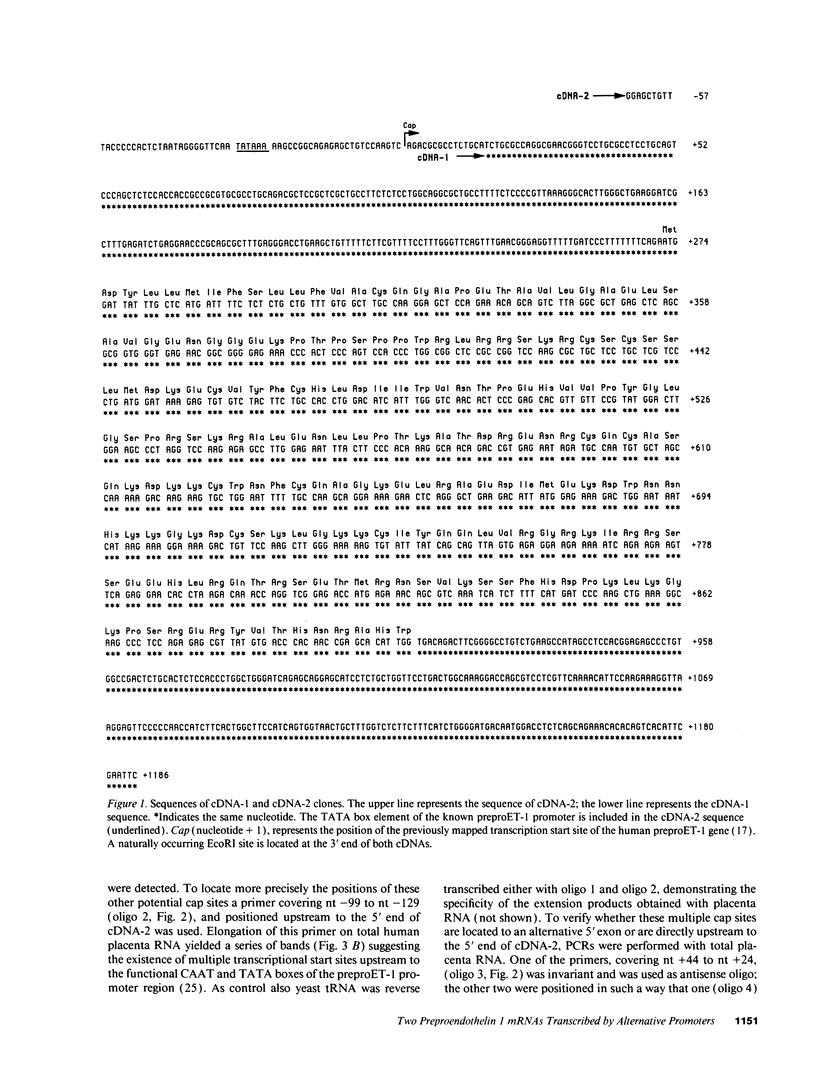
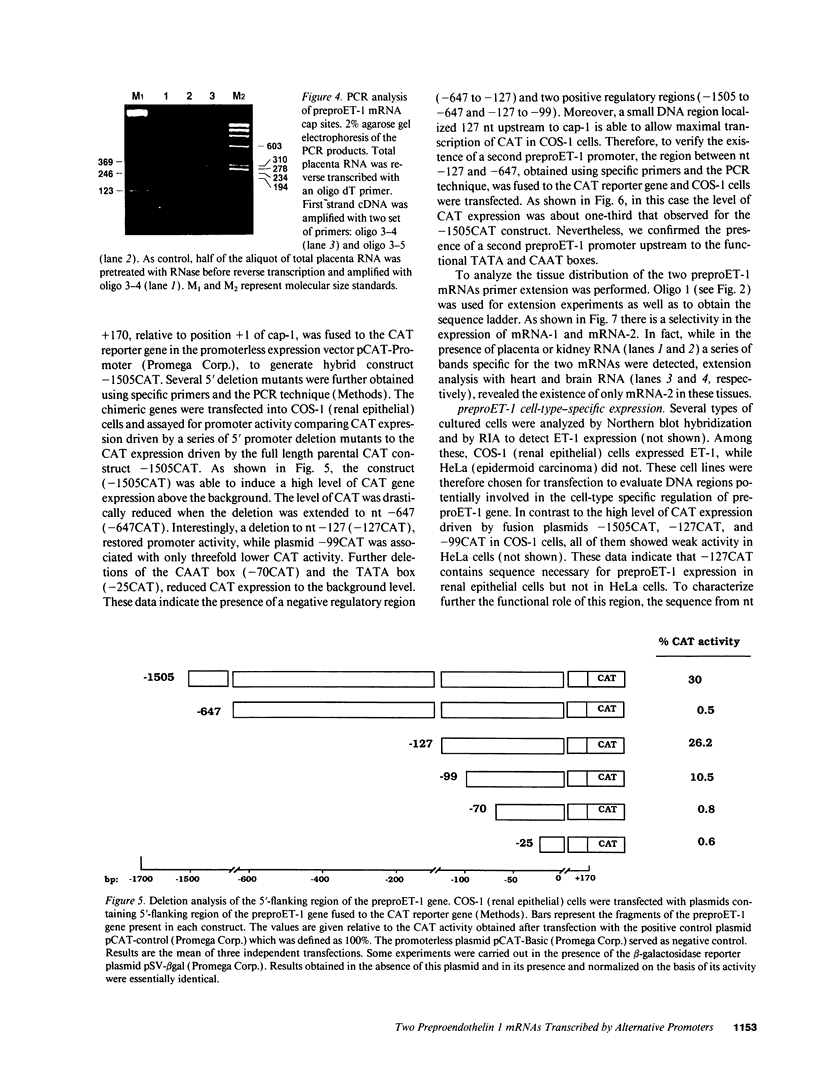
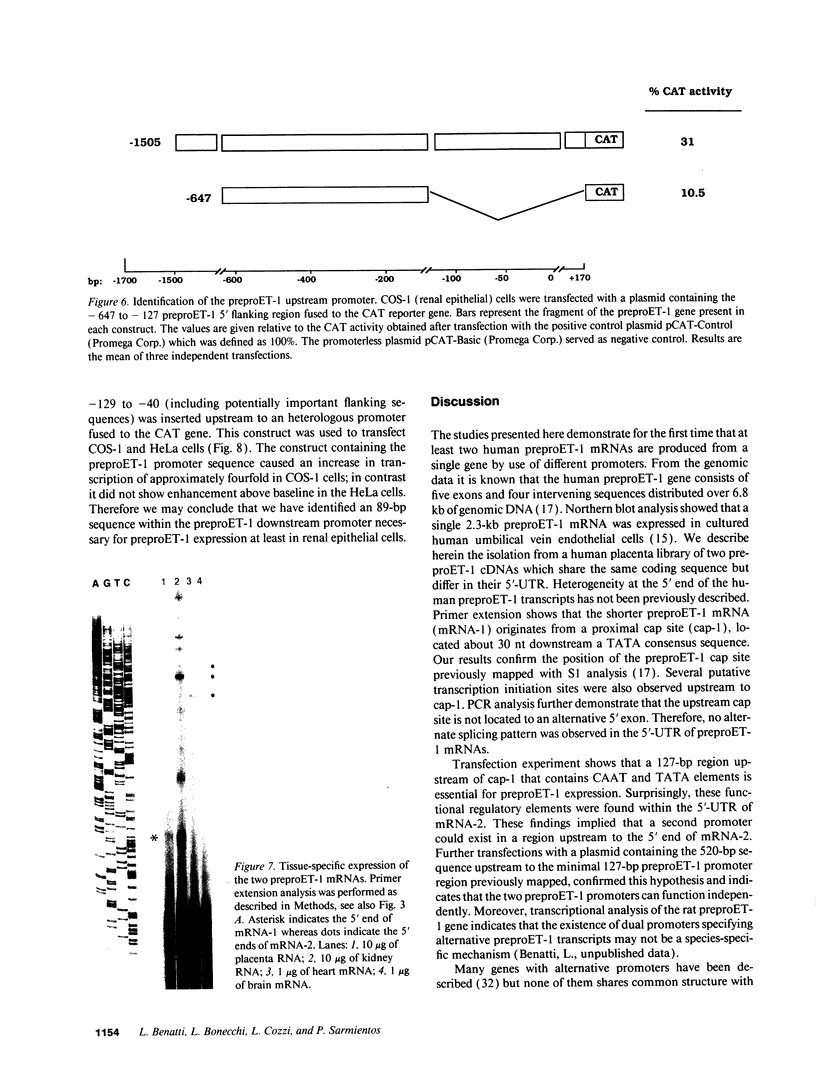
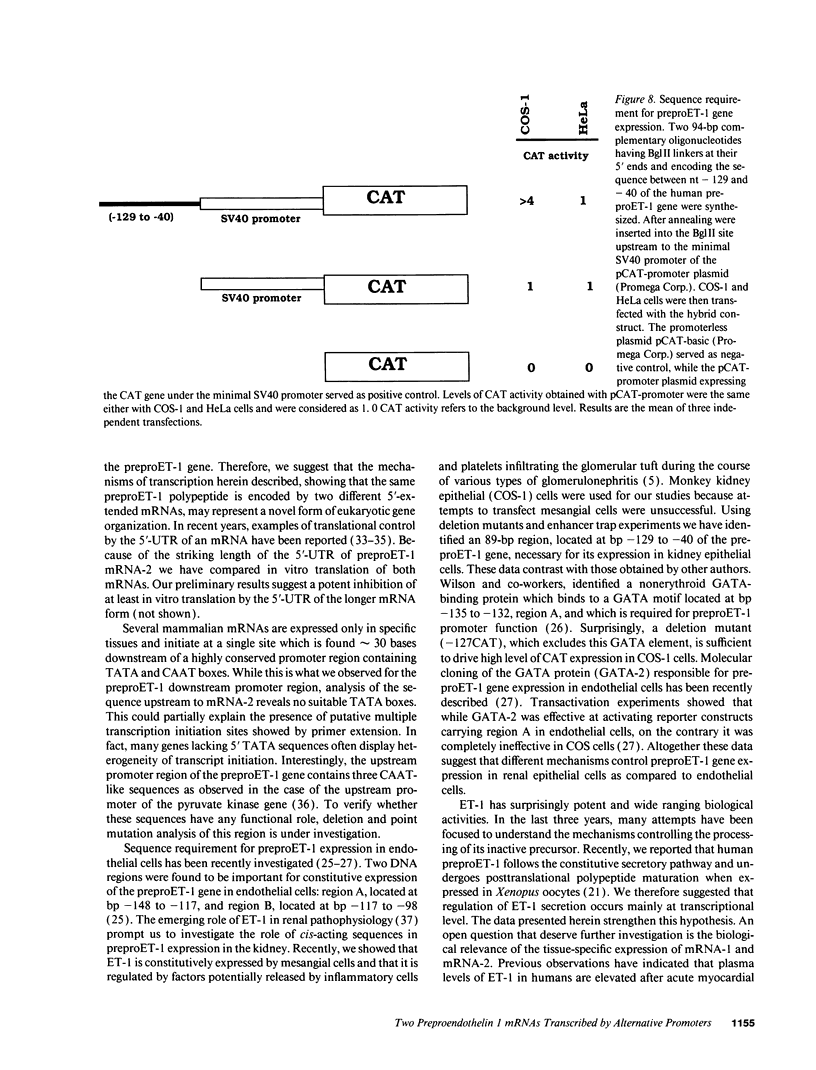
Abstract
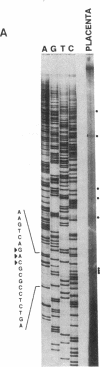
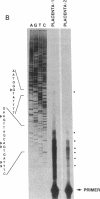
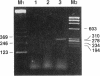
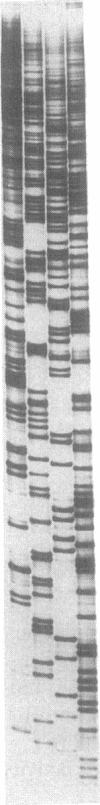
Endothelin-1, initially identified as potent vasoconstrictor secreted by vascular endothelial cells, was subsequently found to have many effects on both vascular and nonvascular tissues. We have identified from a human placenta cDNA library a clone (cDNA-2) which corresponds to a novel 5'-extended preproendothelin 1 (preproET-1) mRNA. Comparison with the known preproET-1 mRNA (cDNA-1), showed that the two molecules share the same coding sequence but differ in the 5'-untranslated region. Interestingly, cDNA-2 extends upstream of promoter regions previously shown to be essential for full preproET-1 expression. Primer extension and PCR analysis of human placenta RNA demonstrated the presence of additional transcription initiation sites located upstream of the previously identified preproET-1 CAP site. Moreover, the two mRNAs show different pattern of expression. To elucidate the mechanisms controlling the production of alternative transcripts we transfected COS-1 cells with a series of preproET-1 promoter deletion mutants. This analysis revealed that the human preproET-1 gene can be transcribed from a proximal and a distal promoter element which has hitherto been undetected. In addition, we demonstrate the presence of a region in the down-epithelial specific expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian G. S., Korinek B. W., Bowman B. H., Yang F. The human transferrin gene: 5' region contains conserved sequences which match the control elements regulated by heavy metals, glucocorticoids and acute phase reaction. Gene. 1986;49(2):167–175. doi: 10.1016/0378-1119(86)90277-5. [DOI] [PubMed] [Google Scholar]
