Abstract
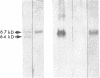
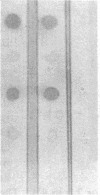
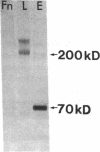
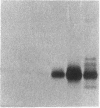
We and others have previously shown that a 67-kD cell surface elastin/laminin-binding protein (EBP) is responsible for cell adhesion to elastin and laminin and for mediating the process of elastin fiber assembly, but the nature of this protein was unknown. In this report we provide evidence that a 67-kD catalytically inactive form of beta-galactosidase produced by alternative splicing demonstrates immunological and functional similarity and sequence homology to the 67-kD EBP, suggesting that the two might be the same. Antibody prepared to a synthetic peptide, N-Ac-GSPSAQDEASPL, corresponding to a frame-shift-generated sequence unique to the alternatively spliced form of human beta-galactosidase, also recognized sheep EBP both on Western blotting and in aortic tissue. Furthermore, this synthetic peptide (S-GAL) binds to elastin and laminin, but not to fibronectin, collagen I, or collagen III. Moreover, both tropoelastin and laminin which bind to S-GAL peptide affinity columns can be specifically eluted from them with an excess of free S-GAL peptides. In addition, sequence homology among this splice variant of human beta-galactosidase, sheep EBP, and NH2-terminal sequences of some elastases suggests that these proteins share a common ligand-binding motif that has not been previously recognized.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- D'Agrosa R. M., Hubbes M., Zhang S., Shankaran R., Callahan J. W. Characteristics of the beta-galactosidase-carboxypeptidase complex in GM1-gangliosidosis and beta-galactosialidosis fibroblasts. Biochem J. 1992 Aug 1;285(Pt 3):833–838. doi: 10.1042/bj2850833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F. Reconstructing the evolution of vertebrate blood coagulation from a consideration of the amino acid sequences of clotting proteins. Cold Spring Harb Symp Quant Biol. 1987;52:869–874. doi: 10.1101/sqb.1987.052.01.095. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Similar amino acid sequences revisited. Trends Biochem Sci. 1989 Jul;14(7):244–245. doi: 10.1016/0968-0004(89)90055-8. [DOI] [PubMed] [Google Scholar]
- Hinek A., Mecham R. P., Keeley F., Rabinovitch M. Impaired elastin fiber assembly related to reduced 67-kD elastin-binding protein in fetal lamb ductus arteriosus and in cultured aortic smooth muscle cells treated with chondroitin sulfate. J Clin Invest. 1991 Dec;88(6):2083–2094. doi: 10.1172/JCI115538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A., Wrenn D. S., Mecham R. P., Barondes S. H. The elastin receptor: a galactoside-binding protein. Science. 1988 Mar 25;239(4847):1539–1541. doi: 10.1126/science.2832941. [DOI] [PubMed] [Google Scholar]
- Hubbes M., D'Agrosa R. M., Callahan J. W. Human placental beta-galactosidase. Characterization of the dimer and complex forms of the enzyme. Biochem J. 1992 Aug 1;285(Pt 3):827–831. doi: 10.1042/bj2850827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. S., Mahuran D., Lowden J. A., Callahan J. W. Human placental beta-galactosidase: structural and immunological observations. Can J Biochem Cell Biol. 1984 Jun;62(6):529–534. doi: 10.1139/o84-070. [DOI] [PubMed] [Google Scholar]
- LaBourene J. I., Coles J. G., Johnson D. J., Mehra A., Keeley F. W., Rabinovitch M. Alterations in elastin and collagen related to the mechanism of progressive pulmonary venous obstruction in a piglet model. A hemodynamic, ultrastructural, and biochemical study. Circ Res. 1990 Feb;66(2):438–456. doi: 10.1161/01.res.66.2.438. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McKenzie H. A., White F. H., Jr Lysozyme and alpha-lactalbumin: structure, function, and interrelationships. Adv Protein Chem. 1991;41:173–315. doi: 10.1016/s0065-3233(08)60198-9. [DOI] [PubMed] [Google Scholar]
- Mecham R. P., Hinek A., Cleary E. G., Kucich U., Lee S. J., Rosenbloom J. Development of immunoreagents to ciliary zonules that react with protein components of elastic fiber microfibrils and with elastin-producing cells. Biochem Biophys Res Commun. 1988 Mar 15;151(2):822–826. doi: 10.1016/s0006-291x(88)80355-3. [DOI] [PubMed] [Google Scholar]
- Mecham R. P., Hinek A., Entwistle R., Wrenn D. S., Griffin G. L., Senior R. M. Elastin binds to a multifunctional 67-kilodalton peripheral membrane protein. Biochemistry. 1989 May 2;28(9):3716–3722. doi: 10.1021/bi00435a014. [DOI] [PubMed] [Google Scholar]
- Mecham R. P., Hinek A., Griffin G. L., Senior R. M., Liotta L. A. The elastin receptor shows structural and functional similarities to the 67-kDa tumor cell laminin receptor. J Biol Chem. 1989 Oct 5;264(28):16652–16657. [PubMed] [Google Scholar]
- Mecham R. P., Whitehouse L., Hay M., Hinek A., Sheetz M. P. Ligand affinity of the 67-kD elastin/laminin binding protein is modulated by the protein's lectin domain: visualization of elastin/laminin-receptor complexes with gold-tagged ligands. J Cell Biol. 1991 Apr;113(1):187–194. doi: 10.1083/jcb.113.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morreau H., Galjart N. J., Gillemans N., Willemsen R., van der Horst G. T., d'Azzo A. Alternative splicing of beta-galactosidase mRNA generates the classic lysosomal enzyme and a beta-galactosidase-related protein. J Biol Chem. 1989 Dec 5;264(34):20655–20663. [PubMed] [Google Scholar]
- Shotton D. M., Hartley B. S. Amino-acid sequence of porcine pancreatic elastase and its homologies with other serine proteinases. Nature. 1970 Feb 28;225(5235):802–806. doi: 10.1038/225802a0. [DOI] [PubMed] [Google Scholar]
- Sinha S., Watorek W., Karr S., Giles J., Bode W., Travis J. Primary structure of human neutrophil elastase. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2228–2232. doi: 10.1073/pnas.84.8.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G., Shinnick T. M., Green N., Liu F. T., Niman H. L., Lerner R. A. Chemical synthesis of a polypeptide predicted from nucleotide sequence allows detection of a new retroviral gene product. Nature. 1980 Oct 30;287(5785):801–805. doi: 10.1038/287801a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrenn D. S., Hinek A., Mecham R. P. Kinetics of receptor-mediated binding of tropoelastin to ligament fibroblasts. J Biol Chem. 1988 Feb 15;263(5):2280–2284. [PubMed] [Google Scholar]