Abstract
The indications for free muscle transfer in brachial plexopathies are prolonged denervation time or inadequate upper extremity function after primary nerve reconstruction. The purpose of this study is to analyze the outcomes of free muscle transfer for elbow flexion and extension in brachial plexopathies in relation to the different muscles used and the respective motor donors. Seventy-three muscles were transferred for elbow flexion and ten for elbow extension. Latissimus dorsi (LD) was used in 37 cases, gracilis in 28, rectus femoris (RF) in seven, and vastus lateralis in one. Five LD and five gracilis were transferred for elbow extension. Patients younger than 15 years yielded better results than older patients for elbow flexion. When LD was transferred, the mean muscle grading (MG) was 3.33 ± 0.25 when the neurotization was from intercostals; these outcomes were statistically significant when compared with outcomes of free gracilis transfer (MG 2.25 ± 0.6). There was also a statistically significant difference when free LD was neurotized with three intercostals as compared with two intercostals nerves. RF yielded also good results when neurotized from contralateral C7 (cC7; MG 3.67 ± 0.6). For elbow extension, the better outcomes of LD were not statistically significant. Among all the free muscle transfers for upper extremity reconstruction, elbow reanimation yielded the most rewarding outcomes. The selection of powerful muscle units was more important than the effect of neurotization which was not as strong as it was in muscle transfers for facial or hand reanimation.
Keywords: Free muscle transfer, Elbow reanimation, Elbow flexion, Elbow extension, Plexopathy, Latissimus dorsi, Gracilis
Introduction
Brachial plexus injuries are devastating lesions. In cases of multiple avulsions, there is paucity of adequate motor donors. The philosophy in our center is to dynamically reanimate proximal to distal joints. Thus, shoulder stability, shoulder abduction, and external rotation take priority, prior to elbow reanimation. In late cases with atrophy of biceps/brachialis or in patients in which the final outcome of primary nerve reconstruction is judged inadequate, functioning free muscle transfer is a reliable option. Free muscle transfers were initially developed for facial reanimation [22] or to reconstruct hand function in Volkman’s contractures [28,31]. For brachial plexus reconstruction, free muscles were used initially for elbow flexion [24], and since then, different muscles have been tried and different techniques have been proposed. Rectus femoris (RF) [1,13,17,18,39,41], latissimus dorsi (LD) [5,18,19,39], and gracilis [4,11,13–16,19,39] were used with or without concomitant finger reanimation.
The evolution of free muscle transplantation for elbow animation in our center involved three periods: In the early period (1981–1986), the gracilis muscle was used alone. This technique was replaced with the combined transfer of gracilis and adductor longus as a double free muscle for elbow flexion and shoulder abduction (middle period 1987–1991). Since 1992 (late period), large muscle units have been transferred.
The purpose of this study was to determine the final outcome of elbow flexion or extension in relation to the chosen muscle and the supplying motor nerve. The current study was conducted under the guidelines and the approval of the Institutional Review Board Committee of Eastern Virginia Medical School.
Materials and Methods
Since 1981, 73 free muscles were transferred in our center for elbow flexion and ten for elbow extension (Tables 1, 2, 3, 4, 5, and 6). The most common mechanism of injury was high-velocity motor vehicle accident. There were 85% male and 15% female patients. The mean age at the time of surgery was 22.9 years.
Table 1.
Elbow flexion-gracilis, early period (1981–1986), single transfer.
| Neurotizations | No. of patients | Muscle grade | ||
|---|---|---|---|---|
| Preop | Postop | |||
| IC | 5 | 0.8 ± 0.44 | 2.25 ± 0.6 | p = 0.003 |
| XI | 1 | 1.33 | 2.66 | |
| C5 | 1 | 0 | 2.33 | |
| TOTAL | 7 | 0.76 ± 0.53 | 2.33 ± 0.54 | p < 0.001 |
Table 2.
Elbow flexion-gracilis and adductor longus, middle period (1987–1991), double free muscle transfer.
| Neurotizations | No. of neurotizations | Muscle grade | ||
|---|---|---|---|---|
| Preoperative | Postoperative | |||
| XI | 5 | 1.47 ± 0.5 | 3.2 ± 1.17 | p = 0.016 |
| IC | 4 | 1.08 ± 0.16 | 2.0 ± 0.5 | p = 0.018 |
| Contralateral lateral pectoral | 4 | 1 ± 0.8 | 2.91 ± 0.5 | p = 0.007 |
| Cervical Motors | 2 | 1.33 ± 0.33 | 2.66 ± 0.33 | |
| C5 | 1 | 1 | 4.33 | |
| Phrenic | 1 | 0 | 2.33 | |
| Total | 16 muscles | 1.1 ± 0.56 | 2.8 ± 0.91 | p < 0.001 |
| 17 neurotizations | ||||
Table 3.
Elbow flexion-gracilis, late period (1992 to date), single transfer.
| Neurotizations | No. of patients | Muscle grade | ||
|---|---|---|---|---|
| Preoperative | Postoperative | |||
| cC7 | 4 | 1.75 ± 0.56a | 3.33 ± 0.77b | p = 0.016 |
| XI | 1 | 2a | 4b | |
| TOTAL | 5 | 1.8 ± 0.5 | 3.46 ± 0.73b | p = 0.003 |
Mean muscle strength for Gracilis when it was transferred as a single muscle was 2.8 ± 0.83 (early and late period, Tables 1 and 3)
aInitial muscle strength from the transferred LD or rectus femoris was not sufficient to flex elbow against gravity
bFree gracilis has been transferred to augment weak elbow flexion over a previous LD or rectus femoris transfer. The postoperative muscle strength for elbow flexion was the muscle strength from both transferred muscles: gracilis and LD or rectus femoris
Table 4.