- Badr K. F., Murray J. J., Breyer M. D., Takahashi K., Inagami T., Harris R. C. Mesangial cell, glomerular and renal vascular responses to endothelin in the rat kidney. Elucidation of signal transduction pathways. J Clin Invest. 1989 Jan;83(1):336–342. doi: 10.1172/JCI113880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baley P. A., Resink T. J., Eppenberger U., Hahn A. W. Endothelin messenger RNA and receptors are differentially expressed in cultured human breast epithelial and stromal cells. J Clin Invest. 1990 Apr;85(4):1320–1323. doi: 10.1172/JCI114570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benatti L., Cozzi L., Zamai M., Tamburin M., Vaghi F., Caiolfa V. R., Fabbrini M. S., Sarmientos P. Human preproendothelin-1 is converted into active endothelin-1 by baculovirus-infected insect cells. Biochem Biophys Res Commun. 1992 Jul 31;186(2):753–759. doi: 10.1016/0006-291x(92)90810-8. [DOI] [PubMed] [Google Scholar]
- Bloch K. D., Friedrich S. P., Lee M. E., Eddy R. L., Shows T. B., Quertermous T. Structural organization and chromosomal assignment of the gene encoding endothelin. J Biol Chem. 1989 Jun 25;264(18):10851–10857. [PubMed] [Google Scholar]
- Emori T., Hirata Y., Ohta K., Shichiri M., Marumo F. Secretory mechanism of immunoreactive endothelin in cultured bovine endothelial cells. Biochem Biophys Res Commun. 1989 Apr 14;160(1):93–100. doi: 10.1016/0006-291x(89)91625-2. [DOI] [PubMed] [Google Scholar]
- Fabbrini M. S., Valsasina B., Nitti G., Benatti L., Vitale A. The signal peptide of human preproendothelin-1. FEBS Lett. 1991 Jul 29;286(1-2):91–94. doi: 10.1016/0014-5793(91)80948-3. [DOI] [PubMed] [Google Scholar]
