Abstract
The properties of control and 370Asn-->Ser glucocerebrosidase, the frequently encountered mutated form of the enzyme in type 1 Gaucher disease, were studied in vitro as well as in situ. The catalytic properties of purified 370Asn-->Ser glucocerebrosidase were highly dependent on the assay conditions. The enzyme was deficient in activity towards substrate and in reactivity with the irreversible inhibitor conduritol B-epoxide (CBE) when activated by the bile salt taurocholate. In the presence of more physiological activators, the lysosomal activator protein saposin C and phosphatidylserine, the 370Asn-->Ser enzyme was near normal in kinetic properties at pH values approximately 5, but not at higher pH. In intact fibroblasts, the enzymic activity of the 370Asn-->Ser glucocerebrosidase and its reactivity with CBE were found to be clearly deficient. However, in intact lymphoblasts from the same patients, the behavior of the mutant enzyme was near normal. The catalytic efficiency of 370Asn-->Ser glucocerebrosidase in situ was also found to be highly pH dependent. When intact lymphoblasts were cultured in the presence of permeant weak bases, which increase the pH of acidic intracellular compartments, the catalytic efficiency of the mutant enzyme, as assessed by its reactivity with CBE, became markedly impaired. Our findings indicate that the intralysosomal pH in the intact cell can be expected to have a critical influence on the activation state of 370Asn-->Ser glucocerebrosidase and its ability to hydrolyse substrate. This phenomenon may partly underly the marked heterogeneity in clinical manifestation of Gaucher disease among patients with this mutated form of glucocerebrosidase.
Full text
PDF
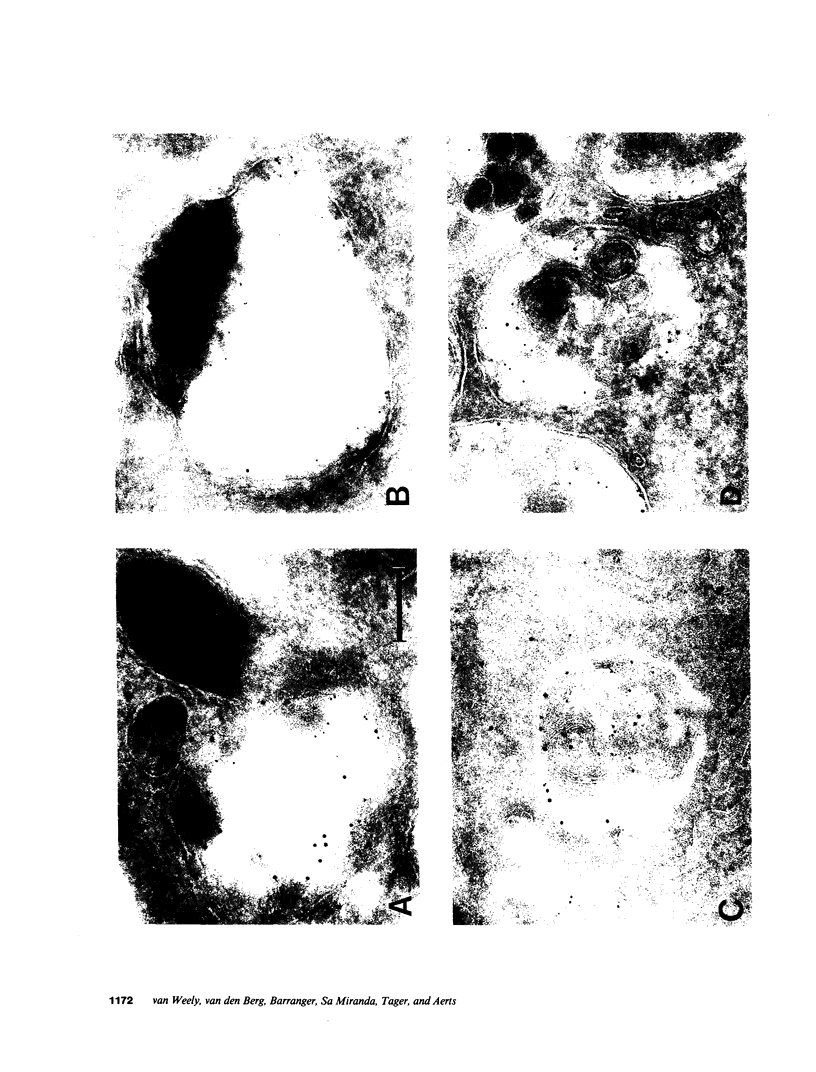

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aerts J. M., Donker-Koopman W. E., Brul S., Van Weely S., Sa Miranda M. C., Barranger J. A., Tager J. M., Schram A. W. Comparative study on glucocerebrosidase in spleens from patients with Gaucher disease. Biochem J. 1990 Jul 1;269(1):93–100. doi: 10.1042/bj2690093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aerts J. M., Donker-Koopman W. E., Murray G. J., Barranger J. A., Tager J. M., Schram A. W. A procedure for the rapid purification in high yield of human glucocerebrosidase using immunoaffinity chromatography with monoclonal antibodies. Anal Biochem. 1986 May 1;154(2):655–663. doi: 10.1016/0003-2697(86)90043-6. [DOI] [PubMed] [Google Scholar]
- Aerts J. M., Donker-Koopman W. E., van Laar C., Brul S., Murray G. J., Wenger D. A., Barranger J. A., Tager J. M., Schram A. W. Relationship between the two immunologically distinguishable forms of glucocerebrosidase in tissue extracts. Eur J Biochem. 1987 Mar 16;163(3):583–589. doi: 10.1111/j.1432-1033.1987.tb10907.x. [DOI] [PubMed] [Google Scholar]
