Abstract
Background/Aims
Helicobacter pylori (H. pylori) infection appears to subvert the human iron regulatory mechanism and thus upregulates hepcidin, resulting in unexplained iron-deficiency anemia (IDA). We evaluated serum prohepcidin levels before and after eradication of H. pylori in IDA patients to assess whether it plays a role in IDA related to H. pylori infection.
Methods
Subjects diagnosed with unexplained IDA underwent upper gastrointestinal endoscopy and colonoscopy to confirm H. pylori infection and to exclude gastrointestinal bleeding. Blood was sampled before treatment to eradicate H. pylori and again 1 month later. Serum prohepcidin levels were measured using a commercial enzyme-linked immunosorbent assay kit.
Results
Serum prohepcidin levels decreased significantly after oral iron replacement combined with H. pylori eradication (p = 0.011). The reduction ratio of serum prohepcidin levels after the treatment did not differ among the combined oral iron replacement and H. pylori eradication groups, the H. pylori eradication only group, and the iron replacement only group (p = 0.894).
Conclusions
Serum prohepcidin levels decrease after both H. pylori eradication and oral iron administration, with improvement in IDA. Serum concentration of prohepcidin is related to the anemia status, rather than to the current status of H. pylori infection, in IDA patients.
Keywords: Prohepcidin; Anemia, iron-deficiency; Helicobacter pylori
INTRODUCTION
Helicobacter pylori (H. pylori) requires iron to survive and may potentially be associated with unexplained iron-deficiency anemia (IDA) [1]. The prevalence of H. pylori infection in refractory IDA is higher than in the normal population [2], and an association between low serum ferritin levels and a high prevalence of H. pylori-specific IgG has been noted [3]. H. pylori-related refractory IDA can be reversed by eradication of H. pylori [4], and some H. pylori-infected IDA patients achieve normal serum hemoglobin levels after H. pylori eradication, even without oral iron treatment [5]. Additionally, it has been shown that combined H. pylori eradication and iron replacement produces a faster response than iron therapy alone [6]. Based on these findings, eradication of H. pylori is recommended in unexplained IDA patients [7], although no association between the pathogenesis of IDA and H. pylori infection has yet been established. A key pathogenic connection must exist to explain the relationship between H. pylori infection and IDA, as not all H. pylori-infected patients develop unexplained IDA.
Hepcidin, a 25-amino-acid disulfide-rich peptide that is synthesized in the liver, acts as a systemic iron regulatory hormone and plays an important role in duodenal iron absorption [8]. Hepcidin mRNA is induced by interleukin-6 [9], and its expression is enhanced by factors such as lipopolysaccharides and Streptococcus iniae infection [10]. Hepcidin binds to cell-surface ferroportin to trigger its tyrosine phosphorylation, internalization, and ubiquitin-mediated degradation in lysosomes, resulting in the retention of iron in enterocytes and the interruption of iron release from macrophages [11]. In murine models, parenchymal iron deposition has been reported in hepcidin knockout mice [12], whereas transgenic mice that overexpress hepcidin exhibited decreased body iron levels and presented with severe IDA [13]. Additionally, H. pylori subverts the human iron regulatory mechanism in a manner that is beneficial to H. pylori, but deleterious to the host, by inducing the up-regulation of hepcidin and/or down-regulation of ferroportin [14].
Attempts to produce correctly folded synthetic hepcidin have proved difficult. Thus, prohepcidin, the 60-amino acid product of the cleavage of the signal peptide from the hepcidin precursor, is measured with a currently available commercial assay for bioactive hepcidin analysis [15]. To determine the feasibility of serum prohepcidin as a useful biomarker in IDA, it is first necessary to determine the precise molecular mechanism underlying the action of prohepcidin under various conditions. In this study, we evaluated changes in serum prohepcidin levels before and after H. pylori eradication in H. pylori-infected IDA patients to establish whether it normalized after treatment.