- Fabbrini M. S., Vitale A., Patrono C., Zamai M., Vaghi F., Caiolfa V., Monaco L., Benatti L. Heterologous in vivo processing of human preproendothelin 1 into bioactive peptides. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8939–8943. doi: 10.1073/pnas.88.20.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes D. M., Mullis N. T., Comeau C. M., Crabtree G. R. Potential basis for regulation of the coordinately expressed fibrinogen genes: homology in the 5' flanking regions. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2313–2316. doi: 10.1073/pnas.81.8.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y., Hirata Y., Yoshimi H., Kojima T., Kobayashi Y., Yanagisawa M., Masaki T. Endothelin is a potent secretagogue for atrial natriuretic peptide in cultured rat atrial myocytes. Biochem Biophys Res Commun. 1988 Aug 30;155(1):167–172. doi: 10.1016/s0006-291x(88)81064-7. [DOI] [PubMed] [Google Scholar]
- Giaid A., Gibson S. J., Ibrahim B. N., Legon S., Bloom S. R., Yanagisawa M., Masaki T., Varndell I. M., Polak J. M. Endothelin 1, an endothelium-derived peptide, is expressed in neurons of the human spinal cord and dorsal root ganglia. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7634–7638. doi: 10.1073/pnas.86.19.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A. W., Resink T. J., Scott-Burden T., Powell J., Dohi Y., Bühler F. R. Stimulation of endothelin mRNA and secretion in rat vascular smooth muscle cells: a novel autocrine function. Cell Regul. 1990 Aug;1(9):649–659. doi: 10.1091/mbc.1.9.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G. Involvement of an initiation factor and protein phosphorylation in translational control of GCN4 mRNA. Trends Biochem Sci. 1990 Apr;15(4):148–152. doi: 10.1016/0968-0004(90)90215-w. [DOI] [PubMed] [Google Scholar]
