Abstract
Malignant melanomas are often infiltrated by T lymphocytes. It is postulated that the presence of tumor-infiltrating lymphocytes (TIL) reflects ongoing immune responses against transformed cells. Such "responses" appear generally inefficient with the potential exception of infrequent clinical situations characterized by spontaneous tumor regression. We have characterized here the molecular structure of the T cell receptor beta chain expressed by TILs in a case of regressive melanoma. Advantage was taken of the PCR technology to study T lymphocytes directly without cell culture. Experimentally validated V beta subfamily specific primers were used to evaluate the V beta usage in TILs and control samples. Our results reveal that clonal T cell populations, precisely defined by their V-D-J junctional sequences, are amplified at the tumor site. The existence of such local antigen-driven selections support the hypothesis that antitumor responses may indeed take place in regressive melanoma.
Full text
PDF



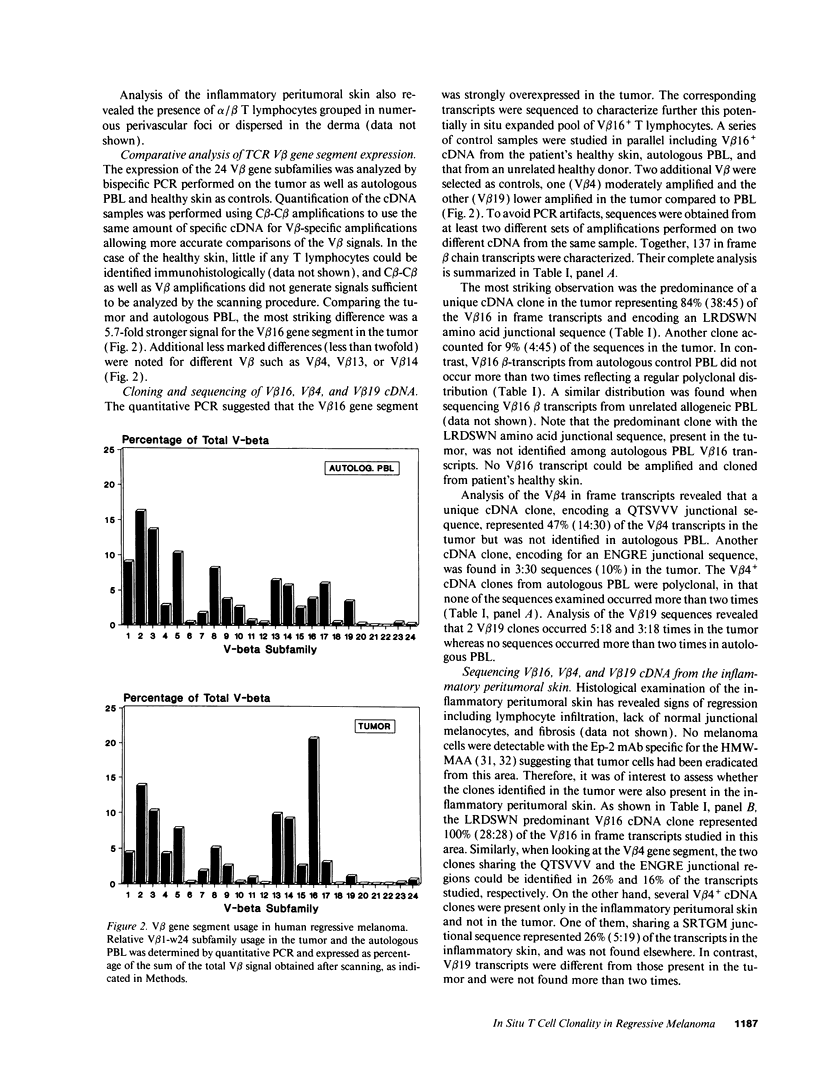
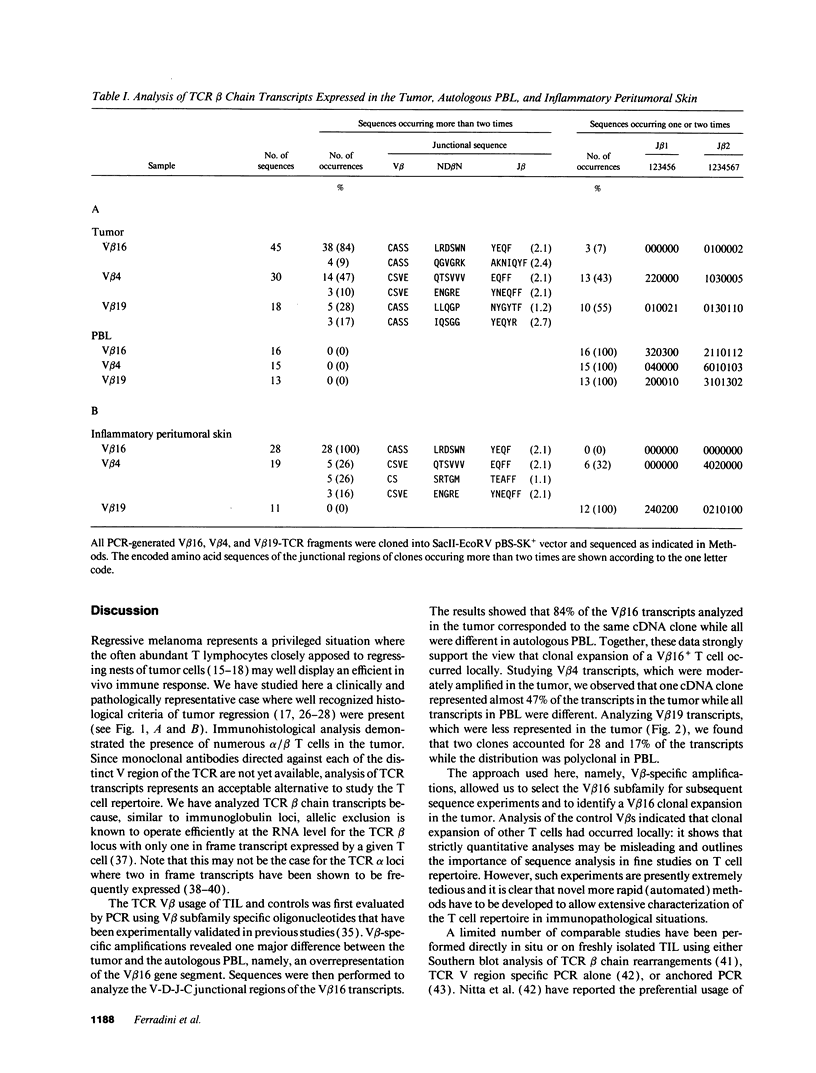


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anichini A., Fossati G., Parmiani G. Clonal analysis of cytotoxic T-lymphocyte response to autologous human metastatic melanoma. Int J Cancer. 1985 May 15;35(5):683–689. doi: 10.1002/ijc.2910350518. [DOI] [PubMed] [Google Scholar]
- Band H., Hochstenbach F., McLean J., Hata S., Krangel M. S., Brenner M. B. Immunochemical proof that a novel rearranging gene encodes the T cell receptor delta subunit. Science. 1987 Oct 30;238(4827):682–684. doi: 10.1126/science.3672118. [DOI] [PubMed] [Google Scholar]
- Belldegrun A., Kasid A., Uppenkamp M., Topalian S. L., Rosenberg S. A. Human tumor infiltrating lymphocytes. Analysis of lymphokine mRNA expression and relevance to cancer immunotherapy. J Immunol. 1989 Jun 15;142(12):4520–4526. [PubMed] [Google Scholar]
- Blüthmann H., Kisielow P., Uematsu Y., Malissen M., Krimpenfort P., Berns A., von Boehmer H., Steinmetz M. T-cell-specific deletion of T-cell receptor transgenes allows functional rearrangement of endogenous alpha- and beta-genes. Nature. 1988 Jul 14;334(6178):156–159. doi: 10.1038/334156a0. [DOI] [PubMed] [Google Scholar]
- Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970 Nov;172(5):902–908. doi: 10.1097/00000658-197011000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardi G., Mastrangelo M. J., Berd D. Depletion of T-cells with the CD4+CD45R+ phenotype in lymphocytes that infiltrate subcutaneous metastases of human melanoma. Cancer Res. 1989 Dec 1;49(23):6562–6565. [PubMed] [Google Scholar]
