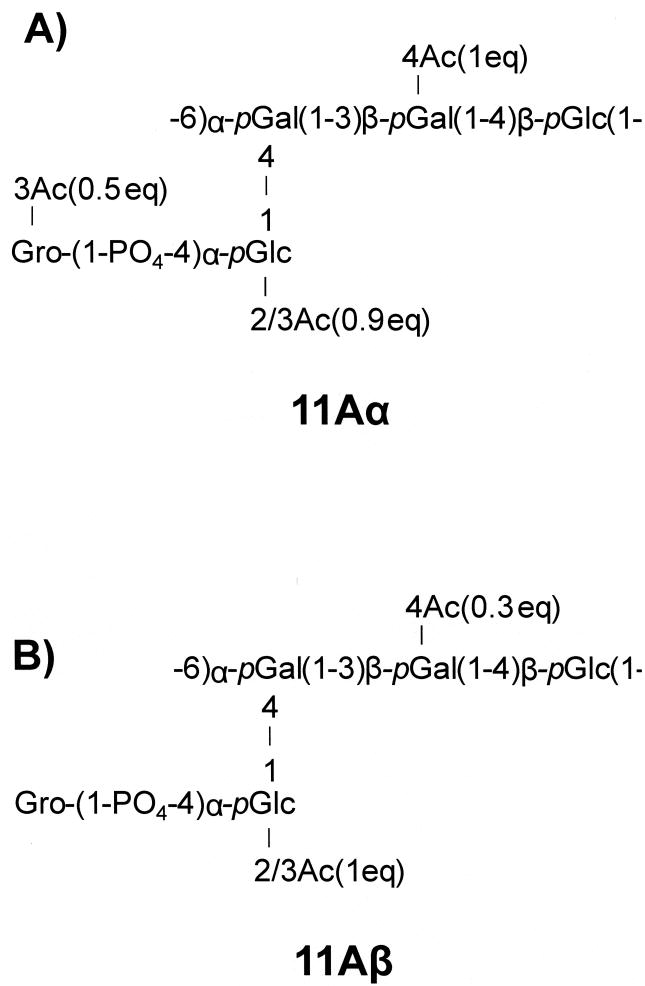
Figure 2. O-acetylated 1-P-glycerol is the major distinction between the biochemical capsule structures of 11Aα (A) and 11Aβ (B) subtypes.
pGlc, glucose pyranose; pGal, galactose pyranose; Gro, glycerol; Ac, O-acetyl group [13].
Materials and Methods
Bacterial isolates and lysates
S. pneumoniae strains used in this study are listed on Table 1 and were serotyped as 11A according to Quellung reaction. In addition to the 11Aβ isolates from Brazil and the CDC reported earlier [18, 19], one new isolate (MNZ265) was obtained at UAB in 2006. Frozen stocks were streaked on blood agar plates (BAP) and grown overnight at 37°C in 5% CO2. Cultures were grown in THY broth, consisting of Todd-Hewitt broth (BD Biosciences, San Jose, California) with 0.5% yeast extract, at 37°C in 5% CO2 up to an OD600 between 0.6-1.0. Lysates were created by suspending pneumococci in THY with 0.013% sodium deoxycholate, 0.0013% SDS, 0.02 M sodium citrate, and incubating at 37°C for 10 minutes. Strains MNZ269 and MNZ270 were established from subcloning the clinical isolate 4011-06. TIGR-J is a nonencapsulaed TIGR4-derived strain whose cps locus has been replaced with a Janus cassette [20].
Table 1.