Elbow flexion, latissimus dorsi (late period: 1992 to date).
| Neurotizations | No. of patients | Muscle grade | ||
|---|---|---|---|---|
| Preoperative | Postoperative | |||
| IC | 16 | 1.17 ± 0.62 | 3.33 ± 0.25 | p < 0.001 |
| Distal spinal accessory | 7 | 1.44 ± 0.45 | 3.05 ± 0.1 | p < 0.001 |
| cC7 | 5 | 1.42 ± 0.42 | 3.22 ± 0.2 | p < 0.001 |
| Cervical motor donors | 4 | 1.08 ± 0.16 | 2.8 ± 0.2 | p < 0.001 |
| C5 or C6 | 4 | 0.75 ± 0.5 | 2.66 ± 0.27 | p < 0.001 |
| Contralateral lateral pectoral | 1 | 0 | 2 | |
| Total | 37 | 1.18 ± 0.52 | 3.25 ± 0.2 | p < 0.001 |
Table 5.
Elbow flexion – rectus femoris (late period: 1992 to date).
| Neurotizations | No. of patients | Muscle grade | ||
|---|---|---|---|---|
| Preoperative | Postoperative | |||
| cC7 | 4 | 1.33 ± 0.4 | 3.67 ± 0.6 | p < 0.001 |
| IC | 2 | 1 | 2.77 ± 1.5 | |
| Cervical motors | 1 | 1 | 2.33 | |
| Total | 7 | 1.19 ± 0.37 | 3.28 ± 1.06 | p < 0.001 |
Table 6.
Elbow extension.
| Motor donors | Latissimus dorsi | Gracilis (single transfer) | Gracilis (transfer with adductor longus) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Muscle grade | No. of patients | Muscle grade | No. of patients | Muscle grade | ||||
| Preoperative | Postoperative | Preoperative | Postoperative | Preoperative | Postoperative | ||||
| IC | 5 | 0.92 ± 0.54 | 3.15 ± 0.95 | 2 | 0.83 ± 0.2 | 3.06 ± 0.9 | – | – | – |
| cC7 | – | – | – | 1 | 1 | 3 | – | – | – |
| Cervical Motor | – | – | – | – | – | – | 2 | 1.33 ± 0.33 | 3.33 ± 0.33 |
| Total | 5 | p = 0.0024 | 3 | p = 0.011 | 2 | p = 0.0018 | |||
Evaluation Methods
Preoperative evaluation included detailed clinical examination, angiography, electromyography, and nerve conduction velocity study [26]. Muscle grading was based on the British Medical Research Council Grading System, as expanded further with intermediate grades of + and − (e.g., M2, M2+, M3−, M3) by Terzis and Vekris [39]. Postoperative functional video assessment and grading of all the cases was carried out by three independent reviewers who were not involved in any surgeries and had no information on any patient.
Surgical Procedures—Elbow Flexion
The gracilis muscle was used alone for both elbow flexion and extension (early period 1981–1986; Table 1). The double free muscle transfer for elbow flexion and shoulder abduction (Middle period 1987–1991) is based on the common vascular pedicle of these muscles (Table 2). The technique is difficult, and interposition vein grafts are needed to reach the recipient artery (common carotid, transverse cervical, or subclavian artery) or the recipient vein (subclavian, jugular, or basilic veins), with end-to-side anastomoses. Proximally, both muscles are inserted through bone holes to the clavicle and acromion, the adductor longus on the acromion to restore shoulder abduction and the gracilis on the lateral clavicle for elbow flexion. Distally, the adductor longus is anchored on the lateral humerus at the deltoid groove with bone compression screws and the gracilis on the biceps tuberosity of the radius. An 11% failure rate was encountered due to the need for long interposition vein grafts and 5:1 cross-sectional vascular discrepancy. These difficulties gave rise to the late period (1992–to date). In this latter period, the LD, RF, or vastus lateralis have been used as powerful muscles for elbow flexion (Tables 4 and 5). The skin envelope in a denervated extremity is usually tight and nonpliable. Because the contralateral LD is quite bulky, it is transferred as a myocutaneous flap. The overlying skin flap is used to monitor capillary refill representing the microcirculation of the transferred muscle. The muscle origin is attached to the clavicle and acromion with nonabsorbable 2-0 Ethibond sutures, while the tendinous insertion is attached to the radius with two compression screws with washers.
Elbow Extension
The technique of free muscle transfer for elbow extension is more difficult than that of free muscle transfer for elbow flexion because the vascular pedicle needs to reach medially to the brachial artery for an end-to-side anastomosis. Proximally, the muscle is anchored to the acromion and/or to the lateral scapula and distally onto the olecranon with two compression screws. Postoperatively, the patient is immobilized in a custom-made brace with the shoulder in 90° anterior flexion and the elbow in extension. The postoperative care is similar to that previously described [5,16].
Statistical Analysis
Statistical analysis was undertaken using Sigma-Stat v2.0 statistical software (Jandel Corp., Richmond, CA, USA). To compare different motor donors, a Kruskal–Wallis test was performed for strength of the transferred muscle data. Wilcoxon signed rank test was used to compare preoperative and postoperative results. Values of p < 0.05 were considered significant.
Results
Elbow Flexion
In our series, LD (n = 37), gracilis (n = 28), RF (n = 7), and vastus lateralis (n = 1) were transferred for elbow flexion neurotized by different motor donors (Tables 1, 2, 3, 4, and 5).
