Abstract
Background
The instantaneous inhibitory potential (IIP), a measure of antiviral activity that incorporates the slope of the dose-response curve, has been proposed as a better predictor of clinical efficacy than the inhibitory quotient (IQ). However there are no quantitative analyses supporting this hypothesis.
Methods
The correlation between differences in (Δ) log10(IQ) or IIP and percent of subjects with plasma HIV-1 RNA below 50 copies/mL at week 48 was determined for antiretroviral drugs compared in 17 randomized clinical trials. The Δ log10(IQmin), log10(IQmax), IIPmin, IIPmax log10(IQ12), log10(IQ24), IIP12 and IIP24 for comparative drugs were correlated with Δ percent of subjects with HIV < 50 copies/mL in each trial. Log10(IQ24), log10(IQ12), IIP24 and IIP12 were calculated using published median effect model slope values, and t1/2 values; r2 values from linear regression and Spearman correlation coefficients were calculated for each analysis and correlations coefficients were compared between Log10(IQ) and IIP.
Results
The r2 values were greatest for the Δlog10(IQ12) and Δlog10(IQ24) comparisons using ITT outcomes from the 17 trials. Differences in r2 values between Δlog10(IQ24) and ΔIIP24, and between Δlog10(IQ12) and ΔIIP12 were 0.05 and 0.18 respectively. Differences in Spearman rank correlation coefficients between log10(IQ) and IIP at each drug concentration were not significantly different with the exception of Δlog10(IQmax) and ΔIIPmax; the Δlog10(IQmax) correlation was significantly stronger than the ΔIIPmax correlation.
Conclusions
The IIP was not substantially better than log(IQ) in describing the modest relationship between antiviral activity, pharmacokinetics and virologic outcomes for antiretroviral drugs.
Keywords: HIV infection, antiviral therapy, pharmacology, inhibitory quotient, instantaneous inhibitory potential
INTRODUCTION
Reliable laboratory and mathematical models to predict the antiretroviral activity of candidate drugs could aid the selection of novel agents for clinical development. Widely used measures to quantify antiviral activity include the IC50 (amount of drug required to inhibit 50 percent of viral activity in vitro) and the inhibitory quotient (IQ), which is the trough drug concentration divided by the IC50. The IQ shows a modest correlation with clinical outcome [1-2], but outcomes can vary in relation to relatively minor changes in maximum inhibition that are not reflected in the IQ. Moreover, the shape of the dose-response curve can vary substantially between drugs with similar IC50’s due to differences in slope. There has been interest in designing and implementing new laboratory measures that overcome existing limitations. A recent study described an index for comparing antiviral activity of different drugs using classic dose-response relationships that incorporate the slope of the inhibition curve, or Hill coefficient, for each drug [3]. The Hill coefficient was first used to describe cooperative binding in hemoglobin [4], and has a role in modeling synergy between different drugs [3, 5-6]. This index, termed the instantaneous inhibitory potential (IIP), is defined by Equation 1:
| (1) |
in which fu represents the fraction of viruses in a single-round infectivity assay unaffected by drug, Ct represents antiviral concentrations at specific time (t), and m represents the slope from the median effect model of mass action.
The IIP has been proposed as a better predictor of antiviral activity in vivo than IQ because it takes into account the intrinsic antiviral potency of a drug and potentially the cooperativity of drugs used in combination [3, 7-8]. It was also proposed that the slope parameter of the IIP is class-specific and define intrinsic limitations on antiviral activity in some classes [3]. However, the IIP has not been compared rigorously to traditional pharmacologic measures such as the IQ, and it is unclear that IIP is a better indicator of virologic response. Correlating in vitro antiviral activity with virologic outcomes has been challenging given the diverse ways in which virologic response data are analyzed and the complexities of extrapolating antiviral activity to predict outcome measures. To test the hypothesis that IIP provides a better measure of clinical outcome than previous pharmacodynamic metrics, we compared the correlation between differences in IIP or IQ and differences in virologic response between pairs of antiretroviral agents using data from 17 randomized clinical trials of antiretroviral drugs.