List of strains used in this study
| Strain | Hyp11AM1 inhibtion | Hyp11AM9 inhibition | Subtype (serotype) designation | Tissue of origin | Location of isolation | Source (reference) |
|---|---|---|---|---|---|---|
| 4011-06 | +/- | + | 11Aαβ | Blood | CDC | isolate F |
| MNZ741 | + | - | 11Aβ (11E) | Blood | CDC | 3056-06 [18] |
| MNZ264 | + | - | 11Aβ (11E) | Blood | CDC | 3455-06 [18] |
| MNZ265 | + | - | 11Aβ (11E) | Middle ear effusion | Birmingham, AL | this study |
| MNZ266 | + | - | 11Aβ (11E) | Cerebral spinal fluid | Sao Paulo, Brazil | BZ435 [19] |
| MNZ267 | + | - | 11Aβ (11E) | Blood | CDC | 3954-06 [18] |
| MNZ268 | + | - | 11Aβ (11E) | Blood | CDC | 3151-06 [18] |
| MNZ269 | + | - | 11Aβ (11E) | Derived from 4011-06 | n/a | This study |
| MNZ270 | - | + | 11Aα (11A) | Derived from 4011-06 | n/a | This study |
| MNZ271 | - | + | 11Aα (11A) | Blood | CDC | isolate C [18] |
| MNZ272 | - | + | 11Aα (11A) | Blood | CDC | isolate D [18] |
| MNZ273 | - | + | 11Aα (11A) | Blood | CDC | isolate E [18] |
| MNZ786 | + | - | 11Aβ (11E) | MNZ270 wcjE∷JS | n/a | This study |
| TIGR-J | - | - | Nonencapsulated | TIGR4 cps∷JS | n/a | [20] |
Inhibition ELISA for serotype detection
Subtype/serotype designation was done using an inhibition-type ELISA (iELISA) as described before [18]. Briefly, ELISA plates were coated with 5 μg/mL of purified 11Aα (American Type Culture Collection, Manassas, VA) or 11Aβ polysaccharide purified in our laboratory, and serial dilutions of cell lysates and monoclonal antibody were added to each well. After incubation and washing of unbound monoclonal antibodies (mAb), bound antibodies were detected with alkaline phosphatase-conjugated anti-mouse immunoglobulin antibody and nitrophenyl phosphate substrate. Strains whose cell lysates competitively inhibited Hyp11AM9 mAb were typed as 11Aα; those that inhibited Hyp11AM1 mAb were typed as 11Aβ [18, 19]. The mAb Hyp11AM9 was used in this study instead of Hyp11AM2 mAb [18, 19] because Hyp11AM9 displays identical specificity, but higher sensitivity, than Hyp11AM2.
PCR sequencing and genetic analysis
Genomic DNA was obtained from the 11Aα and 11Aβ bacterial lysates through phenol:chloroform extraction and used as a template for PCR. PCRs were performed in 50 μL aqueous solutions containing the following: 1X Ex Taq Buffer, 3.0 U of Ex Taq, 100 μM of each deoxynucleoside triphosphate (Takara Bio Inc., Japan), 0.1 μM each of forward and reverse primer, 0.2 μL of purified DNA template solution. Primers (Table 2) were designed according to the published 11A cps locus sequence (Figure 1A) [8]. Sequencing was performed at the Heflin Genomics Core at the University of Alabama at Birmingham School of Medicine. DNA and predicted amino acid sequences were analyzed on Lasergene v. 5. Software (DNASTAR, Madison, WI) or A Plasmid Editor v1.17 (http://www.biology.utah.edu/jorgensen/wayned/ape/). For this study, nucleotide numbers were assigned as shown on Figure 3. Prediction of gene transmembrane regions was performed using Dense Alignment Surface (“DAS”) – Transmembrane Prediction server (http://www.sbc.su.se/∼miklos/DAS/).
Table 2.
List of primers used in this study
| Primer | Sequence | Description |
|---|---|---|
| For PCR products* | ||
| Forward primers | ||
| 5301 | AAGGCAGGTGAAACAAAACG | 11A cps region 1 PCR amplification |
| 5302 | GAGCTTGGACGAGCTACCAC | 11A cps region 2 PCR amplification |
| 5303 | GGTCAATGGCTTTTTGAGGA | 11A cps region 3 PCR amplification |
| 5304 | TTCGGTGGCAAACCTTTATC | 11A cps region 4 PCR amplification |
| 5305 | GATCCGATTTCTTTGGGTGA | 11A cps region 5 PCR amplification |
| 5306 | GGGAGGCAAAACGTTTGTTA | 11A cps region 6 PCR amplification |
| 5307 | CCCTCGGGCAATGTAGATAA | 11A cps region 7 PCR amplification |