Gracilis
Seven gracilis were transferred as single units in the early period (1981–1986; Table 1). Eighteen double muscle transfers of gracilis and adductor longus were performed between 1987 and 1991. Elbow flexion (n = 16) or elbow extension (n = 2) was substituted simultaneously with shoulder abduction (n = 18; Table 2). More recently (from 1992 to date), five gracilis were transferred to reinforce inadequate elbow flexion (Table 3).
Preoperative and postoperative outcomes were statistically significant (Tables 1, 2, 3, 4, and 5).
No significant difference in muscle strength was found when gracilis was transferred alone (Tables 1 and 2, MG 2.8 ± 0.83) or as a double muscle transfer together with adductor longus (Table 2, MG = 2.8 ± 0.91), since in both instances elbow animation was dependent on the functional recovery of the gracilis muscle.
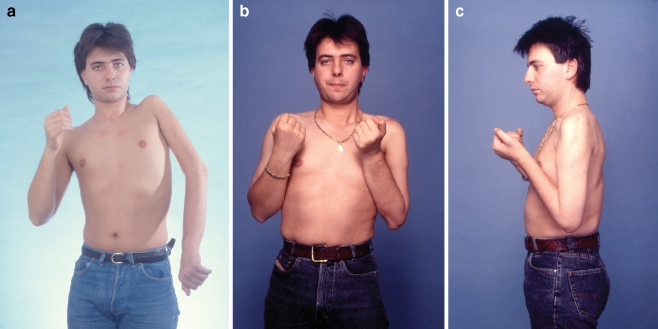
Different motor donors were used for neurotization of single vs combined transfer. For single gracilis transfer, neurotizations were from distal accessory or intercostals (first period 1981–1986) or cC7 (1992–to date). For double muscle transfers, neurotizations were from distal accessory (n = 5), intercostals (n = 4), cervical plexus (n = 2), contralateral pectoral (n = 4), and ipsilateral C5 (n = 1). The distal accessory yielded better results. For different motor donors, differences were not statistically significant either for single muscle transfer (p = 0.13) or for double transfer (p = 0.44). C5 was used once with exceptional results (MG 4+) in a double transfer case (Fig. 1).
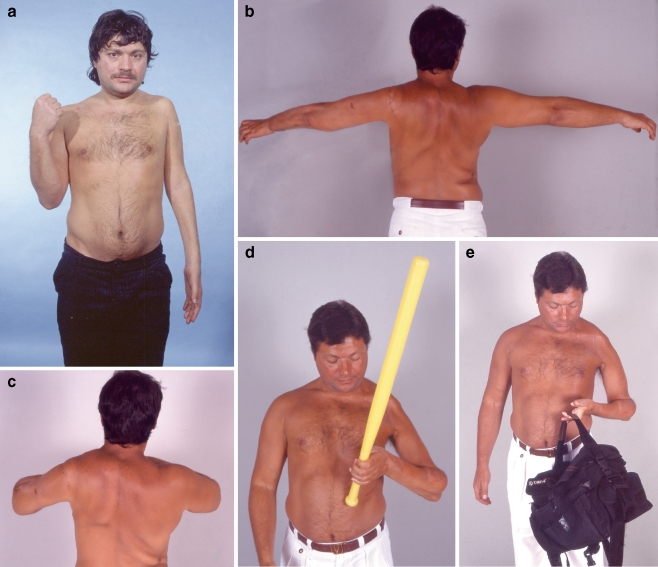
Figure 1.
A 21-year-old male from overseas suffered left global plexopathy following a high-velocity motor vehicle accident. At 4 months postinjury, he underwent brachial plexus exploration elsewhere. The intraoperative findings were C5 rupture and C6 to C8 root avulsion. T1 root was spared. Reconstruction elsewhere involved repair of the upper trunk from C5 root with interposition nerve grafts. He presented to our center at 15 months postinjury with some finger flexion from the T1 root that had escaped injury and inadequate function in the shoulder and elbow (a). In our center, the first stage of brachial plexus reconstruction involved exploration of the left cervical and left brachial plexus and the right lateral pectoral nerve. Neurotizations were performed for elbow extension (from XI), and deltoid (from XI and cervical motors) and banked nerves were placed from dorsal scapular, C4 motor, C5, distal accessory, and contralateral lateral pectoral nerve. One year after the first stage, a double muscle transfer of gracilis and adductor longus was performed. The neurotization of the adductor longus was from the contralateral lateral pectoral nerve and C5 root and of gracilis for elbow flexion from C5 via an interposition nerve graft. Three years after the free double muscle transfer, the patient recovered excellent elbow flexion (muscle grade 4; b and c).
Latissimus Dorsi
LD was neurotized from intercostals (n = 15), distal accessory (n = 7), cervical plexus (n = 4), ipsilateral plexus (n = 4), cC7 (n = 5), and contralateral lateral pectoral (n = 1).