METHODS
Data source
Table 1 lists the clinical trials included in the analysis. Published phase 2b or phase 3 randomized trials were included for analysis if they reported the proportion of subjects with plasma HIV-1 RNA below 50 copies/mL at 48 weeks or one year (with the exception of ACTG A5142, which reported HIV-1 RNA at 96 weeks) and if the pharmacokinetic data required to calculate IQs and IIPs were available [9-28]. Our analysis incorporated data from 11 clinical trials originally addressed by Shen et al. [3], as well as data from 6 additional trials that compared antiretroviral drugs from various classes [9-27].
TABLE 1.
Differences in log10(IQ24), IIP24, and differences in percent of subjects with VL < 50 copies/ml at 48 weeks for trials included in analysisa
| Trial | Drugs Compared | First Regimen | ΔLog10(IQ24) b | ΔIIP24b | Δ % Subjects with VL < 50 copies/mlc |
|---|---|---|---|---|---|
| GSK934d [12] | TDF vs. AZT | Yes | 0.1 | 0.2 | 9 |
| GSK903d [13] | D4T vs. TDF | Yes | −2.1 | −0.7 | 3 |
| ACTG A5095 [14] | EFV vs. ABC | Yes | 1.0 | 3.3 | 22 |
| BMS034 [20] | EFV vs. ATV | Yes | 1.8 | 1.7 | 5 |
| 2NN [22] | EFV vs. NVP | Yes | 1.0 | 2.0 | 5e |
| DMP006 [21] | EFV vs. IDV | Nof | 5.1 | 5.4 | 21 |
| A5142 a [28] | EFV vs. LPV/r | Yes | 1.3 | 1.4 | 12 |
| NEAT [19] | fAMP vs. NFV | Yes | 1.0 | 1.5 | 14 |
| KLEAN [11] | fAMP/r vs. LPV/r | Yes | −0.9 | −1.8 | 1 |
| ARTEMIS [18] | DRV/r vs. LPV/r | Yes | 0.4 | 4.4 | 6 |
| TITAN [16] | DRV/r vs. LPV/r | No | 0.4 | 4.4 | 11 |
| M96-863 [24] | LPV/r vs. NFV | Yes | 2.1 | 3.8 | 15 |
| CASTLE [17] | ATV/r vs. LPV/r | Yes | −0.1 | 0.9 | 2 |
| GEMINId [23] | SQV/r vs. LPV/r | Yes | −1.2 | −1.1 | 1 |
| MaxCmin1d [9, 25] | SQV/r vs. IDV/r | No | 0.7 | 2.4 | 11 |
| MaxCmin2d [10, 25] | LPV/r vs. SQV/r | No | 1.1 | 1.1 | 7 |
| STARTMRKd [26] | RAL vs. EFV | Yes | −1.5 | −3.5 | 4 |
NOTE: VL = viral load, r = ritonavir boosted protease inhibitor, Δ = difference between values for antivirals compared, TDF = tenofovir, AZT = zidovudine, D4T = stavudine, EFV = efavirenz, NVP = nevirapine, ABC = abacavir, ATV = atazanavir, IDV = indinavir, LPV = lopinavir, fAMP = fosamprenavir, DRV = darunavir, NFV = nelfinavir, SQV = saquinavir, RAL = raltegravir
ACTG 5142 VL data from 96 week time-point
Rounded to nearest decimal
Outcomes based on intent to treat analyses (switch/discontinuation/non-completion = failure, when available), rounded to nearest integer
Trial not included in original analysis by Shen et al.[3]
difference in viral load calculated from results of twice-daily NVP dosing
Subject were excluded from this trial if any prior exposure to lamivudine or any NNRTI or PI
Results of intention-to-treat (ITT) analyses of virologic outcomes were used in the primary analysis; if several analyses were reported, data from the switch or discontinuation equals failure analysis were used, whenever possible. Data from ITT analyses were reported most consistently, and were similar to those presented in the original description of the IIP [3].