| 5308 | TGCCATCTCGGTTTATTTCC | 11A cps region 8 PCR amplification |
| 5310 | ACCTTTGATTTGCTTCATTATGG | 11A cps region 9 PCR amplification |
| Reverse primers | ||
| 3301 | GTTAAGGTTGGCGCATCAAT | 11A cps region 1 PCR amplification |
| 3302 | TCCTCAAAAAGCCATTGACC | 11A cps region 2 PCR amplification |
| 3303 | CCCAAAGAAATCGGATCAAA | 11A cps region 3 PCR amplification |
| 3304 | TGCGCTGCCTTTCTTTTTAT | 11A cps region 4 PCR amplification |
| 3305 | CCTCAATAATCGCACCACCT | 11A cps region 5 PCR amplification |
| 3306 | GCTTTCATCCCGACAGACAT | 11A cps region 6 PCR amplification |
| 3307 | TATTTGAAAGAGCCGCACCT | 11A cps region 7 PCR amplification |
| 3308 | CTCACAGAAGCACCAGCAAG | 11A cps region 8 PCR amplification |
| 3309 | TTAGCGATCGAACCTGATCC | 11A cps region 9 PCR amplification |
| Additional primers for sequencing | ||
| 5311 | CTTGCTGGTGCTTCTGTGAG | 11A cps region 9 sequencing |
| 5400 | AACGCCGCTACTGTCGTTAT | sp-IS1380 sequencing |
| 5401 | TGACGGACATACGCATGATT | IS1515 sequencing |
| 5409 | GGAGTTGGTAGCCGTCAGTG | Sequencing 11A cps region 1 |
| 5410 | TTTGGCGCTAAGACAGTCTAC | Sequencing 11A cps region 2 |
| 5411 | CTGCTAAAGTTGCTGGTATCC | Sequencing 11A cps region 3 |
| 5415 | CCGTTACGAAGAAATGTCTGTC | Sequencing 11A cps region 7 |
| 3398 | CCCCTGACCTCATGAG | ISSpn5 sequencing |
| 3409 | CTGTACCTACATTACCATTCCCTC | Sequencing 11A cps region 2,3 |
| Additional primers for transformation assayˆ | ||
| 5405 | GGTACCTCGTTAGTTTCCACAGGTGC | 5′ fragment amplification |
| 3405 | TCTAGATCACAGAAGCACCAGCAAGC | 5′ fragment amplification, includes XbaI |
| 5112 | CTAGTCTAGAGTTTGATTTTTAATGG | Janus amplification, includes XbaI |
| 3112 | CGCGGATCCGGGCCCCTTTCCTTATGCTTTTGG | Janus amplification, includes BamH1 |
| 5417 | AGATCTCTTTGTCAACTAACATCTGGAGAG | 3′ fragment amplification, includes BglII |
| 3402 | AACATCCTTCCATTCATCCCCATA | 3′ fragment amplification |
| 3399 | CAAATGTTGGTGGCACAAAG | Complete insert amplification |
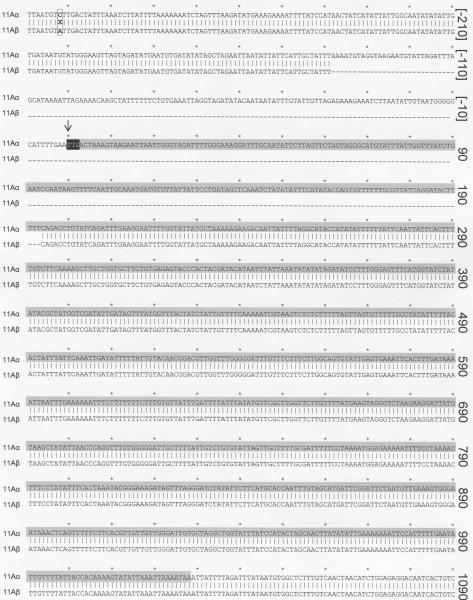
Figure 3. Sequencing of the cps loci of 11A subtypes reveals disruption of wcjE in 11Aβ.
Sequence alignment of a portion of MNZ272 (11Aα) and MNZ264 (11Aβ) cps locus revealing a 332 bp deletion in MNZ264, which includes the start codon (black box) of the wcjE gene (highlighted in gray). The single nucleotide polymorphism [-302] C∷A is highligthed in a box. Nucleotide numbers are assigned according to distance from the wcjE start codon, with the initial thymine being nucleotide ‘0’ (arrow). Nucleotide ‘0’-T corresponds to nucleotide 16325-T of the published 11A cps locus, GenBank accession #CR931653 [8].
Production of wcjE-disrupted strains
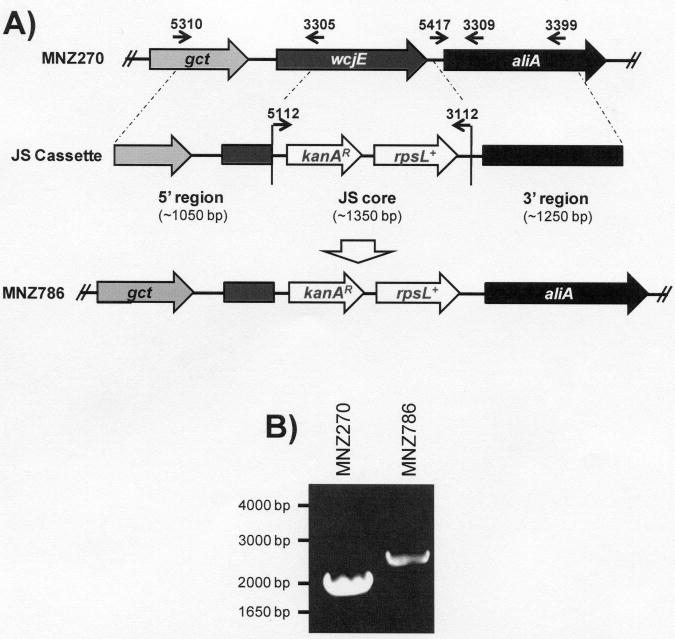
wcjE was disrupted in the wild-type 11Aα strain MNZ270 by using a Janus construct [22]. The construct is composed of a Janus cassette containing kanamycin resistance (kanAR) and streptomycin sensitivity (rpsL+) genes, flanked by 1000-1300 bp regions homologous to the sequence flanking the wcjE gene in MNZ270 (Figure 5). The Janus cassette was PCR amplified using TIGR-J genomic DNA and the primers 3112 and 5112. The 5′ flank was PCR amplified using primers 5405 and 3405, while the 3′ flank was PCR amplified with the primers 5417 and 3402. Fragments were digested with the appropriate restriction enzymes and followed by ligation with T4 DNA ligase (Takara Bio Inc., Japan). Final product was amplified using the primers 5310 and 3399, resulting in an approximately 3.7 kilobasepair Janus cassette (JS) construct (Figure 5). The construct was transformed into MNZ270 using competence stimulating peptide-2 competence induction. Transformants were selected by growth on media containing 100 μg/mL kanamycin. Successful recombination was confirmed by PCR and sequencing using primers 5310 and 3309. Other primers used for this assay are listed in Table 2.