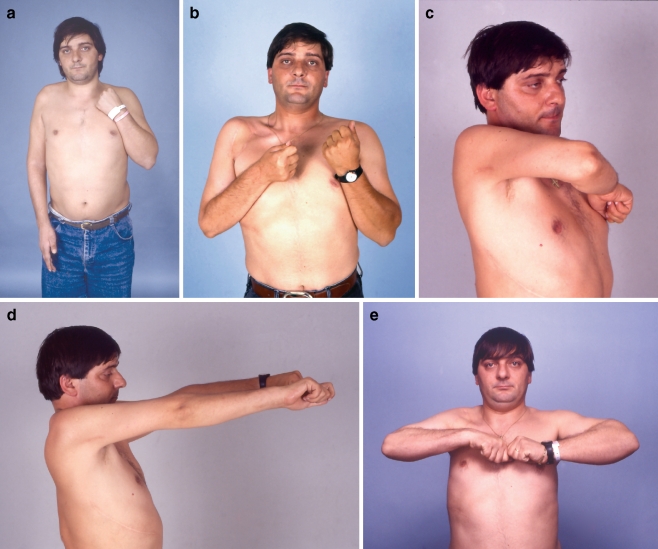
The mean muscle grade of LD was found to be 3.25 ± 0.2. Statistically significant (p = 0.047) difference was found when LD was neurotized with three intercostals (MG 3.87 ± 0.41) as compared with two intercostals (MG 3.02 ± 0.35). An exemplary case of free LD transfer for elbow flexion is shown in Fig. 2.
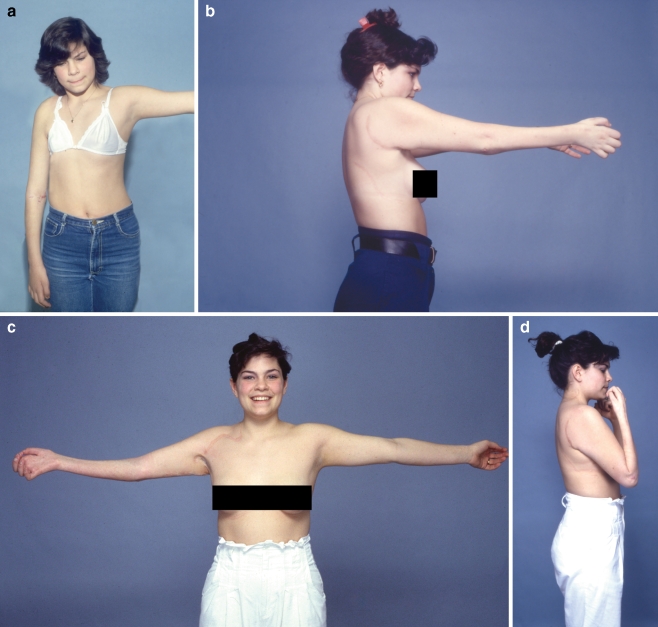
Figure 2.
A 22-year-old man from overseas sustained a right brachial plexus paralysis in a high-velocity motorcycle accident. During the preoperative examination, the patient presented with shoulder and elbow paresis, but the hand was spared (a). Five months after the brachial plexus lesion, he underwent exploration of the right brachial plexus. Intraoperative findings disclosed C5 rupture, C6 rupture/avulsion, C7 avulsion, C8 rupture/traction, and T1 traction. The suprascapular nerve was neurotized by the distal accessory, and the axillary nerve was reinnervated from the C5 and C6 roots. Microneurolysis on both C8 and T1 roots was performed, and in addition C8 root underwent partial nerve grafting. The lateral cord was also neurotized from the proximal stump of the C5 root. Two and half years after the initial injury, he underwent contralateral free latissimus dorsi transfer with direct neurotization from three intercostals T6, T7, and T8. Four years postinjury, a gracilis muscle was transferred for finger extension and was neurotized from the posterior division of cC7. Five years after surgery, the patient presented with a powerful elbow flexion (b and c) and elbow extension (d) and satisfactory shoulder abduction (e). Also, note that his right wrist is in excellent functional position from the free gracilis transfer (d).
Rectus Femoris
RF was used only for elbow flexion and was neurotized from cC7 (n = 4), intercostals (n = 2), and cervical plexus (n = 1; Table 5). The results were better with cC7 (MG 3.67 ± 0.6) as compared to intercostals (MG 2.77 ± 1.5), but the difference was not statistically significant (p = 0.32).
Gracilis vs LD vs RF
Comparison of the muscle strength was based on the same neurotizations. Intercostals (n = 28), distal accessory (n = 14), and cC7 (n = 14) were used more than the other motor donors and allow comparison among the muscles (Tables 1, 2, 3, 4, and 5). cC7 was used only in the late period (1992–to date; Tables 3, 4, and 5).
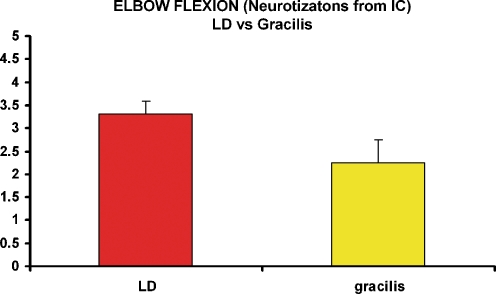
LD was stronger (Table 4, MG 3.33 ± 0.25) as compared to the gracilis (Table 1, MG 2.25 ± 0.6) when it was neurotized from intercostals, and this difference was statistically significant (p = 0.045; Fig. 3). When neurotizations were from cC7, RF yielded better results (Table 5, MG 3.67 ± 0.6) as compared to LD and gracilis, but differences were not statistically significant. Vastus lateralis did not yield the expected function (MG 2+).
Figure 3.
After free latissimus dorsi transfer for elbow flexion restoration, the mean muscle grading when the neurotization was from three intercostals was 3.33 ± 0.25 compared with 2.25 ± 0.6 which was obtained when free gracilis muscle was neurotized from three intercostals, and this difference was statistically significant (p = 0.045).