Study design
The difference in the percentage of study subjects achieving virologic suppression was plotted against the difference between log10(IQ) or IIP values for the drugs being compared in a particular trial (Δlog10[IQ] or ΔIIP, respectively). Separate analyses were performed using minimum and maximum steady-state drug concentrations to calculate Δlog10(IQmin) and ΔIIPmin, or Δlog10(IQmax) and ΔIIPmax, respectively. In addition, Δlog10(IQ12) and Δlog10(IQ24) were compared with ΔIIP12 andΔIIP24, respectively, using drug concentrations 12 and 24 hours after Cmax. For trials included in the analysis by Shen et al., IQmin, IQmax, IIPmin and IIPmax values were based on those reported in their paper, as were values for slope (m) and IC50 [3]. For the remaining trials, IQ24, IQ12, IIP24 and IIP12 were calculated based on published virologic and pharmacokinetic data or data from the FDA-approved prescribing information (package insert). Drug concentrations 12 and 24 hours after Cmax were calculated using the classic decay equation (formula 2) [3]:
| (2) |
where k = ln(2)/t1/2, and t is the time after Cmax.
Statistical analyses
Linear regression curve-fitting was performed using Graphpad v5 (Graphpad Software, CA). Slopes, 95% confidence intervals (CI) for the slope, r2 values and 95% CI for the regression line from best-fit values were calculated. Spearman (rank based) correlation coefficients and P values were also calculated using Graphpad v5. Dependent inter-correlation comparisons using t-tests were performed using SISA. The nominal level of significance was defined as p = 0.05.
RESULTS
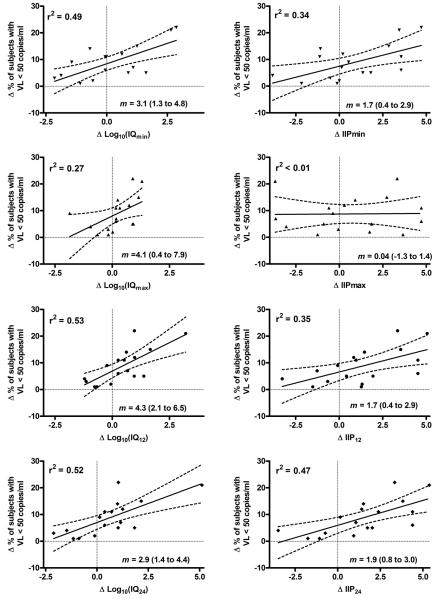
Table 1 lists the antiviral drugs compared in each trial, along with Δlog10(IQ24) and ΔIIP24 for the comparator drugs and differences in virologic suppression between the study arms. The ITT data were the most consistent among the trials compared. Thirteen of the 17 trials compared initial antiretroviral regimens in treatment-naïve patients (the DMP006 trial was open to patients with prior NRTI treatment [except lamivudine], but excluded patients previously treated with any NNRTI or PI) [21]. Figure 1 shows scatter plots and linear regressions of the difference in virologic outcome versus the Δlog10(IQ), or ΔIIP at Cmin, Cmax C12, and C24. Each plot yielded a modest positive correlation between Δlog10(IQ) or ΔIIP and the difference in virologic outcome between treatment arms with the exception of ΔIIPmax (ΔIIP at Cmax). The highest r2 values were obtained for correlations between the difference in virologic outcome and Δlog10(IQ12), Δlog10(IQ24), and Δlog10(IQmin), respectively. Differences in r2 values between analyses using Δlog10(IQ24) and ΔIIP24, or between Δlog10(IQ12) and ΔIIP12 were 0.05 and 0.18, respectively and slopes for these correlations had overlapping 95% confidence limits (Figure 1).
FIGURE 1.
Scatter plots of Δ percent patients in ITT analysis that demonstrated HIV viral load <50 copies/ml at week 48 (week 96 for ACTG A5142) versus Δlog10(IQ) and IIP at different concentrations. Linear regression lines and 95% confidence intervals are shown. R2 and slope (m) with 95% confidence intervals are also shown. Min, max = minimum and maximum steady-state antiviral concentrations; IQ12, IIP12, and IQ24, IIP24 calculated from concentrations 12 and 24 hours after Cmax respectively.