- Aerts J. M., Donker-Koopman W. E., van der Vliet M. K., Jonsson L. M., Ginns E. I., Murray G. J., Barranger J. A., Tager J. M., Schram A. W. The occurrence of two immunologically distinguishable beta-glucocerebrosidases in human spleen. Eur J Biochem. 1985 Aug 1;150(3):565–574. doi: 10.1111/j.1432-1033.1985.tb09058.x. [DOI] [PubMed] [Google Scholar]
- Aerts J. M., Sa Miranda M. C., Brouwer-Kelder E. M., Van Weely S., Barranger J. A., Tager J. M. Conditions affecting the activity of glucocerebrosidase purified from spleens of control subjects and patients with type 1 Gaucher disease. Biochim Biophys Acta. 1990 Oct 18;1041(1):55–63. doi: 10.1016/0167-4838(90)90122-v. [DOI] [PubMed] [Google Scholar]
- Beutler E. Gaucher disease: new molecular approaches to diagnosis and treatment. Science. 1992 May 8;256(5058):794–799. doi: 10.1126/science.1589760. [DOI] [PubMed] [Google Scholar]
- Beutler E., Gelbart T., Kuhl W., Sorge J., West C. Identification of the second common Jewish Gaucher disease mutation makes possible population-based screening for the heterozygous state. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10544–10547. doi: 10.1073/pnas.88.23.10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E., Gelbart T., Kuhl W., Zimran A., West C. Mutations in Jewish patients with Gaucher disease. Blood. 1992 Apr 1;79(7):1662–1666. [PubMed] [Google Scholar]
- Bieberich E., Legler G. Intracellular activity of lysosomal glucosylceramidase measured with 4-nonylumbelliferyl beta-glucoside. Biol Chem Hoppe Seyler. 1989 Aug;370(8):809–817. doi: 10.1515/bchm3.1989.370.2.809. [DOI] [PubMed] [Google Scholar]
- Dahl N., Lagerström M., Erikson A., Pettersson U. Gaucher disease type III (Norrbottnian type) is caused by a single mutation in exon 10 of the glucocerebrosidase gene. Am J Hum Genet. 1990 Aug;47(2):275–278. [PMC free article] [PubMed] [Google Scholar]
- Firon N., Eyal N., Kolodny E. H., Horowitz M. Genotype assignment in Gaucher disease by selective amplification of the active glucocerebrosidase gene. Am J Hum Genet. 1990 Mar;46(3):527–532. [PMC free article] [PubMed] [Google Scholar]
- Fujibayashi S., Wenger D. A. Studies on a sphingolipid activator protein (SAP-2) in fibroblasts from patients with lysosomal storage diseases, including Niemann-Pick disease Type C. Clin Chim Acta. 1985 Mar 15;146(2-3):147–156. doi: 10.1016/0009-8981(85)90053-1. [DOI] [PubMed] [Google Scholar]
- Ginns E. I., Brady R. O., Pirruccello S., Moore C., Sorrell S., Furbish F. S., Murray G. J., Tager J., Barranger J. A. Mutations of glucocerebrosidase: discrimination of neurologic and non-neurologic phenotypes of Gaucher disease. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5607–5610. doi: 10.1073/pnas.79.18.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glew R. H., Gopalan V., Hubbell C. A., Beutler E., Geil J. D., Lee R. E. A case of nonneurologic Gaucher's disease that biochemically resembles the neurologic types. J Neuropathol Exp Neurol. 1991 Mar;50(2):108–117. doi: 10.1097/00005072-199103000-00002. [DOI] [PubMed] [Google Scholar]