Age
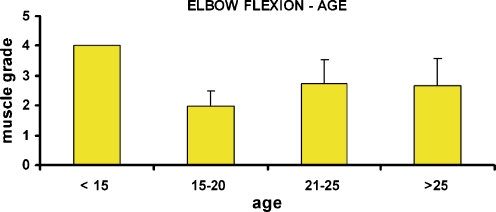
Patients with traumatic brachial plexus were divided into four groups on the basis of their age at surgery: younger than 15 years, between 15 and 20, 21 and 25, and older than 25 years. Statistically significant differences were found between the youngest group and the remaining three groups (Fig. 4).
Figure 4.
Patients with free muscle transfers for elbow reanimation were divided into four separate groups on the basis of their age at the time of surgery: younger than 15 years old, between 15 and 20, 20 and 25, and older than 25 years old. Statistically significant differences were found between the youngest group and each of the other three groups.
Failures
For elbow flexion and extension, the failure rate was 4.7% for LD (two cases in 42 transfers) and 0% for the other single muscle transfers (gracilis, RF, vastus lateralis). For the double muscle transfer, the failure rate was 11% (two failures in 18 transfers). No failures were identified for elbow extension. The total failure rate in our Institute for free muscles transfers for brachial plexopathy was 2.8%.
Elbow Extension
Ten patients underwent free muscle transfer for elbow extension. The mean age was 23.3 years old (range 10 to 34). The initial presentation of the patients was 5 months to 7 years postinjury. There were nine males with left upper extremity injury and one female with right-sided brachial plexus injury. Five patients had a free LD, and five patients had a free gracilis transfer. All LD muscles were neurotized by two intercostals (T6, T7, or T8.) Neurotization of gracilis involved two intercostals, cC7 posterior division, cervical plexus, and distal spinal accessory nerve (Table 6). Exemplary cases of free muscle for elbow extension are shown in Figs. 5 and 6.
Figure 5.
A 32-year-old man from overseas sustained a right global avulsion plexopathy and severe postavulsion pain secondary to a high-velocity motor vehicle accident. Preoperatively, he presented with a flail, anesthetic arm (a). At 5 months postinjury, he had a DREZ procedure (cervical laminectomy of C6–T1 levels) by a neurosurgical colleague. Postoperatively, he was pain free and could easily concentrate on his rehabilitation. A left brachial plexus exploration took place at 6 months postinjury. Intraoperative findings showed avulsion of all the roots except C5. Brachial plexus reconstruction included distal accessory to axillary neurotization, C4 motor to suprascapular nerve, and neurotization via a vascularized ulnar nerve graft from C5 root to left MC, median, and radial nerves. The patient had also wrist fusion and banked nerves placed from C2 to C4 motors for future free muscle neurotization. Two years postinjury, a free gracilis was transferred for finger extension neurotized by cervical motor donors. Three years after the initial reconstruction, the patient demonstrated excellent shoulder abduction and elbow flexion but he recovered only a weak triceps. The right free latissimus dorsi was transferred to the left upper extremity neurotized by two intercostals for elbow extension. Patient seen 6 years after injury, demonstrated powerful elbow extension (b) and flexion (c) with full range of motion. The wrist fusion stabilized his wrist, and now he has a useful left “assist” extremity (d and e).
Figure 6.
A 13-year-old female suffered a right brachial plexus palsy following a high-velocity motorcycle accident. Preoperative appearance of the right upper extremity (a). She underwent primary brachial plexus reconstruction at 8 months postinjury. Exploration of the brachial plexus revealed C5 and C6 roots rupture and C7–T1 root avulsion. Reconstruction involved use of ipsilateral vascularized ulnar nerve graft for neurotization of MC, axillary, median, and radial nerves from C6 root and placement of banked nerves from C5, cervical motors, and T4 for future free muscles. She recovered good elbow flexion, but shoulder abduction and elbow extension were weak. She underwent a double free muscle transfer of gracilis for elbow extension and adductor longus for shoulder abduction at 3 years postinjury. Neurotizations were from cervical motors. The patient is shown 5 years after the double muscle transfer with excellent elbow extension (b) and shoulder abduction from the double muscle (c) and powerful elbow flexion from the C6–vascularized ulnar–MC neurotization (d).
The muscle grading of LD (3.15 ± 0.95) was stronger than gracilis (3.06 ± 0.9), but the difference was not statistically significant. Data could not be analyzed for each muscle versus motor donor neurotization as all LD had the same motor donors.
Discussion
Elbow Flexion
One of the most important functions of the upper extremity is bringing the hand to the mouth for nourishment. For this reason, during the primary reconstruction, the best motor donor is used to neurotize the musculocutaneous nerve. For secondary elbow reconstruction, techniques such as the pedicled muscles transfers [21,39] or Steindler flexoplasty [9,32] can be used. Triceps transfer [1,2,6,23] is not recommended in our center. Instead, in global plexopathies, free muscle transfer is the only way to improve the function of the extremity if the initial neurotization failed.
Choice of Muscle
RF [1,13,18,39,41], LD [5,18,19,39], and gracilis [4,11,13–16,19,39] have been used as free muscle transfers for elbow flexion with or without concomitant finger reanimation.
In our institution, during the early period (1981–1986), the gracilis muscle was used for elbow flexion reconstruction. However, the resultant force of contraction was weak for the typical Caucasian patient. The double muscle transfer followed (1987–1991), and the major advantage of this technique was reconstruction of shoulder and elbow with one procedure. However, the failure rate was 11% as compared to the overall failure rate in our center for brachial plexopathy (2.8%). This was the reason that in the later period (1992–to date) larger single muscle units such as the LD have been used (MG 3.25 ± 0.2). When neurotizations were from three intercostals, LD transfer for elbow flexion yielded better results (MG 3.33 ± 0.25) versus gracilis (MG 2.25 ± 0.6), and this difference was statistically significant (p = 0.045). RF was also a powerful muscle (MG 3.28 ± 1.06). When neurotization was from cC7, the rectus femoris yielded better results. Experience with vastus lateralis did not yield the anticipated function.
