BACKGROUND
Otosclerosis was first described in 1735 by Valsalva as ankylosis of the stapes to the margins of the oval window.1 Later in 1841, Toynbee described osseous anchylosis of the stapes to the fenestra ovalis as one of the common causes of deafness.2 The term sclerosis was introduced in 1881 by von Troltsch with the belief that sclerosis of the tympanic mucosa caused the fixation of the stapes,3 and even though in 1893 Politzer discovered the true pathology to be in the labyrinthine capsule,4 the term otosclerosis has remained in use.
Otosclerosis (Ous, ear; skleros, hard; osis, condition) is a distinctive bone dyscrasia known only to affect humans. It consists of one or several circumscribed foci of new, softer, and more vascular bone that replace the remarkably avascular endochondrial bone of the adult labyrinthine capsule.5 The process can spread across the stapedial annular ligament and fix the stapes, producing a conductive loss, or it can surround the cochlea and parts of the labyrinth, causing a sensorineural hearing loss, referred to as “labyrinthine otosclerosis”.6 Otosclerosis is one of the most common causes of conductive hearing loss (clinical otosclerosis) yet most cases of otosclerosis are asymptomatic and only diagnosed as incidental findings in temporal bone autopsies (histological otosclerosis). These changes can occur at many locations in the inner ear. The majority of cases consist of changes in the oval window, but they have also been described in the round window, cochlear apex, posterior to the oval window, posterior and anterior wall of the internal auditory canal (IAC), cochlear aqueduct, semicircular canals, and within the stapes footplate.7
Histopathologically, the disease is composed of active and inactive foci. Under light microscopy, active lesions (otospongiosis) can be recognized by their spongy structure and immature osseous tissue, by the size of the marrow spaces that contain a very cellular connective tissue with numerous osteoclastic giant cells, and most importantly by an increased and dilated vascularity. This corresponds to the areas undergoing active resorption by numerous multinucleated osteoclastic giant cells. Within the perivascular connective tissue, fibroblasts take the characteristics of the osteoblasts, osteocytes are more numerous, large and plump compared to normal bone, and a new immature basophilic fiber bone is laid down in a web-like pattern, which takes a bluish stain with H&E. Inactive lesions (otosclerosis) are characterized by solid compact mosaic-like osseous tissue that contains few and small marrow spaces as well as infrequent and small blood vessels. This corresponds to the resorption of immature bone and its replacement by an osseous tissue with less ground substance and more collagen fibers, which take a reddish color when stained with H&E. Here, the osteocytes are fewer, smaller and more mature. These steps go on continuously and irregularly within a focus.8
Cochlear otosclerosis (CO) is defined as a sensorineural hearing loss in the presence of, and presumably due to, an otosclerotic focus in the cochlear capsule without associated stapes fixation.7 CO is well known and has been described in the literature, but so far there has been no mention of cavity formations in CO foci. However, Schuknecht has shown histological sections that satisfy the criteria for bilateral cochlear otosclerosis in which both temporal bones exhibit severe cavitating otosclerosis with bilateral severe sensorineural hearing loss.9
METHODS
The temporal bone bank at our institution has 11 cases of neurosensory hearing loss that histopathologically fulfill criteria for a diagnosis of otosclerosis with cavitation. Of these bones, 3 cases present the cavitation in a pericochlear otosclerotic focus, 4 in a focus related to the IAC, and 4 cases present with both. We describe 2 cases in which the cavitating otosclerosis presents with clinical and surgical relevance.
The temporal bones that were donated to the House Ear Institute were decalcified in EDTA for 8–12 months and later processed for celloidin embedding. The bones were then cut with a width of 20μm, and every 10th section was stained with Hematoxylin & Eosin. These sections were placed on slides and described under light microscopy. Histological findings were recorded and stored with the samples in the Institute’s temporal bone bank.
Following HIPPA regulations for protection of patient privacy, medical records were analyzed for a clear clinical and surgical history.