METHODS
Patients
Subjects diagnosed with unexplained IDA at the Division of Hematology at Konkuk University Medical Center between October 2006 and March 2008 were asked to participate in the study. Upper gastrointestinal endoscope and colonoscope examinations were performed to exclude any source of gastrointestinal bleeding. Other exclusion criteria were: 1) patients with chronic inflammatory disease, infection, recent bleeding, or malignancy, 2) subjects who had previously undergone H. pylori eradication, or 3) subjects who had taken regular iron supplement therapy within the most recent 3 months.
Blood was sampled before treatment to eradicate H. pylori and again 1 month later for hemoglobin, hematocrit, iron (Fe), total iron binding capacity (TIBC), ferritin, and serum pro-hepcidin levels.
Blood was sampled before treatment to eradicate H. pylori and again 1 month later for hemoglobin, hematocrit, iron (Fe), total iron binding capacity (TIBC), ferritin, and serum pro-hepcidin levels.
Diagnosis and treatment for H. pylori infection
Positivity for H. pylori infection was recorded when one of the following tests was positive: 1) histology with hematoxylin and eosin staining and Giemsa staining, 2) rapid urease by a CLOtest, or 3) a serum antibody test. For the first eradication, amoxicillin 1 g, clarithromycin 500 mg, and a proton pump inhibitor 20 mg were given twice a day for 7 days. Four weeks after eradication, a urease breath test was carried out; if it was positive, a second eradication was performed with tetracycline 500 mg and bismuth 300 mg four times a day, metronidazole 500 mg three times a day, and a proton pump inhibitor twice a day for a week. The urease breath test was performed again after 4 weeks of eradication.
Measurement of serum prohepcidin levels
Whole blood samples were centrifuged (2,000 × g, 5 minutes), aliquoted, and kept frozen at -80℃ until assayed. Levels of serum prohepcidin were determined by an enzyme-linked immunosorbent assay based on a competitive principle (DRG International Inc., Mountainside, NJ, USA) according to the manufacturer's protocol. Optical densities were read with a Bio-Rad PhDTM system (Bio-Rad Laboratories, Hercules, CA, USA). A standard curve was automatically constructed using a 4parameter logistics curve fit with Bio-Rad PhDTM system (Bio-Rad Laboratories), and the corresponding concentrations of patient samples were calculated from the standard curve. All standards, subject samples, and controls were run in duplicate, and individual samples with a coefficient of variation for paired replicates of more than 15.0% were retested. The reduction ratio was determined by dividing the prohepcidin level after the treatment by the initial prohepcidin level.
Statistical analyses
Differences between pre-treatment and post-treatment levels were analyzed using the Wilcoxon signed rank test. Differences between H. pylori-infected and noninfected groups were analyzed using the chi-squared test and Student's t test, and comparisons among three groups were done by analysis of variance (ANOVA). Fe and ferritin were analyzed using the Kruskal-Wallis test and are presented as median values. Age, hemoglobin, hematocrit, TIBC, prohepcidin, and reduction ratio are expressed as means ± standard deviations (SD). All increment and decrement ratios were determined by dividing the serum level after 4 weeks of treatment by the initial serum level before treatment. Analysis of covariance was carried out to compare the changes of prohepcidin levels before and after treatment among the groups. A probability value of p < 0.05 was deemed to indicate statistical significance.
RESULTS
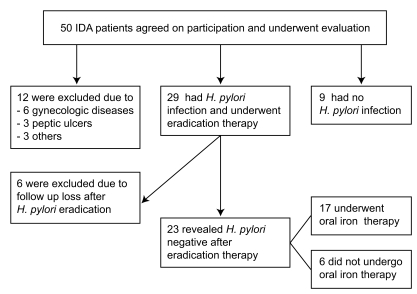
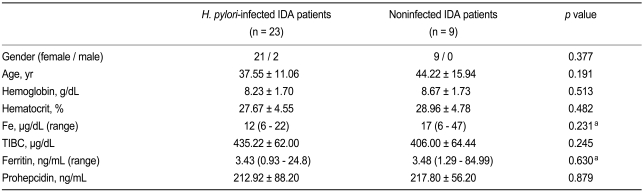
Of the 50 initially enrolled subjects, 12 were excluded because the cause of their IDA was established (Fig. 1). Of the 38 remaining IDA subjects, 29 subjects (76.3%) revealed H. pylori infection. Eradication was conducted in these 29 subjects, but six refused to participate in the study after the treatment. Baseline characteristics did not differ between the remaining 23 H. pylori-infected subjects (212.9 ± 88.2 ng/mL) and nine non-infected subjects (217.8 ± 56.2 ng/mL, p = 0.879, Table 1). Of 23 H. pylori-infected subjects, six patients preferred to undergo H. pylori eradication without iron replacement based on their past experiences of severe adverse effects during iron intake. Thus, only 17 H. pylori-infected IDA subjects underwent combined H. pylori eradication and iron replacement.