Gracilis muscle was used to augment weak elbow flexion but is never our first choice for biceps substitution in a Caucasian patient. Instead, larger muscles such as the contralateral LD or RF are strongly recommended.
The Effect of Neurotization
Better results were encountered when gracilis was neurotized from the distal spinal accessory (Tables 1, 2, and 3), and this is in agreement with the outcomes for hand reanimation [37]. But, in the present study, dealing with Caucasian patients, different neurotizations for gracilis transfers did not reveal statistically significant differences, possibly because of the inadequate bulk of the muscle for elbow flexion.
Latissimus dorsi, a more powerful muscle, showed statistically significant difference when neurotized with three intercostals versus two intercostals. Rectus femoris, also a powerful muscle, showed differences from neurotizations from cC7 versus IC, but these differences were not statistically significant.
Others recommend the use of three intercostals for gracilis neurotization for elbow flexion. They claim that the use of two intercostals lengthens the rehabilitation period and achieves an inferior result [13].
With the exception of LD, where axonal loading from three intercostals was significant versus two intercostals, we could not detect significant differences with other neurotizations for elbow flexion. In contrast, free muscle transfers for hand reanimation and for facial reanimation were more affected from the type and quantity of neurotization [27,37,40]. It is reasonable to assume that for delicate functions such as hand and facial reanimation the degree of axonal load and type of motor donor play a greater role than for movements of proximal joints. Thus, for elbow flexion, the transferred muscle has to generate enough power to overcome the weight of the forearm and hand, and the differences from different motor donors could not be detected in the final outcomes.
The Effect of Age on Outcome
Patients younger than 15 years yielded statistically significant better outcomes as compared to the older age groups (Fig. 4). There is more brain plasticity in younger patients who require less rehabilitation and retraining [3]; regeneration distances are shorter, and the paralyzed extremity is probably lighter.
One Muscle Transfer for Simultaneous Reconstruction of Two Functions vs One Muscle Transfer for Single Function
The gracilis transfer for reconstruction of two functions takes advantage of the length of the gracilis muscle and proximal location of its neurovascular pedicle to promote rapid muscle reinnervation while allowing for distal joint function [4]. However, the importance of restoring the original tension of the muscle transplant was clearly shown by Terzis [38] and Manktelow [29]. When the gracilis is transferred for elbow flexion and hand reanimation, the distance from the clavicle to the hand is much longer than the original length of the muscle in the thigh. This forces the elbow into flexion, resulting in a tenodesis effect rather than muscle contraction. This is probably the reason why, even when acceptable muscle strength was achieved, the range of motion for elbow flexion was limited [13].
Another advantage is the use of limited motor donors for simultaneous movement of multiple joints [16,18]. However, Landsmeer [25] showed that to control two joints of a multiarticular chain in all positions at least three muscles are necessary; all three may cross two joints, or two may be biarticular and one monoarticular.
Studies confirmed that more reliable results are obtained with the transfer of a single muscle compared to two function reconstructions [15]. This technique was unable to restore prehension consistently [4], and it was suggested that only elbow flexion should be reconstructed [7]. In contrast, Chuang [11], in spite of his initial good results with single muscle transfer, shifted later to gracilis for double function [12]. It seems that gracilis in Asian patients may recover double function, but these outcomes cannot be duplicated in the Caucasian population [8].
Differences between the outcomes of the present study with free gracilis transfer for single function and the transfer technique for double function [33] probably are related to these racial differences. Differences in limb length [10] and weight between our group (Caucasian) and Oriental patients were previously recognized [35, 36].
Elbow Extension
Elbow extension is a very important function because it stabilizes the elbow joint in space and prevents flexion contracture to occur from unopposed flexors. Most authors have ignored elbow extension; they do not reconstruct it and often sacrifice the triceps to substitute for biceps function [1,6]. Elbow extension is more difficult to be restored with microsurgery than elbow flexion [30], and if it is present, it should be maintained [20,34]. A reliable strategy during primary reconstruction is the neurotization of the triceps from two or three intercostals if biceps neurotization took place from intraplexus donors. Neurotization of both triceps and biceps with intercostals should be avoided as it will yield crippling cocontraction in the adult.
In our center, after ten free muscle transfers for elbow extension, no significant differences between LD versus gracilis were identified, despite a trend for stronger elbow extension with the LD muscle.
This can be explained because elbow extension is helped by gravity and because the LD transfer for elbow extension was neurotized with only two intercostals.
To our knowledge, free muscle transfer for elbow extension as a single function has not been used systematically elsewhere. Elbow extension was also important in Doi’s double function of the transferred muscle, and for this reason triceps was neurotized primarily, but since the triceps alone was not strong enough to antagonize elbow flexion, patients were able to stabilize the elbow with the aid of gravity [20]. In our center, restoration of elbow extension is considered important, and efforts are made to restore it with primary and/or secondary reconstruction. Each muscle that was transferred for elbow function acted as single unit for a single function and, in this way, could simulate the normal function of the upper extremity without the aid of gravity.