Results of Spearman (rank based) correlation analyses were consistent with those of the linear regression models. All of the comparisons revealed modest, statistically significant correlations between differences in virologic outcome and Δlog10(IQ) or ΔIIP with the exception of ΔIIPmax. The correlation coefficients are listed in Table 2. There were no significant differences between correlation coefficients for Δlog10(IQ) and ΔIIP at each drug concentration (e.g. Δlog10[IQ12] and ΔIIP12) with the exception of Cmax (P = 0.03). The Δlog10(IQmax) correlation was significantly stronger than that of the ΔIIPmax. Sample size for these comparisons was relatively small (N=17).
TABLE 2.
Spearman rank correlations of differences in percent of subjects demonstrating VL < 50 copies/ml for trials included in analysis and differences in log10(IQ) and IIPa
| Spearman Correlation Coefficient | P | |
|---|---|---|
| ΔLog10(IQmin) | 0.63 | 0.007 |
| ΔIIPmin | 0.54 | 0.025 |
| ΔLog10(IQmax) | 0.67 | 0.004 |
| ΔIIPmax | 0.07 | 0.802 |
| ΔLog10(IQ12) | 0.72 | 0.001 |
| ΔIIP12 | 0.56 | 0.020 |
| ΔLog10(IQ24) | 0.73 | 0.001 |
| ΔIIP24 | 0.72 | 0.001 |
NOTE: VL = viral load, Δ = difference between values for antivirals compared
Virologic outcomes from intent to treat analysis
DISCUSSION
In this study, we explored the correlation between the instantaneous inhibitory potential of antiretroviral drugs and their virologic efficacy, and sought to determine whether differences in IIP might predict the outcome of randomized clinical trials comparing two antiretroviral regimens better than differences in the inhibitory quotient. A previous study suggested that by incorporating the slope, or steepness, of the drug inhibition curve the IIP represented a more accurate pharmacodynamic measure of in vivo antiviral activity than traditional parameters and may play a major role in determining virologic outcomes [3]. This new measure has generated a great deal of interest since its introduction, especially because it has been purported to predict differences in potency of different antiviral classes; the IIP has also been proposed as a tool for selecting new drugs for clinical development [3, 7-8]. Our analysis of data from 17 randomized clinical trials of antiretroviral drugs (ITT analysis) found that differences in IIP between drugs did show a modest correlation with differences in virologic outcome, but that these correlations were not significantly stronger than the correlations obtained using the IQ. The strongest correlations between difference in pharmacokinetic variables and difference in percent virologic suppression from linear regression modeling were for Δlog10(IQ12), Δlog10(IQ24), and Δlog10(IQmin), respectively, whereas the strongest Spearman correlations were for Δlog10(IQ24), ΔIIP24, and Δlog10(IQ12). Differences between analyses were modest and Spearman correlations were not significantly different between Δlog10(IQ12) and ΔIIP12, and Δlog10(IQ24) and ΔIIP24; these results are not surprising given the relatively small sample size used in the correlation analysis.
Despite positive correlations between the difference in virologic outcome and Δlog10(IQ) or ΔIIP overall, many individual studies fell well outside the 95% confidence limits of the linear regression plots. In some cases, small differences in treatment outcome between arms were observed despite substantial differences in IIP or IQ between the comparator drugs; conversely, in other studies significant differences in outcome were observed despite modest differences in IIP or IQ. Although this finding may reflect the uncertainty in the estimated difference in both virologic and pharmacokinetic effect sizes, it suggests that variables other than intrinsic drug activity and pharmacokinetics, such as adherence, tolerability, dosing convenience, and emergence of resistance are important contributors to overall clinical effectiveness of a drug or regimen. The predictive capacity of both IIP and IQ are therefore inherently limited by the complex factors encountered during the extrapolation of in vitro measures to virologic outcome in clinical trials.