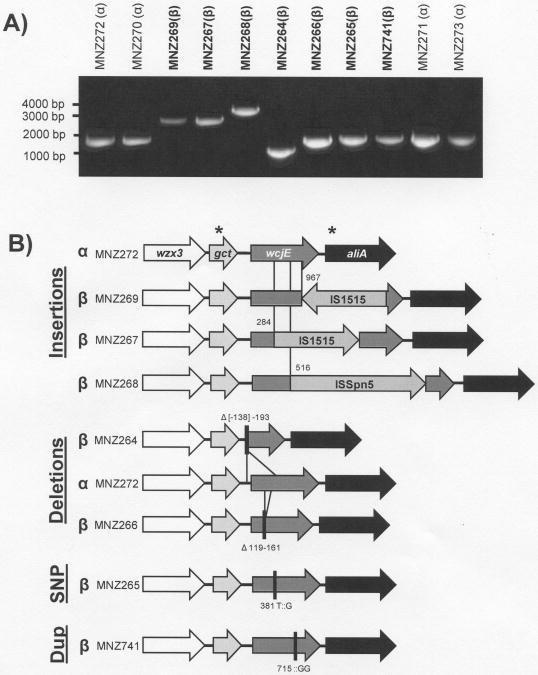
Figure 5. 11Aβ strains display high heterogeneity in disruption of wcjE.
A) PCR products of region 9 from seven 11Aβ strains (β, bold) and in four 11Aα strains (α) were run on 1% agarose gel for size comparison. B) Depiction of the unique mutations affecting wcjE, including transposable element insertions, multiple-nucleotide deletions, a single nucleotide polymorphism (SNP) and nucleotide duplication (Dup). The 11Aα strain MNZ272 is depicted for comparison. Asterisks mark primer binding sites for region 9 PCR amplification. Values correspond to nucleotide distance from the wcjE start codon.
Results
Monoclonal antibodies identified only two antigenic phenotypes within the 11A serotype strains
11Aβ strains used in this study were identified by iELISA with mAb specific for the 11A subtypes, Hyp11AM1 and Hyp11AM9 (Table 1). Lysates from 11Aα strains inhibited Hyp11AM9, but not Hyp11AM1, whereas 11Aβ lysates inhibited Hyp11AM1, but not Hyp11AM9. This was shown with the two control strains, MNZ272 (a known 11Aα strain) and MNZ264 (a known 11Aβ strain). In addition to verifying previous assignments [18] of the four strains MNZ264, MNZ266, MNZ267, and MNZ268 to 11Aβ, we identified two new 11Aβ strains, MNZ741 and MNZ265, which clearly inhibited Hyp11AM1 but not Hyp11AM9. The 11Aα strains MNZ271 and MNZ273 inhibited Hyp11AM9, but not Hyp11AM1 as previously published [18]. TIGR-J is a nonencapsulated strain, and its lysate inhibited neither antibody.
The clinical isolate 4011-06 is shown to inhibit mAbs to both 11A subtypes and its phenotype was labeled 11Aαβ [18]. To investigate its nature, subclones of the isolate were examined for antigenicity. The parent isolate strongly inhibited Hyp11AM9 and somewhat less strongly Hyp11AM1 (Table 1). However, all subclones of 4011-06 inhibited only one of the two mAbs. For instance, MNZ269, a subclone of 4011-06, inhibited Hyp11AM1, but not Hyp11AM9 characteristic of an 11Aβ strain. In contrast, another subclone MNZ270 inhibited Hyp11AM9 without inhibiting Hyp11AM1 characteristic of an 11Aα strain. Both subclones belong to the pneumococcal multilocus sequence typing (MLST) type 62 (unpublished data). Thus, the 11Aαβ phenotype was not a third “hybrid” subtype, and 4011-06 was composed of two strains with similar genetic backgrounds expressing either 11Aα or 11Aβ subtypes. It is likely that one strain was derived from the other during infection.