- Inoue A., Yanagisawa M., Takuwa Y., Mitsui Y., Kobayashi M., Masaki T. The human preproendothelin-1 gene. Complete nucleotide sequence and regulation of expression. J Biol Chem. 1989 Sep 5;264(25):14954–14959. [PubMed] [Google Scholar]
- Itoh Y., Yanagisawa M., Ohkubo S., Kimura C., Kosaka T., Inoue A., Ishida N., Mitsui Y., Onda H., Fujino M. Cloning and sequence analysis of cDNA encoding the precursor of a human endothelium-derived vasoconstrictor peptide, endothelin: identity of human and porcine endothelin. FEBS Lett. 1988 Apr 25;231(2):440–444. doi: 10.1016/0014-5793(88)80867-6. [DOI] [PubMed] [Google Scholar]
- Komuro I., Kurihara H., Sugiyama T., Yoshizumi M., Takaku F., Yazaki Y. Endothelin stimulates c-fos and c-myc expression and proliferation of vascular smooth muscle cells. FEBS Lett. 1988 Oct 10;238(2):249–252. doi: 10.1016/0014-5793(88)80489-7. [DOI] [PubMed] [Google Scholar]
- Kon V., Badr K. F. Biological actions and pathophysiologic significance of endothelin in the kidney. Kidney Int. 1991 Jul;40(1):1–12. doi: 10.1038/ki.1991.172. [DOI] [PubMed] [Google Scholar]
- Lee M. E., Bloch K. D., Clifford J. A., Quertermous T. Functional analysis of the endothelin-1 gene promoter. Evidence for an endothelial cell-specific cis-acting sequence. J Biol Chem. 1990 Jun 25;265(18):10446–10450. [PubMed] [Google Scholar]
- Lee M. E., Temizer D. H., Clifford J. A., Quertermous T. Cloning of the GATA-binding protein that regulates endothelin-1 gene expression in endothelial cells. J Biol Chem. 1991 Aug 25;266(24):16188–16192. [PubMed] [Google Scholar]
- Lidbury P. S., Thiemermann C., Korbut R., Vane J. R. Endothelins release tissue plasminogen activator and prostanoids. Eur J Pharmacol. 1990 Sep 21;186(2-3):205–212. doi: 10.1016/0014-2999(90)90435-9. [DOI] [PubMed] [Google Scholar]