RESULTS
CASE 1
A 41-year-old male presented with a conductive hearing loss in his right ear. A right stapes mobilization was done 5 years later with some improvement, but failed to close the air-bone gap. Because of the persistent gap, a stapedectomy with a polyethylene strut on a vein graft was done, but again a 20 dB gap remained. Another revision with a piston also failed to close the gap, and he was diagnosed with “inner ear conductive hearing loss.”
Histological examination of the right temporal bone (Fig. 1) shows an opening through the obliterated footplate with a space for the prosthesis. There is a large otospongiotic and sclerotic lesion at the posterior apex of the cochlea and also involving the usual site of the fissula antefenestram. It extends to the wall of the cochlea, particularly the basal turn where there is hyalinization of the spiral ligament. There is a large cavitation of the anterior inferior internal auditory canal by the otosclerotic process which extends as far anteriorly as the edge of the basal turn of the cochlea. There is no bone between this excavation and the scala tympani, leaving only periosteum separating the two.
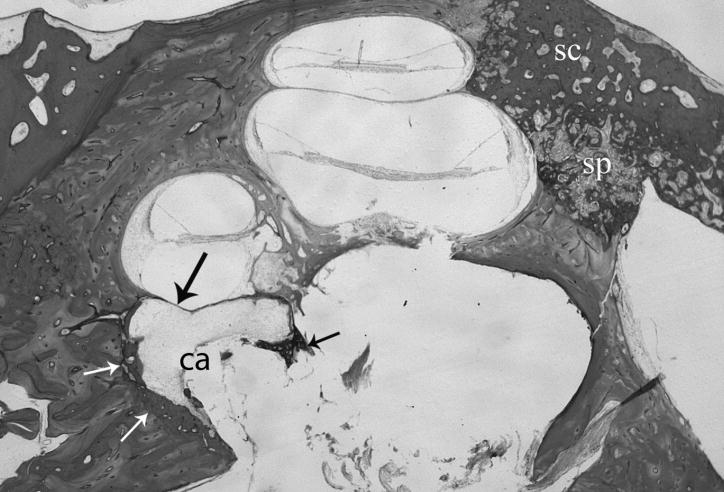
Fig. 1.
Histological section of right temporal bone shows otosclerosis (small arrows) surrounding a cavity (ca) that is continuous with the internal auditory canal and contains some loose fibrous tissue. The process has eroded the endosteal bone leaving only a fibrous membrane between the cochlear basal segment and the cavity (large arrow). (sc) otosclerosis. (sp) otospongiosis. H&E × 15.
CASE 2
A 47-year-old male suffered progressive hearing loss bilaterally over several years. He had a left stapedectomy and a subsequent revision. He did well with a left hearing aid until he became acutely deaf in the left ear following coughing fits. Preoperative CT showed essentially symmetric, bilateral, extensive multifocal and coalescent hypoattenuations in the cochlear capsules consistent with otosclerosis, as well as a focus approximating cerebrospinal fluid (CSF) in attenuation value (Fig. 2A). A left cochlear implantation encountered a CSF leak, which the surgeon sealed with a muscle plug, and concluded as partial implantation. An immediate postimplant CT showed the tip of the electrode array curving anteriorly out of the inferior segment of the basal turn into the otic capsule (Fig. 2B). Postoperative audiology reports showed the patient’s inability to hear on the implanted side.
Fig. 2.
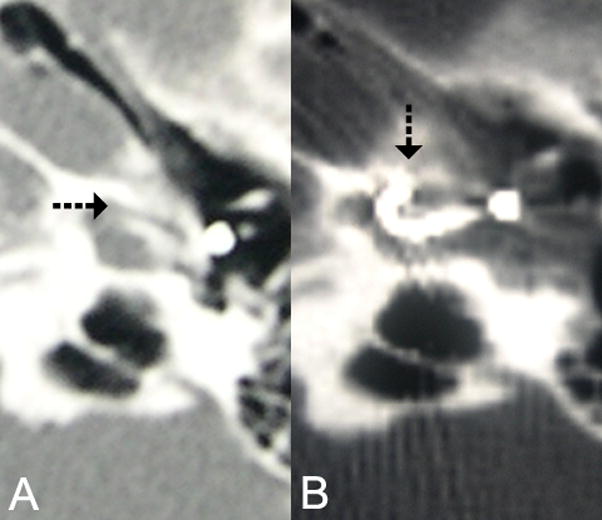
(A) Pre cochlear implant CT shows pericochlear cavity. (B) CT of left cochlear implant shows electrode array traversing inferior segment of basal turn and curving anteriorly into a pericochlear cavity.