PCR and sequencing analysis identified disruption of wcjE in an 11Aβ cps locus
To identify the genetic basis of the 11Aα and 11Aβ subtypes, we used PCR to compare the sizes of the genes found in an approximately 11.5 kb region of the capsule gene loci of an 11Aα (MNZ272) and 11Aβ (MNZ264) strains (Figure 1). Since the four upstream cps genes are highly conserved among serotypes [8] they were not addressed in this study. The regions amplified by PCR were labeled 1 through 9 as shown in Figure 2A. Comparisons of PCR products showed no difference in the size of regions 1-7 between 11Aα and 11Aβ strains. However, regions 8 and 9 were smaller in MNZ264 than in MNZ272 (Figure 1B).
To better define this size discrepancy, the nucleotide sequences of the PCR products from MNZ272 and MNZ264 were determined (GenBank Accession #GU074952, GU074953). Comparison of regions 1-7 showed that all the capsule synthesis genes, including the two putative O-acetyltransferases wcwC and wcwT (also known as cps11aI and cps11AL, respectively), had identical sequences between 11Aα and 11Aβ. However, the comparison of regions 8 and 9 showed that MNZ264 had a 332 basepair deletion (Figure 3) resulting is disruption of wcjE, a putative member of the OAcT Pfam family PF01757 [9]. The deletion began 138 bases upstream of the wcjE putative start codon (Figure 3, highlighted in black) and ended at nucleotide 193. The only other sequence difference identified in regions 1-9 was a single nucleotide polymorphism, [-302] C(x02237)A, located in a non-coding region upstream of wcjE (Figure 3, highlighted with a box).
In vitro disruption of wcjE converts the 11Aα phenotype to the 11Aβ phenotype
While the above findings strongly suggested that wcjE inactivation is responsible for the 11Aβ phenotype, they did not exclude the participation of genes outside the cps locus. To confirm whether wcjE disruption alone is enough to switch 11A subtype antigenicity, we created a wcjE knock-out strain using a JS construct (Figure 4). As expected, the transformant MNZ786 exhibited inhibitory binding of Hyp11AM1, but did not bind Hyp11AM9 in contrast to its parent strain MNZ270 (Table 1). Comparison of the PCR products of region 9 showed that MNZ786 had a larger product than MNZ270 (Figure 4B) and sequencing confirmed insertion of JS at nucleotide 321 resulting in recombinational deletion of basepairs 322-1053 (Figure 3, GenBank Accession #GU074962). This demonstrated that wcjE disruption in an 11Aα cps locus is the basis for the 11Aβ phenotype.
Figure 4. wcjE disruption was achieved using a Janus (JS) cassette construct.
A) This diagram depicts JS cassette construction using the 5′ and 3′ regions flanking wcjE, and a JS core containing the genes kanAR (kanamycin resistance) and rpsL+ (streptomycin sensitivity). The cassette was transformed into MNZ270 (11Aα), resulting in MNZ786 (11Aβ). Smaller arrows indicate corresponding binding sites of primers used in this assay (Table 2). B) Primers 5310 and 3309 PCR products from MNZ270 and MNZ786.
Genetic analysis of multiple 11Aβ strains showed variable disruptive mutations in wcjE
To confirm the changes in 11Aβ, region 9 was PCR-amplified and sequenced in the remaining 11Aβ clinical strains as well as two additional 11Aα strains, MNZ271 and MNZ270 (Figure 5). All nine sequences have been deposited in GenBank (Accession # GU074954 - GU074961). The two 11Aα strains had PCR product sizes and sequences identical to MNZ272. In contrast, the sizes of the PCR products of the 11Aβ strains were variable.
Three 11Aβ strains had longer region 9 PCR products than 11Aα strains (Figure 5A). Determination of their sequences revealed transposable elements inserted into wcjE (Figure 5B). MNZ267 and MNZ269 contained an 873-basepair-long insertion sequence (IS), IS1515, inserted in different orientations. The transposable elements were inserted in different ATT sequences within wcjE – 282-ATT-284 and 965-ATT-967, respectively – and resulted in a duplication of the triplet. IS1515 sequences from both strains were identical to the previously published sequence (accession #Z86112) [23], except that both shared an adenosine duplication that results in truncation of the putative transposase gene and a G(x02237)A single nucleotide polymorphism (SNP). MNZ268 had a different putative transposable element, ISSpn5 (IS1380 family) [24], starting at position 516 and resulting in duplication of the sequence 512-TTTTA-516. The insert was 1708 bases long.
Two 11Aβ strains had shorter PCR products than 11Aα strains (Figure 5A). As noted above, MNZ264 had a 332 basepair deletion including the gene's initiation sequence. MNZ266 had a 43 basepair deletion (starting at nucleotide position 119 of wcjE) and results in a premature stop codon at 211-TGA-213.