- Chakraborty N. G., Twardzik D. R., Sivanandham M., Ergin M. T., Hellstrom K. E., Mukherji B. Autologous melanoma-induced activation of regulatory T cells that suppress cytotoxic response. J Immunol. 1990 Oct 1;145(7):2359–2364. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Couez D., Malissen M., Buferne M., Schmitt-Verhulst A. M., Malissen B. Each of the two productive T cell receptor alpha-gene rearrangements found in both the A10 and BM 3.3 T cell clones give rise to an alpha chain which can contribute to the constitution of a surface-expressed alpha beta dimer. Int Immunol. 1991 Jul;3(7):719–729. doi: 10.1093/intimm/3.7.719. [DOI] [PubMed] [Google Scholar]
- Darrow T. L., Slingluff C. L., Jr, Seigler H. F. The role of HLA class I antigens in recognition of melanoma cells by tumor-specific cytotoxic T lymphocytes. Evidence for shared tumor antigens. J Immunol. 1989 May 1;142(9):3329–3335. [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Ferradini L., Roman-Roman S., Azocar J., Avril M. F., Viel S., Triebel F., Hercend T. Analysis of T-cell receptor alpha/beta variability in lymphocytes infiltrating a melanoma metastasis. Cancer Res. 1992 Sep 1;52(17):4649–4654. [PubMed] [Google Scholar]
- Furutani M., Yanagi Y., Fujisawa I., Nakayama T., Kishimoto H., Kuida K., Asano Y., Tada T. Post-transcriptional allelic exclusion of two functionally rearranged T cell receptor alpha genes. Int Immunol. 1989;1(3):281–288. doi: 10.1093/intimm/1.3.281. [DOI] [PubMed] [Google Scholar]
- Genevée C., Diu A., Nierat J., Caignard A., Dietrich P. Y., Ferradini L., Roman-Roman S., Triebel F., Hercend T. An experimentally validated panel of subfamily-specific oligonucleotide primers (V alpha 1-w29/V beta 1-w24) for the study of human T cell receptor variable V gene segment usage by polymerase chain reaction. Eur J Immunol. 1992 May;22(5):1261–1269. doi: 10.1002/eji.1830220522. [DOI] [PubMed] [Google Scholar]
- Gervois N., Heuze F., Diez E., Jotereau F. Selective expansion of a specific anti-tumor CD8+ cytotoxic T lymphocyte clone in the bulk culture of tumor-infiltrating lymphocytes from a melanoma patient: cytotoxic activity and T cell receptor gene rearrangements. Eur J Immunol. 1990 Apr;20(4):825–831. doi: 10.1002/eji.1830200417. [DOI] [PubMed] [Google Scholar]
- Giacomini P., Segatto O., Natali P. G. Multiple epitope recognition: an approach to improved radioimmunodetection of tumor-associated antigens. Int J Cancer. 1987 Jun 15;39(6):729–736. doi: 10.1002/ijc.2910390613. [DOI] [PubMed] [Google Scholar]
- Hérin M., Lemoine C., Weynants P., Vessière F., Van Pel A., Knuth A., Devos R., Boon T. Production of stable cytolytic T-cell clones directed against autologous human melanoma. Int J Cancer. 1987 Mar 15;39(3):390–396. doi: 10.1002/ijc.2910390320. [DOI] [PubMed] [Google Scholar]