While it is tempting to conclude that molecules with a steeper dose response curve make better drugs, clinical experience suggests that other properties such as the pharmacokinetic profile, tolerability and dosing convenience may be more important determinants of drug efficacy. For example, the slope of the inhibition curve for indinavir is substantially greater than that for efavirenz (Figure 1d and Supplementary Table 1 in [3]), but efavirenz is more effective than indinavir due to its longer half-life and better tolerability [21]. Likewise, lopinavir and nelfinavir have similar slope values [3], but comparative trials show that lopinavir/ritonavir is superior to nelfinavir [24]. The generally similar correlation between IIP and IQ with virologic outcome in the trials we studied suggests that drug susceptibility (as measured by IC50), pharmacokinetic parameters (as measured by Cmin, Cmax, t½ etc.), which are common to IIP and IQ, dominate the slope in determining drug efficacy.
The 48 week endpoint (ITT, switch or non-completion = failure when available) was chosen for primary analysis for 3 major reasons: 1) similar data were used in the original description of the IIP [3], 2) it was the primary endpoint of most of the studies incorporated in our analysis, and 3) there are limitations to publically available as-treated data or from earlier time-points. Factors such as drug toxicity, tolerability, baseline drug resistance, variations in background antiviral therapies and loss to follow-up no doubt contributed to overall regimen efficacy, but are not captured by either the IIP or IQ. However, the aim of this exploratory analysis was to provide a quantitative estimate of the association between pharmacodynamic properties of different drugs and virologic outcomes, and was consistent with the approach taken in the original IIP report. However, that report did not formally test the relative strength of association of the IIP and other traditional pharmacodynamic metrics with virologic outcomes in clinical trials [3], making it difficult to infer superiority of one laboratory measure over another.
Although utilizing as-treated or per-protocol data may partially control for potentially confounding factors such as drug toxicity, tolerability and medication cross-over, there are limitations with this potential approach. As-treated results may involve an endpoint in which subjects are censored at treatment discontinuation, which would be informative in our comparison between IIP and IQ. However, many trials do not define early discontinuation of patients, and the as-treated results represent a cross-sectional analysis of patients still randomized at week 48. This analysis discounts early treatment discontinuation for inadequate virologic outcome and leads to informative censoring. Although a method for carrying forward the last HIV-1 RNA level to subsequent time-points of a patient who discontinues treatment early during a study protocol may overcome some of the informative censoring, this type of analysis is not possible without access to raw data for all trials. Furthermore, this manipulation does not necessarily overcome the problem of bias created by early discontinuations before a study subject has had a chance to have a response to medication.
A method of quantifying treatment differences for both a major clinical outcome and a surrogate marker and for measuring the strength of association between these values has been described [29]. This approach requires that measurements of the surrogate marker and the clinical endpoint come from one group of individuals or from a single study, or requires an allowance of precision to be assessed for treatment differences across studies [29]. We could not apply this approach to our analysis as it requires access to raw data from each of the respective clinical trials. Moreover, in the current analysis the treatment effect was estimated across trials with different sample sizes and different degrees of precision, which were not taken into consideration. Furthermore, few of these studies included measurement of both pharmacokinetic parameters and longer term clinical outcomes, which would be necessary for a more complete analysis. However, all the virologic studies included in our analysis were based on robust clinical trials with relatively large study populations. Spearman rank correlations were chosen to reduce emphasis on individual trial size and the strength of each correlation data point as well as to compensate for potential lack of linearity between variables.
Despite these limitations, this study provides an in-depth, quantitative comparison of the relationship between log10IQ, IIP, and virologic outcome. Our analysis shows that the insights the IIP provides into the kinetics of drug activity at the cellular and biochemical level do not translate into substantially better predictions of efficacy from primary outcomes of major clinical trials than traditional measures such as the IQ. The slope of the dose response curve used in calculation of the IIP may be an important factor influencing in vivo antiviral activity, but better models are needed for predicting the relative efficacy of antiretroviral regimens that incorporate these additional pharmacokinetic and cooperative drug parameters.