Unlike the five 11Aβ strains mentioned above, region 9 PCR products from MNZ741 and MNZ265 were comparable in size to the 11Aα controls (Figure 5A). Sequencing showed that MNZ741 contained two additional guanines at residue 715 in wcjE and, as a result, had a premature stop codon at 742-TAG-744. MNZ265 had a 382 T(x02237)G SNP that resulted in a trp138gly in the predicted amino acid sequence. This tryptophan residue is highly conserved among the Pfam family PF01757 acetyltransferases [25] and is at the margin of a predicted transmembrane region in wcjE according to DAS analysis (data not shown).
Discussion
We have previously shown that pneumococcal strains typed as 11A by the classical Quellung reaction can be separated into two serologically and biochemically distinct subtypes, 11Aα and 11Aβ [18]. The capsular PS of the 11Aα subtype has O-acetylation of the 1-p-Gro, but the capsular PS of 11Aβ does not [13]. In this study we show that the genetic basis of 11A subtypes is wcjE, with wcjE being disrupted in all isolates of the 11Aβ subtype, and that an experimental disruption of wcjE converts an 11Aα strain to an 11Aβ strain. This finding is consistent with genomic analysis that suggested that wcjE encodes a membrane-associated O-acetyltransferase [9]. Having determined the serologic, chemical, and genetic bases for the subtypes, we propose labelling the more common subtype, 11Aα, as the previously established serotype 11A, and naming 11Aβ as a new serotype, 11E.
The capsule gene locus of serotype 11A includes three genes (wcjE, wcwC, and wcwT) encoding putative OAcT, but their enzymatic specificities have been unclear [9]. We have recently identified the exact location of the three O-acetylation sites of serotype 11A PS (Figure 2) [13]. Our current study shows that wcjE encodes an OAcT of the 1-P-Gro residue present on the 11A PS but absent in 11E PS. The functional assignment of wcjE permits identifying enzymatic functions for the remaining two OAcT genes. Since wcwC is only found in the capsule gene loci of the members of serogroup 11 that have O-acetylated galactose in their capsule (11F, 11A and 11E)[8, 13, 26], the wcwC gene product should O-acetylate galactose. By elimination, O-acetylation of the glucose residue should then be mediated by wcwT. Indeed, all serogroup 11 cps loci contain wcwT, and all known serogroup 11 capsule structures exhibit O-acetylation of the corresponding glucose or N-acetylglucosamine [8, 26].
The cps loci of eleven other pneumococcal serotypes, including 9V, 11D, 15F, 20 and 33A, contain wcjE genes that display ≥80% sequence homology to the wcjE gene of serotype 11A [8]. However, with the possible exception of 11D (whose PS structure is unknown but has a cps locus similar to 11A), the PS capsule of these serotypes do not contain 1-p-Gro moieties, and their wcjE products may target substrates other than 1-p-Gro. Interestingly, in all eleven serotypes, wcjE is invariably located at the downstream margin of the cps locus, which is an area shown by observations in this and previous genetic studies to be susceptible to disruptive genetic alterations, i.e., transposable element insertion and recombinational deletion [8]. Serotypes 9V and 33A have partner serotypes - 9A and 33F - which have almost identical cps loci except for the disrupted wcjE. Furthermore, O-acetylation is known to be the only biochemical difference between the serotype 9V and 9A PSs. The above considerations raise the possibility that wcjE active serotypes, such as 11D and 20, may have yet unidentified partner serotypes with inactive wcjE genes and that more specific serotyping methods would be critical in the search for the unidentified partner serotypes since the insufficient specificity of the current typing antisera may have been the reason for their being unidentified.
One interesting observation in our study was that every 11E isolate exhibits nonrelated means of wcjE gene disruption, implying that all seven 11E strains emerged independently. To our knowledge, this degree of genetic heterogeneity has not been previously observed for any serotype. Thus, we initially considered that wcjE inactivation occurred in vitro during laboratory culture. However, we found that 11A isolates stably maintain their serotypes in vitro (unpublished observation), and one 11E strain (e.g., MNZ265) was freshly obtained from a patient. Moreover, serotype 9A and 33F isolates appear to show heterogeneous disruptions in their wcjE genes (unpublished data), echoing the example of 11E. Since a certain percentage of individuals target most of their antibodies to the O-acetyl group, an interesting interpretation is that the wcjE disruption in 11E is not selected for in early colonization of the nasopharynx and is, instead, a mechanism to escape an in vivo pressure during advanced infection. This would also explain the apparent lack of host-to-host dissemination by a single clone of 11E. Therefore, MNZ270 and MNZ269, which share an MLST lineage, may have been co-isolated due to the seroswitching of 11A to 11E within a patient. To assess this hypothesis, we plan to perform additional studies, including the search for the 11E serotype among nasopharyngeal isolates which can spread to other hosts. Nevertheless, our studies highlight the involvement of the wcjE gene as a novel and dynamic mechanism for increasing pneumococcal capsule diversity with interesting biological implications.