Conclusion
Among all the muscle transfers for upper extremity reconstruction, elbow reanimation gave us the most promising outcomes. From the three periods of evolution of free muscle transfers for elbow reconstruction, the following conclusions can be made: in the Caucasian patient, gracilis alone did not yield adequate force for a strong elbow flexion. The elbow flexion of the double muscle was primarily based again on the gracilis, and the results were similar to gracilis alone. Finally, larger muscle units such as the LD made the difference for Caucasian patients and represent our first choice today. Neurotization of the LD with three intercostals for elbow flexion significantly improves outcomes.
Elbow extension is important, and efforts should be made to reconstruct it with free muscle transfer if primary reconstruction yielded inadequate function. Also, if elbow extension is present, it should be preserved to guarantee stability of the elbow joint in space.
References
- 1.Akasaka Y, Hara T, Takahashi M. Free muscle transplantation combined with intercostal nerve crossing for reconstruction of elbow flexion and wrist extension in brachial plexus injuries. Microsurgery. 1991;5:346–351. doi: 10.1002/micr.1920120506. [DOI] [PubMed] [Google Scholar]
- 2.Alnot JY. Elbow flexion palsy after traumatic lesions of the brachial plexus in adults. Hand Clin. 1989;5:15–22. [PubMed] [Google Scholar]
- 3.Anastakis DJ, Chen R, Davis KD, et al. Cortical plasticity following upper extremity injury and reconstruction. Clin Plast Surg. 2005;32:617–634. doi: 10.1016/j.cps.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Barrie K, Steinman S, Shin A, et al. Gracilis free muscle transfer for restoration of function after complete brachial plexus avulsion. Neurosurg Focus. 2004;16(5):E8. doi: 10.3171/foc.2004.16.5.9. [DOI] [PubMed] [Google Scholar]
- 5.Berger A, Brenner P. Secondary surgery following brachial plexus injuries. Microsurgery. 1995;16:43–47. doi: 10.1002/micr.1920160112. [DOI] [PubMed] [Google Scholar]
- 6.Berger A, Flory PJ, Schaller E. Muscle transfers in brachial plexus lesions. J Reconstr Microsurg. 1990;6:113–116. doi: 10.1055/s-2007-1006809. [DOI] [PubMed] [Google Scholar]
- 7.Berger AC, Hierner R, Kleinschmidt L. Palliative surgery: the elbow and forearm. In: Gilbert A, editor. Brachial plexus injuries. London: Dunitz Martin; 2001. pp. 123–130. [Google Scholar]
- 8.Bishop AT. Functioning free-muscle transfer for brachial plexus injury. Hand Clin. 2005;21:91–102. doi: 10.1016/j.hcl.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Brunelli G, Monini L, Brunelli GR. Modified Steindler procedure for elbow flexion restoration. J Hand Surg (Am) 1995;20:743–746. doi: 10.1016/S0363-5023(05)80424-1. [DOI] [PubMed] [Google Scholar]
- 10.Chou PH, Shyu JF, Ma HL, et al. Courses of the radial nerve differ between Chinese and Caucasians: clinical applications. Clin Orthop Relat Res. 2008;466:135–138. doi: 10.1007/s11999-007-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chuang DC. Functioning free muscle transplantation for brachial plexus injury. Clin Orthop Relat Res. 1995;314:104–111. [PubMed] [Google Scholar]
- 12.Chuang DC. Neurotization and free muscle transfer for brachial plexus avulsion injury. Hand Clin. 2007;23:91–104. doi: 10.1016/j.hcl.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Chuang DC, Carver N, Wei FC. Results of functioning free muscle transplantation for elbow flexion. J Hand Surg. 1996;21A:1071–1077. doi: 10.1016/s0363-5023(96)80318-2. [DOI] [PubMed] [Google Scholar]
- 14.Chuang DC, Epstein MD, Yeh MC, et al. Functional restoration of elbow flexion in brachial plexus injuries: results in 167 patients (excluding obstetric brachial plexus injury) J Hand Surg (Am) 1993;18:285–291. doi: 10.1016/0363-5023(93)90363-8. [DOI] [PubMed] [Google Scholar]
- 15.Doi K, Hattori Y, Ikeda K, et al. Significance of shoulder function in the reconstruction of prehension with double free-muscle transfer after complete paralysis of the brachial plexus. Plast Reconstr Surg. 2003;112:1596–1603. doi: 10.1097/01.PRS.0000085820.24572.EE. [DOI] [PubMed] [Google Scholar]
- 16.Doi K, Muramatsu K, Hattori Y, et al. Restoration of prehension with the double free muscle technique following complete avulsion of the brachial plexus. Indications and long-term results. J Bone Joint Surg (Am) 2000;82:652–666. doi: 10.1302/0301-620X.82B5.10038. [DOI] [PubMed] [Google Scholar]
- 17.Doi K, Sakai K, Ihara K, et al. Reinnervated free muscle transplantation for extremity reconstruction. Plast Reconstr Surg. 1983;91:872–883. doi: 10.1097/00006534-199304001-00021. [DOI] [PubMed] [Google Scholar]
- 18.Doi K, Sakai K, Kuwata N, et al. Reconstruction of finger and elbow function after complete avulsion of the brachial plexus. J Hand Surg (Am) 1991;16:796–803. doi: 10.1016/S0363-5023(10)80138-8. [DOI] [PubMed] [Google Scholar]