Summary: Article we compares the correlation between differences in IIP or IQ and differences in virologic response between pairs of antiretroviral agents in order to test the hypothesis that IIP provides a better measure of clinical outcome than previous pharmacodynamic metrics.
Acknowledgements
D.R.K. is a consultant to, and/or has received research support or honoraria from Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Merck, Monogram BioScience, Pfizer and Roche. T.J.H is the recipient of a Bristol-Myers-Squibb Virology Fellows grant. H.J.R served on the data and safety monitoring board for Koronis Pharmaceuticals and has received honoraria from Roche Diagnostics.
Grant support: NIH grant K24 RR016482 and U01 AI068636 (to D.R.K.); NRSA training grant T32 AI07387-19 (to T.J.H.); NIH / Harvard Catalyst KL2 MeRIT grant RR025757-01 (to T.J.H.); NIH grant P30 AI060354 (to H.J.R.).
Footnotes
Presented in part: 17th Conference on Retroviruses and Opportunistic Infections, San Francisco, CA, 16-19 February 2010 (abstract 51).
References
- 1.Hoefnagel JG, Koopmans PP, Burger DM, Schuurman R, Galama JM. Role of the inhibitory quotient in HIV therapy. Antiviral therapy. 2005;10:879–92. [PubMed] [Google Scholar]
- 2.Morse GD, Catanzaro LM, Acosta EP. Clinical pharmacodynamics of HIV-1 protease inhibitors: use of inhibitory quotients to optimise pharmacotherapy. The Lancet infectious diseases. 2006;6:215–25. doi: 10.1016/S1473-3099(06)70436-4. [DOI] [PubMed] [Google Scholar]
- 3.Shen L, Peterson S, Sedaghat AR, et al. Dose-response curve slope sets class-specific limits on inhibitory potential of anti-HIV drugs. Nature medicine. 2008;14:762–6. doi: 10.1038/nm1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill AV. The possible effects of the aggregation of the molecules of hemoglobin on its dissociation curves. Proceedings of the Physiological Socieity. 1910:iv–vii. [Google Scholar]
- 5.Chou TC. Derivation and properties of Michaelis-Menten type and Hill type equations for reference ligands. Journal of theoretical biology. 1976;59:253–76. doi: 10.1016/0022-5193(76)90169-7. [DOI] [PubMed] [Google Scholar]
- 6.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in enzyme regulation. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 7.McMahon MA, Shen L, Siliciano RF. New approaches for quantitating the inhibition of HIV-1 replication by antiviral drugs in vitro and in vivo. Current opinion in infectious diseases. 2009;22:574–82. doi: 10.1097/QCO.0b013e328332c54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen L, Rabi SA, Siliciano RF. A novel method for determining the inhibitory potential of anti-HIV drugs. Trends in pharmacological sciences. 2009;12:610–6. doi: 10.1016/j.tips.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dragsted UB, Gerstoft J, Pedersen C, et al. Randomized trial to evaluate indinavir/ritonavir versus saquinavir/ritonavir in human immunodeficiency virus type 1-infected patients: the MaxCmin1 Trial. The Journal of infectious diseases. 2003;188:635–42. doi: 10.1086/377288. [DOI] [PubMed] [Google Scholar]
- 10.Dragsted UB, Gerstoft J, Youle M, et al. A randomized trial to evaluate lopinavir/ritonavir versus saquinavir/ritonavir in HIV-1-infected patients: the MaxCmin2 trial. Antiviral therapy. 2005;10:735–43. [PubMed] [Google Scholar]
- 11.Eron J, Jr., Yeni P, Gathe J, Jr., et al. The KLEAN study of fosamprenavir-ritonavir versus lopinavir-ritonavir, each in combination with abacavir-lamivudine, for initial treatment of HIV infection over 48 weeks: a randomised non-inferiority trial. Lancet. 2006;368:476–82. doi: 10.1016/S0140-6736(06)69155-1. [DOI] [PubMed] [Google Scholar]