- Itoh K., Platsoucas C. D., Balch C. M. Autologous tumor-specific cytotoxic T lymphocytes in the infiltrate of human metastatic melanomas. Activation by interleukin 2 and autologous tumor cells, and involvement of the T cell receptor. J Exp Med. 1988 Oct 1;168(4):1419–1441. doi: 10.1084/jem.168.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuth A., Wölfel T., Klehmann E., Boon T., Meyer zum Büschenfelde K. H. Cytolytic T-cell clones against an autologous human melanoma: specificity study and definition of three antigens by immunoselection. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2804–2808. doi: 10.1073/pnas.86.8.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern V. J., Shaw H. M., Milton G. W. Prognosis in patients with thin malignant melanoma: influence of regression. Histopathology. 1983 Sep;7(5):673–680. doi: 10.1111/j.1365-2559.1983.tb02279.x. [DOI] [PubMed] [Google Scholar]
- McGovern V. J. Spontaneous regression of melanoma. Pathology. 1975 Apr;7(2):91–99. doi: 10.3109/00313027509092702. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Fitzgerald K. A., Hussey R. E., Hodgdon J. C., Schlossman S. F., Reinherz E. L. Clonotypic structures involved in antigen-specific human T cell function. Relationship to the T3 molecular complex. J Exp Med. 1983 Feb 1;157(2):705–719. doi: 10.1084/jem.157.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T., Salmeron M. A., Moser R. P., Ross M. I., Itoh K. Oligoclonal expansion of V beta 8+ cells in interleukin-2-activated T cells residing in subcutaneous metastatic melanoma. Clin Exp Metastasis. 1992 Jan;10(1):69–76. doi: 10.1007/BF00163578. [DOI] [PubMed] [Google Scholar]
- Mukherji B., Guha A., Chakraborty N. G., Sivanandham M., Nashed A. L., Sporn J. R., Ergin M. T. Clonal analysis of cytotoxic and regulatory T cell responses against human melanoma. J Exp Med. 1989 Jun 1;169(6):1961–1976. doi: 10.1084/jem.169.6.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muul L. M., Spiess P. J., Director E. P., Rosenberg S. A. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J Immunol. 1987 Feb 1;138(3):989–995. [PubMed] [Google Scholar]
- Natali P. G., Imai K., Wilson B. S., Bigotti A., Cavaliere R., Pellegrino M. A., Ferrone S. Structural properties and tissue distribution of the antigen recognized by the monoclonal antibody 653.40S to human melanoma cells. J Natl Cancer Inst. 1981 Sep;67(3):591–601. [PubMed] [Google Scholar]
- Nathanson Spontaneous regression of malignant melanoma: a review of the literature on incidence, clinical features, and possible mechanisms. Natl Cancer Inst Monogr. 1976 Nov;44:67–76. [PubMed] [Google Scholar]
- Nitta T., Oksenberg J. R., Rao N. A., Steinman L. Predominant expression of T cell receptor V alpha 7 in tumor-infiltrating lymphocytes of uveal melanoma. Science. 1990 Aug 10;249(4969):672–674. doi: 10.1126/science.2382141. [DOI] [PubMed] [Google Scholar]