- 19.Doi K, Sakai K, Kuwata N, et al. Double muscle technique for reconstruction of prehension after complete avulsion of brachial plexus. J Hand Surg. 1995;20A:408–414. doi: 10.1016/S0363-5023(05)80097-8. [DOI] [PubMed] [Google Scholar]
- 20.Doi K, Shigetomi M, Kaneko K, et al. Significance of elbow extension in reconstruction of prehension with reinnervated free-muscle transfer following complete brachial plexus avulsion. Plast Reconstr Surg. 1997;100:364–372. doi: 10.1097/00006534-199708000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Gutowski KA, Orenstein HH. Restoration of elbow flexion after brachial plexus injury: the role of nerve and muscle transfers. Plast Reconstr Surg. 2000;106:1348–1357. doi: 10.1097/00006534-200011000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Harii K, Ohmori K, Torii S. Free gracilis muscle transplantation with microneurovascular anastomoses for the treatment of facial paralysis. Plast Reconstr Surg. 1976;57:133–143. doi: 10.1097/00006534-197602000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Hoang PH, Mills C, Burke FD. Triceps to biceps transfer for established brachial plexus palsy. J Bone J Surg (Br) 1989;71:268–271. doi: 10.1302/0301-620X.71B2.2647756. [DOI] [PubMed] [Google Scholar]
- 24.Ikuta Y, Yoshioka, Tsuge K. Free muscle graft as applied to brachial plexus injury-case report and experimental study. Ann Acad Med Singapore. 1979;8:454–458. [PubMed] [Google Scholar]
- 25.Landsmeer JMF. Anatomical and functional investigations on the articulations of the human fingers. Acta Anat. 1955;25(suppl 24):1–69. doi: 10.1159/000142158. [DOI] [PubMed] [Google Scholar]
- 26.Liberson WT, Terzis JK. Contribution of clinical neurophysiology and rehabilitation medicine to the management of brachial plexus palsy. In: Terzis JK, editor. Microreconstruction of nerve injuries. Philadelphia: Saunders; 1987. pp. 555–567. [Google Scholar]
- 27.MacQuillan AH, Grobbelaar AO. Functional muscle transfer and the variance of reinnervating axonal load: part I. The facial nerve. Plast Reconstr Surg. 2008;121:1570–1577. doi: 10.1097/PRS.0b013e31816fda3e. [DOI] [PubMed] [Google Scholar]
- 28.Manktelow RT, McKee NM. Free muscle transplantation to provide active finger flexion. J Hand Surg. 1978;3:416–426. doi: 10.1016/s0363-5023(78)80134-8. [DOI] [PubMed] [Google Scholar]
- 29.Manktelow RT, Zuker RM, Mc Kee NH. Functioning free muscle transplantation. J Hand Surg. 1984;9A:32–39. doi: 10.1016/s0363-5023(84)80181-1. [DOI] [PubMed] [Google Scholar]
- 30.Narakas AO, Hentz VR. Neurotization in brachial plexus injuries. Indication and results. Clin Orthop Relat Res. 1988;237:43–56. [PubMed] [Google Scholar]
- 31.Sixth People's Hospital, Microvascular Service, Shanghai Free muscle transplantation by microsurgical neurovascular anastomoses. Chin Med J. 1976;2:47–50. [PubMed] [Google Scholar]
- 32.Steindler A. A muscle plasty for the relief of flail elbow in infantile paralysis. Interstate Med J. 1918;25:235–241. [Google Scholar]
- 33.Takka S, Doi K, Hattori Y, et al. Selection of grip function in double free gracilis transfer procedures after complete paralysis of the brachial plexus. Ann Plast Surg. 2005;54:610–614. doi: 10.1097/01.sap.0000162514.14226.53. [DOI] [PubMed] [Google Scholar]
- 34.Terzis JK. The reconstructive strategy for improving elbow function in late obstetric brachial plexus palsy by David Chwei Chin Chuang, Yasunori Hattori, Haw-Shya Ma and Hung-Chi Chen (articles: discussion) Plast Reconstr Surg. 2002;109:127–129. doi: 10.1097/00006534-200201000-00021. [DOI] [PubMed] [Google Scholar]
- 35.Terzis JK, Kostopoulos VK. The surgical treatment of brachial plexus injuries in adults. Plast Reconstr Surg. 2007;119:73e. doi: 10.1097/01.prs.0000254859.51903.97. [DOI] [PubMed] [Google Scholar]
- 36.Terzis JK, Kostopoulos VK. 151 neurotizations with ulnar vascularized nerve grafts for brachial plexus injuries. Plast Reconstr Surg. 2009;123:1276–1291. doi: 10.1097/PRS.0b013e31819f2afd. [DOI] [PubMed] [Google Scholar]
- 37.Terzis JK, Kostopoulos VK: Functional free muscle transfers for adult plexopathies. Part III: the hand. 2009; in press.
- 38.Terzis JK, Sweet RC, Dykes RW, et al. Recovery of function in free muscle transplants using microneurovascular anastomoses. J Hand Surg. 1978;3:37–59. doi: 10.1016/s0363-5023(78)80116-6. [DOI] [PubMed] [Google Scholar]
- 39.Terzis JK, Vekris MD, Soucacos PN. Outcomes of brachial plexus reconstruction in 204 patients with devastating paralysis. Plast Reconstr Surg. 1999;104:1221–1240. doi: 10.1097/00006534-199910000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Terzis JK, Wang W. Effect of axonal load on the functional and aesthetic outcomes of cross facial nerve graft procedure for facial reanimation. Plast Reconstr Surg. 2009; in press [DOI] [PubMed]
- 41.Wei FC, Chuang DC, Chen HC, et al. The versatility of free rectus femoris muscle flap: an alternative flap. Microsurgery. 1995;16:698–703. doi: 10.1002/micr.1920161008. [DOI] [PubMed] [Google Scholar]