- Grabowski G. A., Dinur T., Osiecki K. M., Kruse J. R., Legler G., Gatt S. Gaucher disease types 1, 2, and 3: differential mutations of the acid beta-glucosidase active site identified with conduritol B epoxide derivatives and sphingosine. Am J Hum Genet. 1985 May;37(3):499–510. [PMC free article] [PubMed] [Google Scholar]
- Grabowski G. A., Gatt S., Horowitz M. Acid beta-glucosidase: enzymology and molecular biology of Gaucher disease. Crit Rev Biochem Mol Biol. 1990;25(6):385–414. doi: 10.3109/10409239009090616. [DOI] [PubMed] [Google Scholar]
- Grabowski G. A., Osiecki-Newman K., Dinur T., Fabbro D., Legler G., Gatt S., Desnick R. J. Human acid beta-glucosidase. Use of conduritol B epoxide derivatives to investigate the catalytically active normal and Gaucher disease enzymes. J Biol Chem. 1986 Jun 25;261(18):8263–8269. [PubMed] [Google Scholar]
- Grace M. E., Berg A., He G. S., Goldberg L., Horowitz M., Grabowski G. A. Gaucher disease: heterologous expression of two alleles associated with neuronopathic phenotypes. Am J Hum Genet. 1991 Sep;49(3):646–655. [PMC free article] [PubMed] [Google Scholar]
- Grace M. E., Graves P. N., Smith F. I., Grabowski G. A. Analyses of catalytic activity and inhibitor binding of human acid beta-glucosidase by site-directed mutagenesis. Identification of residues critical to catalysis and evidence for causality of two Ashkenazi Jewish Gaucher disease type 1 mutations. J Biol Chem. 1990 Apr 25;265(12):6827–6835. [PubMed] [Google Scholar]
- Hollemans M., Elferink R. O., De Groot P. G., Strijland A., Tager J. M. Accumulation of weak bases in relation to intralysosomal pH in cultured human skin fibroblasts. Biochim Biophys Acta. 1981 Apr 22;643(1):140–151. doi: 10.1016/0005-2736(81)90226-1. [DOI] [PubMed] [Google Scholar]
- Hong C. M., Ohashi T., Yu X. J., Weiler S., Barranger J. A. Sequence of two alleles responsible for Gaucher disease. DNA Cell Biol. 1990 May;9(4):233–241. doi: 10.1089/dna.1990.9.233. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Latham T. E., Theophilus B. D., Grabowski G. A., Smith F. I. Heterogeneity of mutations in the acid beta-glucosidase gene of Gaucher disease patients. DNA Cell Biol. 1991 Jan-Feb;10(1):15–21. doi: 10.1089/dna.1991.10.15. [DOI] [PubMed] [Google Scholar]
- Masuno M., Tomatsu S., Sukegawa K., Orii T. Non-existence of a tight association between a 444leucine to proline mutation and phenotypes of Gaucher disease: high frequency of a NciI polymorphism in the non-neuronopathic form. Hum Genet. 1990 Jan;84(2):203–206. doi: 10.1007/BF00208943. [DOI] [PubMed] [Google Scholar]
- O'Brien J. S., Kishimoto Y. Saposin proteins: structure, function, and role in human lysosomal storage disorders. FASEB J. 1991 Mar 1;5(3):301–308. doi: 10.1096/fasebj.5.3.2001789. [DOI] [PubMed] [Google Scholar]