- MacCumber M. W., Ross C. A., Snyder S. H. Endothelin in brain: receptors, mitogenesis, and biosynthesis in glial cells. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2359–2363. doi: 10.1073/pnas.87.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi T., Yanagisawa M., Tomizawa T., Sugishita Y., Suzuki N., Fujino M., Ajisaka R., Goto K., Masaki T. Increased plasma concentrations of endothelin-1 and big endothelin-1 in acute myocardial infarction. Lancet. 1989 Jul 1;2(8653):53–54. doi: 10.1016/s0140-6736(89)90303-6. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Yamada K., Inoue H., Matsuda T., Tanaka T. The L- and R-type isozymes of rat pyruvate kinase are produced from a single gene by use of different promoters. J Biol Chem. 1987 Oct 15;262(29):14366–14371. [PubMed] [Google Scholar]
- Parkin N., Darveau A., Nicholson R., Sonenberg N. cis-acting translational effects of the 5' noncoding region of c-myc mRNA. Mol Cell Biol. 1988 Jul;8(7):2875–2883. doi: 10.1128/mcb.8.7.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruis J., Emeis J. J. Endothelin-1 and -3 induce the release of tissue-type plasminogen activator and von Willebrand factor from endothelial cells. Eur J Pharmacol. 1990 Oct 2;187(1):105–112. doi: 10.1016/0014-2999(90)90345-7. [DOI] [PubMed] [Google Scholar]
- Ratner L. Regulation of expression of the c-sis proto-oncogene. Nucleic Acids Res. 1989 Jun 12;17(11):4101–4115. doi: 10.1093/nar/17.11.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sawamura T., Kimura S., Shinmi O., Sugita Y., Yanagisawa M., Masaki T. Analysis of endothelin related peptides in culture supernatant of porcine aortic endothelial cells: evidence for biosynthetic pathway of endothelin-1. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1287–1294. doi: 10.1016/0006-291x(89)90813-9. [DOI] [PubMed] [Google Scholar]
- Schibler U., Sierra F. Alternative promoters in developmental gene expression. Annu Rev Genet. 1987;21:237–257. doi: 10.1146/annurev.ge.21.120187.001321. [DOI] [PubMed] [Google Scholar]