The patient was later referred for a right cochlear implant. At surgery, the round window was completely obliterated by otosclerotic bone. A CSF gusher was again encountered at cochleostomy and controlled by muscle packing. The surgeon proceeded to drill again in a more superior location and exposed the true cochlear lumen, which did not present with a CSF leak, and was able to introduce the full length of the electrode.
DISCUSSION
The first case describes a patient with conductive hearing loss due to the existence of a “third window”, which is a potential connection to the membranous labyrinth beyond the oval and round window.10 Such a connection can channel the inner ear fluid wave made by sound energy entering through the oval window away from the basilar membrane, but would have no effect on bone conduction. Many causes for a “third window” have been identified and described, including dehiscence of the semicircular canals, large vestibular aqueduct syndrome, DFN-3, dehiscence between the cochlea and carotid canal, and Paget’s disease, the most common being a dehiscence of the superior semicircular canal,11 but there is no description in the literature about this syndrome being caused by a cavity formation within an otosclerotic focus that is continuous with CSF.
The second case shows the relevance of a cavitating otosclerotic focus in the surgical field. A patient suffering from bilateral cavitating otosclerosis presented with a complication specific for a connection between the CSF and cochlear lumen. The cavities were large enough to contain the greater part of an electrode array of a cochlear implant (Fig. 3); they connected with the IAC (Fig. 4) and filled with CSF, which explains the gushers encountered during the procedures.
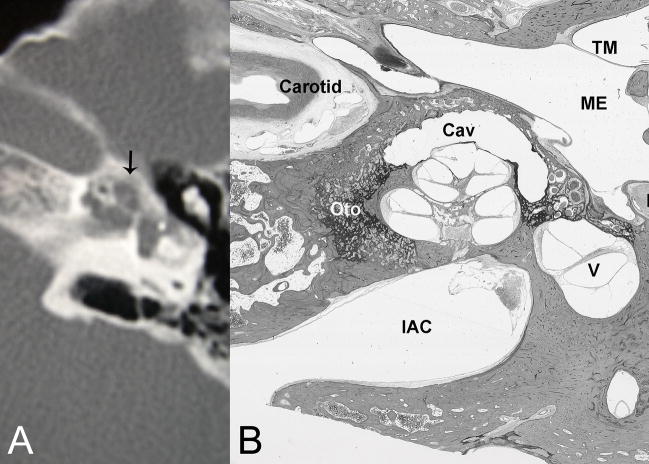
Fig. 3.
(A) CT reveals pericochlear cavities, one of which is in a periapical location. (B) Histological section of right temporal bone of another patient with comparable disease shows substantial otosclerosis foci (Oto) with a large cavity (Cav) capping (anterior and lateral to) the apex of the cochlea. (TM) tympanic membrane, (ME) middle ear, (FN) facial nerve, (V) vestibule, (IAC) internal auditory canal. H&E × 8.
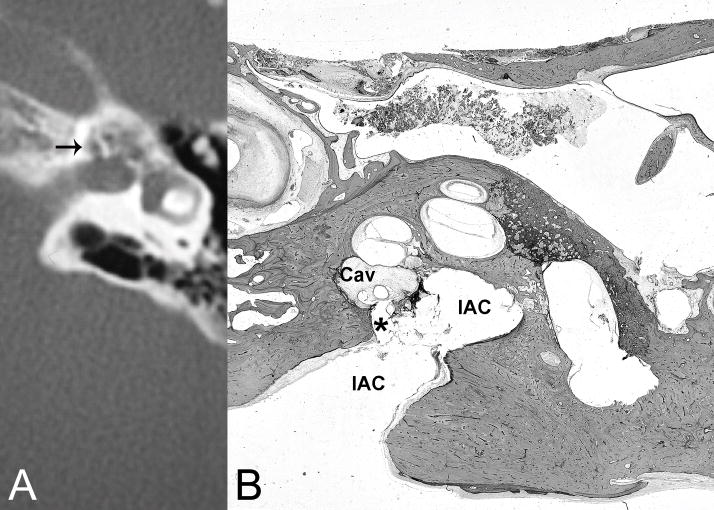
Fig. 4.