Figure 1.
Study flow of the subjects. Of 50 subjects, 12 were excluded because the causes of iron-deficiency anemia were found. Of 29 Helicobacter pylori (H. pylori)-infected subjects, six did not return after 4 weeks of treatment. Thus, only 23 H. pylori-infected subjects and nine non-infected subjects were analyzed. IDA, iron-deficiency anemia.
Table 1.
Baseline characteristics of the Helicobacter pylori-infected and noninfected patients with IDA
Values are presented as mean ± SD.
IDA, iron-deficiency anemia; Fe, iron; TIBC, total iron binding capacity.
aKruskal-Wallis test.
Serum prohepcidin levels before and after treatment
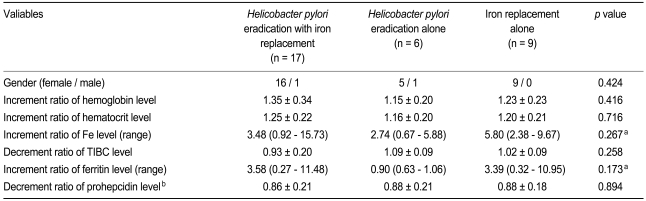
In the 17 subjects who underwent oral iron therapy with successful H. pylori eradication, serum prohepcidin level decreased from 200.8 ± 80.8 ng/mL (mean ± SD) to 170.4 ± 67.6 ng/mL after 4 weeks of treatment (p = 0.011). Of the 17 subjects, eradication of H. pylori failed with the first-line therapy in two, and thus second-line eradication was conducted. At 4 weeks, all showed negative urease breath tests.
Mean serum prohepcidin levels decreased in the six subjects who underwent H. pylori eradication alone (247.2 ± 107.0 ng/mL to 200.1 ± 69.4 ng/mL, p = 0.075) and in the nine noninfected subjects who took oral iron therapy alone (217.8 ± 56.2 ng/mL to 188.7 ± 52.4 ng/mL, p = 0.086). With regard to the reduction ratio of serum prohepcidin levels after treatment, no significant difference among the groups after treatment (p = 0.8936, Table 2). Although the reduction ratio value in the H. pylori eradication alone group (0.86 ± 0.21) was lower than that in the H. pylori eradication group with iron replacement (0.88 ± 0.21) and that in the iron replacement only group (0.88 ± 0.18), the difference was not statistically significant (p = 0.8936).
Table 2.
Changes in laboratory blood tests before and after 4 weeks of treatment
Values are presented as mean ± SD.
Fe, iron; TIBC, total iron binding capacity.
aKruskal-Wallis test.
bDecrement ratio is determined by dividing the serum level after 4 weeks of treatment by the initial serum level before treatment.