- Shubeita H. E., McDonough P. M., Harris A. N., Knowlton K. U., Glembotski C. C., Brown J. H., Chien K. R. Endothelin induction of inositol phospholipid hydrolysis, sarcomere assembly, and cardiac gene expression in ventricular myocytes. A paracrine mechanism for myocardial cell hypertrophy. J Biol Chem. 1990 Nov 25;265(33):20555–20562. [PubMed] [Google Scholar]
- Simonson M. S., Wann S., Mené P., Dubyak G. R., Kester M., Nakazato Y., Sedor J. R., Dunn M. J. Endothelin stimulates phospholipase C, Na+/H+ exchange, c-fos expression, and mitogenesis in rat mesangial cells. J Clin Invest. 1989 Feb;83(2):708–712. doi: 10.1172/JCI113935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi M., Matsuoka H., Atarashi K., Yagi S. Endothelin: a new inhibitor of renin release. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1164–1168. doi: 10.1016/s0006-291x(88)80996-3. [DOI] [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Yanagisawa M., Yamashita K., Masaki T. A novel vasoactive peptide endothelin stimulates mitogenesis through inositol lipid turnover in Swiss 3T3 fibroblasts. J Biol Chem. 1989 May 15;264(14):7856–7861. [PubMed] [Google Scholar]
- Wilson D. B., Dorfman D. M., Orkin S. H. A nonerythroid GATA-binding protein is required for function of the human preproendothelin-1 promoter in endothelial cells. Mol Cell Biol. 1990 Sep;10(9):4854–4862. doi: 10.1128/mcb.10.9.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Zoja C., Orisio S., Perico N., Benigni A., Morigi M., Benatti L., Rambaldi A., Remuzzi G. Constitutive expression of endothelin gene in cultured human mesangial cells and its modulation by transforming growth factor-beta, thrombin, and a thromboxane A2 analogue. Lab Invest. 1991 Jan;64(1):16–20. [PubMed] [Google Scholar]