- Poppema S., Bröcker E. B., de Leij L., Terbrack D., Visscher T., Ter Haar A., Macher E., Thé T. H., Sorg C. In situ analysis of the mononuclear cell infiltrate in primary malignant melanoma of the skin. Clin Exp Immunol. 1983 Jan;51(1):77–82. [PMC free article] [PubMed] [Google Scholar]
- Ronan S. G., Eng A. M., Briele H. A., Shioura N. N., Das Gupta T. K. Thin malignant melanomas with regression and metastases. Arch Dermatol. 1987 Oct;123(10):1326–1330. [PubMed] [Google Scholar]
- Rosenberg S. A., Packard B. S., Aebersold P. M., Solomon D., Topalian S. L., Toy S. T., Simon P., Lotze M. T., Yang J. C., Seipp C. A. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988 Dec 22;319(25):1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Spiess P., Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986 Sep 19;233(4770):1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Jr, Stehlin J. S., Jr Spontaneous regression of primary malignant melanomas with regional metastases. Cancer. 1965 Nov;18(11):1399–1415. doi: 10.1002/1097-0142(196511)18:11<1399::aid-cncr2820181104>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Topalian S. L., Solomon D., Avis F. P., Chang A. E., Freerksen D. L., Linehan W. M., Lotze M. T., Robertson C. N., Seipp C. A., Simon P. Immunotherapy of patients with advanced cancer using tumor-infiltrating lymphocytes and recombinant interleukin-2: a pilot study. J Clin Oncol. 1988 May;6(5):839–853. doi: 10.1200/JCO.1988.6.5.839. [DOI] [PubMed] [Google Scholar]
- Topalian S. L., Solomon D., Rosenberg S. A. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J Immunol. 1989 May 15;142(10):3714–3725. [PubMed] [Google Scholar]
- Uematsu Y., Ryser S., Dembić Z., Borgulya P., Krimpenfort P., Berns A., von Boehmer H., Steinmetz M. In transgenic mice the introduced functional T cell receptor beta gene prevents expression of endogenous beta genes. Cell. 1988 Mar 25;52(6):831–841. doi: 10.1016/0092-8674(88)90425-4. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel T., Klehmann E., Müller C., Schütt K. H., Meyer zum Büschenfelde K. H., Knuth A. Lysis of human melanoma cells by autologous cytolytic T cell clones. Identification of human histocompatibility leukocyte antigen A2 as a restriction element for three different antigens. J Exp Med. 1989 Sep 1;170(3):797–810. doi: 10.1084/jem.170.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino I., Yano T., Yoshikai Y., Murata M., Sugimachi K., Kimura G., Nomoto K. Oligoclonal T lymphocytes infiltrating human lung cancer tissues. Int J Cancer. 1991 Mar 12;47(5):654–658. doi: 10.1002/ijc.2910470504. [DOI] [PubMed] [Google Scholar]