- Ohashi T., Hong C. M., Weiler S., Tomich J. M., Aerts J. M., Tager J. M., Barranger J. A. Characterization of human glucocerebrosidase from different mutant alleles. J Biol Chem. 1991 Feb 25;266(6):3661–3667. [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa Miranda M. C., Aerts J. M., Pinto R., Fontes A., de Lacerda L. W., van Weely S., Barranger J., Tager J. M. Activity of glucocerebrosidase in extracts of different cell types from type 1 Gaucher disease patients. Clin Genet. 1990 Sep;38(3):218–227. doi: 10.1111/j.1399-0004.1990.tb03573.x. [DOI] [PubMed] [Google Scholar]
- Sidransky E., Tsuji S., Martin B. M., Stubblefield B., Ginns E. I. DNA mutation analysis of Gaucher patients. Am J Med Genet. 1992 Feb 1;42(3):331–336. doi: 10.1002/ajmg.1320420315. [DOI] [PubMed] [Google Scholar]
- Sorge J., West C., Westwood B., Beutler E. Molecular cloning and nucleotide sequence of human glucocerebrosidase cDNA. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7289–7293. doi: 10.1073/pnas.82.21.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theophilus B., Latham T., Grabowski G. A., Smith F. I. Gaucher disease: molecular heterogeneity and phenotype-genotype correlations. Am J Hum Genet. 1989 Aug;45(2):212–225. [PMC free article] [PubMed] [Google Scholar]
- Tsuji S., Choudary P. V., Martin B. M., Stubblefield B. K., Mayor J. A., Barranger J. A., Ginns E. I. A mutation in the human glucocerebrosidase gene in neuronopathic Gaucher's disease. N Engl J Med. 1987 Mar 5;316(10):570–575. doi: 10.1056/NEJM198703053161002. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Martin B. M., Barranger J. A., Stubblefield B. K., LaMarca M. E., Ginns E. I. Genetic heterogeneity in type 1 Gaucher disease: multiple genotypes in Ashkenazic and non-Ashkenazic individuals. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2349–2352. doi: 10.1073/pnas.85.7.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Weely S., Van Leeuwen M. B., Jansen I. D., De Bruijn M. A., Brouwer-Kelder E. M., Schram A. W., Sa Miranda M. C., Barranger J. A., Petersen E. M., Goldblatt J. Clinical phenotype of Gaucher disease in relation to properties of mutant glucocerebrosidase in cultured fibroblasts. Biochim Biophys Acta. 1991 Jun 5;1096(4):301–311. doi: 10.1016/0925-4439(91)90066-i. [DOI] [PubMed] [Google Scholar]
- Wiemer E. A., Brul S., Just W. W., Van Driel R., Brouwer-Kelder E., Van Den Berg M., Weijers P. J., Schutgens R. B., Van Den Bosch H., Schram A. Presence of peroxisomal membrane proteins in liver and fibroblasts from patients with the Zellweger syndrome and related disorders: evidence for the existence of peroxisomal ghosts. Eur J Cell Biol. 1989 Dec;50(2):407–417. [PubMed] [Google Scholar]
- Zimran A., Gelbart T., Westwood B., Grabowski G. A., Beutler E. High frequency of the Gaucher disease mutation at nucleotide 1226 among Ashkenazi Jews. Am J Hum Genet. 1991 Oct;49(4):855–859. [PMC free article] [PubMed] [Google Scholar]
- Zimran A., Sorge J., Gross E., Kubitz M., West C., Beutler E. Prediction of severity of Gaucher's disease by identification of mutations at DNA level. Lancet. 1989 Aug 12;2(8659):349–352. doi: 10.1016/s0140-6736(89)90536-9. [DOI] [PubMed] [Google Scholar]