O-acetylation is often used by bacteria to modify their molecular properties. The staphylococcal gene SA2354 (oatA), which has a high homology to wcjE, is responsible for O-acetylation of staphylococcal peptidoglycan and confers resistance to lysozyme [27]. In addition, O-acetylation has been associated with serological alteration and different host immunities. For instance, adults immunized with PS from pneumococcal serotype 15B produce antibodies binding to and opsonizing the 15B serotype but not the 15C serotype, whose capsule structure differs from 15B by the lack of O-acetylation at one PS residue [28, 29]. In 10-20 % of adults or children who were immunized with 9V PS, more than 80% of their antibodies are specific for the O-acetylated capsule and do not bind to 9A PS [30]. If individuals mount a humoral response exclusively against wcjE-dependent epitopes, the 11E serotype may emerge to escape an 11A-specific antibody response, and it is possible that the 11A PS present in the 23-valent PS vaccine may provide insufficient protection against 11E.
The identification of heterogeneity within established serotypes is important for our understanding of pneumococcal pathology and for vaccine development. Historically, serogroup 9V was identified as a new member of serogroup 9 in 1939 when antisera for serogroup 9 available at that time failed to treat a Danish prince [5]. More recently, the perceived rise in serotype 6A incidence following PCV7 introduction was due to the emergence of the recently discovered serotype 6C [14, 15]. Without the discovery of 6C, these data could have been interpreted as indicating the failure of vaccine efforts (which target 6A strains) instead of heralding the emergence of a new serotype. Previous findings suggest that 10-25% of “11A” may be 11E [18], and recent epidemiological studies have suggested that “11A” is filling the void left by widespread vaccination [15-17]. It would be important to determine if diseases caused by either 11A or 11E have preferentially increased in prevalence in recent years.
Acknowledgments
We thank Dr. Marilyn Crane for providing a pneumococcal strain.
This work was funded by the NIH grant AI-31473, the NIH Training Grant T32-AI0007041, and the UAB Medical Scientist Training Program Fund GM-008361.
Footnotes
Neither of the authors has conflicts of interest.
References
- 1.Avery OT, Dubos R. The protective action of a specific enzyme against type III pneumococcus infection in mice. J Exp Med. 1931;54:73–89. doi: 10.1084/jem.54.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole R. Treatment of pneumonia by means of specific serums. JAMA. 1913;61:663–666. [Google Scholar]
- 3.Rennels MB, Edwards KM, Keyserling HL, et al. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics. 1998;101:604–611. doi: 10.1542/peds.101.4.604. [DOI] [PubMed] [Google Scholar]
- 4.Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MC, Nahm MH. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol. 2007;45:1225–1233. doi: 10.1128/JCM.02199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henrichsen J. Six newly recognized types of Streptococcus pneumoniae. J Clin Microbiol. 1995;33:2759–2762. doi: 10.1128/jcm.33.10.2759-2762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llull D, Lopez R, Garcia E. Genetic bases and medical relevance of capsular polysaccharide biosynthesis in pathogenic streptococci. Curr Mol Med. 2001;1:475–91. doi: 10.2174/1566524013363618. [DOI] [PubMed] [Google Scholar]
- 7.Park IH, Park S, Hollingshead SK, Nahm MH. Genetic basis for the new pneumococcal serotype, 6C. Infect Immun. 2007;75:4482–9. doi: 10.1128/IAI.00510-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bentley SD, Aanensen DM, Mavroidi A, et al. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. Pub Lib of SciGenet. 2006;2:e31. doi: 10.1371/journal.pgen.0020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aanensen DM, Mavroidi A, Bentley SD, Reeves PR, Spratt BG. Predicted functions and linkage specificities of the products of the Streptococcus pneumoniae capsular biosynthetic loci. J Bacteriol. 2007;189:7856–76. doi: 10.1128/JB.00837-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mavroidi A, Aanensen DM, Godoy D, et al. Genetic relatedness of the Streptococcus pneumoniae capsular biosynthetic loci. J Bacteriol. 2007;189:7841–55. doi: 10.1128/JB.00836-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bender MH, Cartee RT, Yother J. Positive correlation between tyrosine phosphorylation of CpsD and capsular polysaccharide production in Streptococcus pneumoniae. J Bacteriol. 2003;185:6057–66. doi: 10.1128/JB.185.20.6057-6066.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Selm S, van Cann LM, Kolkman MA, van der Zeijst BA, van Putten JP. Genetic basis for the structural difference between Streptococcus pneumoniae serotype 15B and 15C capsular polysaccharides. Infect Immun. 