- 12.Gallant JE, DeJesus E, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. The New England journal of medicine. 2006;354:251–60. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 13.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. Jama. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 14.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. The New England journal of medicine. 2004;350:1850–61. doi: 10.1056/NEJMoa031772. [DOI] [PubMed] [Google Scholar]
- 15.Lennox J, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive HIV-1 infected patients: STARTMRK Protocol 021; ICAAC/IDSA Annual Meeting; Washington, DC. 2008; Abstract H896a. [Google Scholar]
- 16.Madruga JV, Berger D, McMurchie M, et al. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial. Lancet. 2007;370:49–58. doi: 10.1016/S0140-6736(07)61049-6. [DOI] [PubMed] [Google Scholar]
- 17.Molina JM, Andrade-Villanueva J, Echevarria J, et al. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008;372:646–55. doi: 10.1016/S0140-6736(08)61081-8. [DOI] [PubMed] [Google Scholar]
- 18.Ortiz R, Dejesus E, Khanlou H, et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS (London, England) 2008;22:1389–97. doi: 10.1097/QAD.0b013e32830285fb. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-French A, Boghossian J, Gray GE, et al. The NEAT study: a 48-week open-label study to compare the antiviral efficacy and safety of GW433908 versus nelfinavir in antiretroviral therapy-naive HIV-1-infected patients. Journal of acquired immune deficiency syndromes (1999) 2004;35:22–32. doi: 10.1097/00126334-200401010-00003. [DOI] [PubMed] [Google Scholar]
- 20.Squires K, Lazzarin A, Gatell JM, et al. Comparison of once-daily atazanavir with efavirenz, each in combination with fixed-dose zidovudine and lamivudine, as initial therapy for patients infected with HIV. Journal of acquired immune deficiency syndromes (1999) 2004;36:1011–9. doi: 10.1097/00126334-200408150-00003. [DOI] [PubMed] [Google Scholar]
- 21.Staszewski S, Morales-Ramirez J, Tashima KT, et al. Study 006 Team Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. The New England journal of medicine. 1999;341:1865–73. doi: 10.1056/NEJM199912163412501. [DOI] [PubMed] [Google Scholar]
- 22.van Leth F, Phanuphak P, Ruxrungtham K, et al. Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. Lancet. 2004;363:1253–63. doi: 10.1016/S0140-6736(04)15997-7. [DOI] [PubMed] [Google Scholar]
- 23.Walmsley S, Avihingsanon A, Slim J, et al. Gemini: a noninferiority study of saquinavir/ritonavir versus lopinavir/ritonavir as initial HIV-1 therapy in adults. Journal of acquired immune deficiency syndromes (1999) 2009;50:367–74. doi: 10.1097/QAI.0b013e318198a815. [DOI] [PubMed] [Google Scholar]
- 24.Walmsley S, Bernstein B, King M, et al. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. The New England journal of medicine. 2002;346:2039–46. doi: 10.1056/NEJMoa012354. [DOI] [PubMed] [Google Scholar]
- 25.Youle M, Gerstoft J, Fox Z, et al. The final Week 48 analysis of a phase IV, randomised, open-label, multi-centre trial to evaluate safety and efficacy of lopinavir/ritonavir (400/100 mg bid) versus saquinavir/ritonavir (1000/10); Poster Late Breakers: The 2nd IAS Conference on HIV Pathogenesis and Treatment; Paris. 2003; Abstract no LB23. [Google Scholar]
- 26.Lennox JL, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374:796–806. doi: 10.1016/S0140-6736(09)60918-1. [DOI] [PubMed] [Google Scholar]
- 27.van Leth F, Hassink E, Phanuphak P, et al. Results of the 2NN Study: A Randomized Comparative Trial of First-line Antiretroviral Therapy with Regimens Containing Either Nevirapine Alone, Efavirenz Alone or Both Drugs Combined, Together with Stavudine and Lamivudine; 10th Conf Retrovir Oppor Infect; Boston, USA. abstract no 176. [Google Scholar]
- 28.Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. The New England journal of medicine. 2008;358:2095–106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes MD, DeGruttola V, Welles SL. Evaluating surrogate markers. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10(Suppl 2):S1–8. [PubMed] [Google Scholar]