(A) CT demonstrates cavitating otosclerosis with pericochlear cavities, one of which communicates with the internal auditory canal near the fundus (arrow). (B) Histological section of right temporal bone of another patient with comparable disease shows otosclerosis cavity (Cav) in continuity (*) with the internal auditory canal (IAC) communicating with the cerebral spinal fluid space. H&E × 8.
Otosclerosis/spongiosis, whether fenestral or cochlear, is best demonstrated by high-resolution CT. Sclerotic foci are mildly hypoattenuating compared to normal bone of the otic capsule. Spongiotic foci approach soft-tissues in attenuation. Cavitating foci, which may communicate with the CSF space in the internal auditory canal, as shown in case 2, approximate CSF in attenuation. Rarely, osteogenesis imperfecta or tertiary otosyphilis may simulate florid otosclerosis, but the bone lesions tend to be less confined to the enchondral cochlear capsule. Recognizing cavitating otosclerosis by preimplant CT may help the surgeon prepare for possible CSF leak and avoid electrode misplacement into a pericochlear cavity.
CONCLUSION
Although the pathophysiology of a cavity formation in an otosclerosis focus is not yet understood, the cases described provide evidence that cavitating otosclerosis may be an additional cause of “third window” lesions, and it is a potential cause for CSF gushing and electrode misplacement at cochlear implantation.
References
- 1.Valsalva AM. Pera, Hoc Est, Tractatus de Aure Humana. Venice, Italy: Pitteri; 1741. [Google Scholar]
- 2.Toynbee J. Pathological and surgical observations on the diseases of the ear. Med Chir Tr. 1841;24:190. doi: 10.1177/095952874102400115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Von Troltsch AF. Lehrbuch der Ohrenheilkunde. 7. Leipzig, Germany: Vogel; 1881. [Google Scholar]
- 4.Politzer A. Uber primare erkrankung del knochernen labyrinthkapsel. Ztschr Ohrenh. 1893;25:309. [Google Scholar]
- 5.Wiet RJ, Causse JB, et al. Otosclerosis (Otospongiosis) 2. American Academy of Otolaryngology-Head Neck Surgery Foundation, Inc; 2007. [Google Scholar]
- 6.Linthicum FH. Otosclerosis. In: House JW, editor. The Otolaryngologic Clinics of North America. 3. Vol. 26. Philadelphia: W.B. Saunders Company; 1993. pp. 335–357. [PubMed] [Google Scholar]
- 7.Schuknecht HF. Pathology of the Ear. 2. New York: Lea and Febiger; 1993. pp. 365–379. [Google Scholar]
- 8.Nager GT. Pathology of the Ear and Temporal Bone. 1. Baltimore: Williams & Wilkins; 1993. pp. 943–1010. [Google Scholar]
- 9.Schuknecht HF. Pathology of the Ear. 2. New York: Lea and Febiger; 1993. p. 379. [Google Scholar]
- 10.Bance M. When is a conductive hearing loss not a conductive hearing loss? Causes of a mismatch in air-bone threshold measurements or a “pseudoconductive” hearing loss. J Otolaryngol. 2004 Apr;33(2):135–8. doi: 10.2310/7070.2004.00135. [DOI] [PubMed] [Google Scholar]
- 11.Merchant SN, Rosowski JJ. Conductive Hearing loss caused by third-window lesions of the inner ear. Otol Neurotol. 2008 Apr;29(3):282–9. doi: 10.1097/mao.0b013e318161ab24. [DOI] [PMC free article] [PubMed] [Google Scholar]