2003;71:6192–8. doi: 10.1128/IAI.71.11.6192-6198.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zartler ER, Porambo RJ, Anderson CL, Chen LH, Yu J, Nahm MH. The structure of the capsular polysaccharide of pneumococcal serotype 11A reveals a novel acetyl-glycerol that is the structural basis for 11A subtypes. J Biol Chem. 2009;284:7318–7329. doi: 10.1074/jbc.M807952200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nahm MH, Lin J, Finkelstein JA, Pelton SI. Increase in the prevalence of the newly discovered pneumococcal serotype 6C in the nasopharynx after introduction of pneumococcal conjugate vaccine. J Infect Dis. 2009;199:320–325. doi: 10.1086/596064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang SS, Hinrichsen VL, Stevenson AE, et al. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009;124:e1–11. doi: 10.1542/peds.2008-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richter SS, Heilmann KP, Dohrn CL, Riahi F, Beekmann SE, Doern GV. Changing epidemiology of antimicrobial-resistant Streptococcus pneumoniae in the United States, 2004-2005. Clin Infect Dis. 2009;48:e23–33. doi: 10.1086/595857. [DOI] [PubMed] [Google Scholar]
- 17.Kellner JD, Scheifele D, Vanderkooi OG, Macdonald J, Church DL, Tyrrell GJ. Effects of routine infant vaccination with the 7-valent pneumococcal conjugate vaccine on nasopharyngeal colonization with streptococcus pneumoniae in children in Calgary, Canada. Pediatr Infect Dis J. 2008;27:526–32. doi: 10.1097/INF.0b013e3181658c5c. [DOI] [PubMed] [Google Scholar]
- 18.Yu J, Carvalho Mda G, Beall B, Nahm MH. A rapid pneumococcal serotyping system based on monoclonal antibodies and PCR. J Med Microbiol. 2008;57:171–8. doi: 10.1099/jmm.0.47549-0. [DOI] [PubMed] [Google Scholar]
- 19.Lin J, Kaltoft MS, Brandao AP, et al. Validation of a multiplex pneumococcal serotyping assay with clinical samples. J Clin Microbiol. 2006;44:383–8. doi: 10.1128/JCM.44.2.383-388.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trzcinski K, Thompson CM, Lipsitch M. Construction of otherwise isogenic serotype 6B, 7F, 14, and 19F capsular variants of Streptococcus pneumoniae strain TIGR4. Appl Environ Microbiol. 2003;69:7364–70. doi: 10.1128/AEM.69.12.7364-7370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enright MC, Spratt BG. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology. 1998;144(Pt 11):3049–60. doi: 10.1099/00221287-144-11-3049. [DOI] [PubMed] [Google Scholar]
- 22.Sung CK, Li H, Claverys JP, Morrison DA. An rpsL cassette, janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl Environ Microbiol. 2001;67:5190–6. doi: 10.1128/AEM.67.11.5190-5196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munoz R, Lopez R, Garcia E. Characterization of IS1515, a functional insertion sequence in Streptococcus pneumoniae. J Bacteriol. 1998;180:1381–8. doi: 10.1128/jb.180.6.1381-1388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tettelin H, Nelson KE, Paulsen IT, et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293:498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- 25.Slauch JM, Lee AA, Mahan MJ, Mekalanos JJ. Molecular characterization of the oafA locus responsible for acetylation of Salmonella typhimurium O-antigen: oafA is a member of a family of integral membrane trans-acylases. J Bacteriol. 1996;178:5904–9. doi: 10.1128/jb.178.20.5904-5909.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richards JC, Perry MB, Kniskern PJ. The structure of the specific capsular polysaccharide of Streptococcus pneumoniae type 11F (American type 11) Can J Biochem Cell Biol. 1985;63:953–68. doi: 10.1139/o85-118. [DOI] [PubMed] [Google Scholar]
- 27.Bera A, Biswas R, Herbert S, Gotz F. The presence of peptidoglycan O-acetyltransferase in various staphylococcal species correlates with lysozyme resistance and pathogenicity. Infect Immun. 2006;74:4598–45604. doi: 10.1128/IAI.00301-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajam G, Carlone GM, Romero-Steiner S. Functional antibodies to the O-acetylated pneumococcal serotype 15B capsular polysaccharide have low cross-reactivities with serotype 15C. Clin Vaccine Immunol. 2007;14:1223–7. doi: 10.1128/CVI.00184-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones C, Lemercinier X. Full NMR assignment and revised structure for the capsular polysaccharide from Streptococcus pneumoniae type 15B. Carbohydr Res. 2005;340:403–9. doi: 10.1016/j.carres.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 30.McNeely TB, Staub JM, Rusk CM, Blum MJ, Donnelly JJ. Antibody responses to capsular polysaccharide backbone and O-acetate side groups of Streptococcus pneumoniae type 9V in humans and rhesus macaques. Infect Immun. 1998;66:3705–10. doi: 10.1128/iai.66.8.3705-3710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]