Long-term follow-up after H. pylori eradication
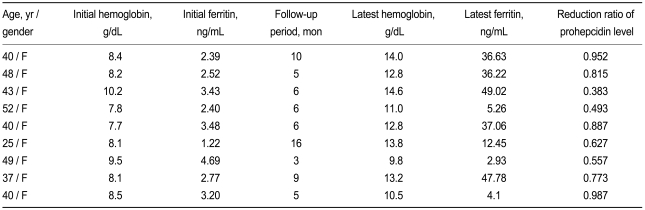
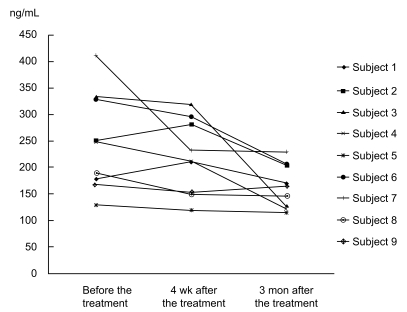
Of the 17 subjects who underwent combined oral iron replacement and H. pylori eradication, nine were followed at the outpatient clinic at least for 3 months (Table 3). All showed a decrease in serum prohepcidin levels (Fig. 2) with improvement in IDA.
Table 3.
Long-term follow-up results of nine subjects after combined Helicobacter pylori eradication and iron replacement therapy
Figure 2.
Serum prohepcidin levels measured over a long term follow up period. Of 17 subjects who underwent successful Helicobacter pylori eradication and iron replacement therapy, nine were followed for more than 3 months. Serum prohepcidin levels continued to decrease in all nine subjects with improvement in iron-deficiency anemia.
DISCUSSION
In this study, we measured only the serum prohepcidin level because of the difficulty inherent in measuring serum hepcidin. Although we were unable to measure hepatic iron accumulation in each case because that would involve an invasive procedure, our data suggest that serum prohepcidin levels were increased in patients with IDA regardless of the presence of H. pylori infection. To our knowledge, this is the first reported study to measure serum prohepcidin levels in IDA patients, comparing levels before and after H. pylori eradication with or without iron replacement.
Serum prohepcidin levels decreased significantly in the H. pylori-infected IDA patients after combined H. pylori eradication and oral iron therapy. Additionally, a tendency toward a decrease in serum prohepcidin levels after H. pylori eradication or iron replacement was observed. Our findings may be explained by the suggestion made in previous work that H. pylori infection results in the regulation of hepcidin, which prevents a response to oral iron and manipulates the host's iron homeostasis to ensure its survival [14,16]. Notably, six H. pylori-infected subjects who declined to take oral iron also exhibited a reduction in serum prohepcidin levels after H. pylori eradiation. It seems that iron therapy is not required in H. pylori-infected IDA patients, as it appears that the IDA can be cured merely by eradicating the H. pylori infection [5]. However, serum prohepcidin levels decreased further with combined H. pylori eradication and oral iron replacement. This is consistent with a previous study that found that combined H. pylori eradication and iron replacement produced a faster response than iron therapy alone [6].
The serum concentration of prohepcidin in Korean female college students has been reported to be 85.1 ± 6.1 ng/mL, with a range of 13.6 to 295.7 mg/mL [17]. Serum prohepcidin levels in our IDA patients were significantly higher than this value, regardless of the presence of H. pylori infection. We are unable to explain the lack of difference in serum pro-hepcidin levels between the H. pylori-infected and noninfected groups before treatment. We suggest that long-term infection with H. pylori leads to homeostasis in IDA patients because most are infected during childhood. Interestingly, serum prohepcidin levels exhibited a tendency to decrease in the noninfected IDA group after oral iron therapy, suggesting that hepcidin is an important mediator of iron homeostasis even in noninfected IDA. During long-term follow-up observation for more than 3 months, the prohepcidin levels in nine subjects decreased further after successful H. pylori eradication therapy as IDA improved with continuous oral iron replacement. This indicates that the serum prohepcidin level does not normalize immediately.
Our study has several limitations. First, no long-term follow-up data were gathered in the H. pylori-eradication only group or the iron-replacement only group. Second, we did not check intragastric acid levels in each subject, although a possibility of achlorhydria exists due to H. pylori-induced chronic pangastritis. Third, most of the subjects were premenopausal women; indeed, only two men were included in the study. Most male subjects were ruled out from unexplained IDA because they showed positive findings in upper or lower gastrointestinal endoscopy.
In summary, our findings suggest that serum prohepcidin levels are related to the severity of IDA, rather than to the presence of H. pylori infection, for the following reasons. First, basal serum prohepcidin levels were higher in our IDA patients than reported for normal healthy Koreans regardless of H. pylori infection status [17]. Second, serum prohepcidin levels decreased after iron-replacement therapy, even in the noninfected group. Third, during longterm followup after successful H. pylori eradication, serum prohepcidin levels decreased further with improvement in the IDA. Fourth, no significant difference in the reduction ratios was found among the three groups, although combined H. pylori eradication and iron replacement therapy seemed to have a synergistic effect. Fifth, either H. pylori eradication or oral iron administration resulted in decreased serum prohepcidin levels after the treatment, accompanied by IDA improvement. Taken together. Taken together, these findings indicate that the serum concentration of prohepcidin is related to the status of anemia rather than to the current status of H. pylori infection in IDA patients, indicating that serum concentration of prohepcidin is a functional measure in IDA.
Acknowledgements
This study was supported by a grant from the Korean Association of Internal Medicine.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Dufour C, Brisigotti M, Fabretti G, Luxardo P, Mori PG, Barabino A. Helicobacter pylori gastric infection and sideropenic refractory anemia. J Pediatr Gastroenterol Nutr. 1993;17:225–227. doi: 10.1097/00005176-199308000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Choe YH, Lee JE, Kim SK. Effect of helicobacter pylori eradication on sideropenic refractory anaemia in adolescent girls with Helicobacter pylori infection. Acta Paediatr. 2000;89:154–157. doi: 10.1080/080352500750028753. [DOI] [PubMed] [Google Scholar]
- 3.Parkinson AJ, Gold BD, Bulkow L, et al. High prevalence of Helicobacter pylori in the Alaska native population and association with low serum ferritin levels in young adults. Clin Diagn Lab Immunol. 2000;7:885–888. doi: 10.1128/cdli.7.6.885-888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annibale B, Marignani M, Monarca B, et al. Reversal of iron deficiency anemia after Helicobacter pylori eradication in patients with asymptomatic gastritis. Ann Intern Med. 1999;131:668–672. doi: 10.7326/0003-4819-131-9-199911020-00006. [DOI] [PubMed] [Google Scholar]
- 5.Hershko C, Ianculovich M, Souroujon M. A hematologist's view of unexplained iron deficiency anemia in males: impact of Helicobacter pylori eradication. Blood Cells Mol Dis. 2007;38:45–53. doi: 10.1016/j.bcmd.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Choe YH, Kim SK, Son BK, Lee DH, Hong YC, Pai SH. Randomized placebo-controlled trial of Helicobacter pylori eradication for iron-deficiency anemia in preadolescent children and adolescents. Helicobacter. 1999;4:135–139. doi: 10.1046/j.1523-5378.1999.98066.x. [DOI] [PubMed] [Google Scholar]
- 7.Malfertheiner P, Megraud F, O'Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krause A, Neitz S, Mägert HJ, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480:147–150. doi: 10.1016/s0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- 9.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 10.Pigeon C, Ilyin G, Courselaud B, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 11.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 12.Nicolas G, Bennoun M, Devaux I, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicolas G, Bennoun M, Porteu A, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci U S A. 2002;99:4596–4601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beutler E. Hepcidin mimetics from microorganisms? A possible explanation for the effect of Helicobacter pylori on iron homeostasis. Blood Cells Mol Dis. 2007;38:54–55. doi: 10.1016/j.bcmd.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulaksiz H, Gehrke SG, Janetzko A, et al. Pro-hepcidin: expression and cell specific localisation in the liver and its regulation in hereditary haemochromatosis, chronic renal insufficiency, and renal anaemia. Gut. 2004;53:735–743. doi: 10.1136/gut.2003.022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pellicano R, Rizzetto M. Is hepcidin the bridge linking Helicobacter pylori and anemia of chronic infection? A research proposal. Panminerva Med. 2004;46:165–169. [PubMed] [Google Scholar]
- 17.Chung J. Relationship between serum pro-hepcidin concentration and body iron status in female college students. Korean J Nutr. 2005;38:750–755. [Google Scholar]