Abstract
Heme oxygenase-1 (HO-1) system catabolizes heme into three products: carbon monoxide, biliverdin/bilirubin and free iron. It is involved in many physiological and pathophysiological processes. A great deal of data has demonstrated the roles of HO-1 in the formation, growth and metastasis of tumors. The interest in this system by investigators involved in gastrointestinal tumors is fairly recent, and few papers on HO-1 have touched upon this subject. This review focuses on the current understanding of the physiological significance of HO-1 induction and its possible roles in the gastrointestinal tumors studied to date. The implications for possible therapeutic manipulation of HO-1 in gastrointestinal tumors are also discussed.
Keywords: Heme oxygenase-1, Gastrointestinal tumors
INTRODUCTION
Heme oxygenase (HO) plays an important role in regulating the intracellular heme level by cleaving heme into carbon monoxide (CO), biliverdin and free iron[1]. Three HO isoforms have been identified to date: HO-1, HO-2, and HO-3, among which the isoforms 1 and 2 are the best known. HO-2 is constitutively and most highly expressed in neuronal tissues contributing to cell homeostasis, whereas HO-1, also referred to as heat shock protein-32 (Hsp32), is an inducible enzyme and relatively lowly expressed in most tissues[2]. HO-3, which is described in the rat brain, has no activity and is not expressed in humans[3].
HO-1 and HO-2 are different gene products. Unlike the constitutively expressed HO-2, HO-1 is exquisitely sensitive, not only to heavy metals[4], but to all kinds of stimuli and agents that cause oxidative stress and pathological conditions, including ischemia[5], hemorrhagic shock[6], heat shock[7], hypoxia[8], UVA radiation[9], reactive oxygen species (ROS)[10]. In fact, there is no other enzyme to date that is affected by so many stimuli of diverse nature as HO-1[2].
HO-1 is involved in many pathophysiological processes, ranging from Alzheimer's disease to cancer. The interest in this system by investigators involved in gastrointestinal tumors is fairly recent, and few reports on HO-1 have focused on this subject.
HO-DERIVANTS AND THEIR EFFECTS
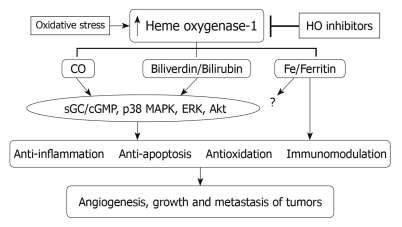
All products of HO activity are now suspected to be biologically active, in which metabolic pathway is involved in a wide variety of physiological and pathophysiological processes[11]. Almost all CO produced in vivo comes from the degradation of heme by HO. Depending on the cell type, CO can activate one or both key signaling pathways in numerous physiological and pathophysiological conditions (Figure 1). One of the pathways is soluble guanylate cyclase (sGC)/cyclic guanosine monophosphate (cGMP), which has been implicated in mediating the effects of CO on vascular contractility, the inhibition of smooth muscle proliferation, neurotransmission[12,13] and preventing apoptosis in endothelial cells[14] and fibroblasts[15]. Another one is p38 mitogen-activated protein kinase (MAPK) pathway, through which CO can mediate the anti-inflammatory actions in a large measure[16-18]. Moreover, Chin et al[19] recently pointed out that CO has played an additional novel role as a host defense molecule agent against microbes (bactericidal agent).
Figure 1.
Schematic demonstration of mechanism underlying the biological actions of HO-1 pathway in tumors. HO-1: Heme oxygenase-1; CO: Carbon monoxide; sGC/cGMP: Soluble guanylate cyclase/cyclic guanosine monophosphate; MAPK: Mitogen-activated protein kinase.
HO-1 catalyzes the rate-limiting step in heme degradation to biliverdin. Biliverdin is, in turn, converted into bilirubin by biliverdin reductase at the expense of NADPH. Biliverdin and bilirubin are potent antioxidants[20,21]. Several studies have demonstrated that the administration of biliverdin and/or bilirubin is potently cytoprotective in a variety of pathophysiological events, including ischemia-reperfusion injury, transplant rejection and inflammatory bowel disease[22-25]. In addition, bilirubin is also known to modulate immune effector functions and suppress inflammatory response[26].
Fe2+, which is also a product of heme degradation, upregulates an iron-transporter pump that removes intracellular Fe2+ from the cell[27] and induces the expression of ferritin, a iron binding protein[28]. Expression of ferritin is originally reported to protect endothelial cell against oxidant damage in vitro[28]. In addition, over-expression of H-ferritin (heavy chain ferritin) has also been shown to protect cultured endothelial cells from undergoing apoptosis and protect livers from transplant-associated ischemia-reperfusion injury[29]. Although the roles of the iron and ferritin in the overall cytoprotective effect of HO-1 are not clear, presumably both contribute in a crucial manner to the overall anti-oxidant effect following increased HO-1 expression in a variety of situations[30].
EXPRESSION OF HO-1 IN GASTROINTESTINAL TUMORS
Expression of HO-1 is usually increased in tumors, compared with surrounding healthy tissues, which was shown in oral squamous cell carcinoma[31], pancreatic cancer[32] and hepatoma[33]. There have been few published reports in gastrointestinal tumors and only one paper to date has investigated HO-1 expression in gastrointestinal tumors. The study investigates the expression of HO-1 in human colon tumor. Focal HO-1 expression in colorectal cancer and colonic adenoma was found to be 41.8% (23/55) and 36.8% (7/19), respectively[34]. Of importance, HO-1 in gastrointestinal tumors can be further upregulated in response to chemotherapy[35] and photodynamic therapy[36].
HO-1 PROMOTER POLYMORPHISM
HO-1 is known as an oxidative stress responsive protein that is upregulated by multiple stimuli, which has been proposed to provide an important cellular response that protects cells against oxidative damage. However, humans differ quantitatively in their ability to mount an HO-1 response. The association between the HO-1 genotype and various gastrointestinal tumors has been studied. Lo et al[37] examined the correlation between the HO-1 gene promoter polymorphism and the clinicopathological characteristics, along with the risk of gastric cancer. Their findings suggest that the long (GT)n repeat of HO-1 gene promoter was associated with a higher frequency of gastric adenocarcinoma, and the medium (GT)n repeat might possess a protective effect against gastric adenocarcinoma with a lower frequency of lymphovascular invasion in tumors. Additionally, the (GT)n-repeat promoter polymorphism was investigated in gastrointestinal stromal tumors (GIST) by Vashist et al[38]. They found that Short GTn allele (SGTn) was significantly associated with metastasis, higher tumor recurrence rates and high-risk GIST. Furthermore, SGTn allele carriers had significantly shorter disease-free and overall survival[38]. Moreover, a higher promoter activity genotype of the HO-1 gene was associated with increased risk in women with gastric cancer[39]. In gastrointestinal tumors, a potential impact of the (GT)n repeat polymorphism has been demonstrated, which might be considered as a potential prognostic marker to allocate patients to different risk groups and customize therapy and follow-up.
HO-1 AND CYTOPROTECTION AND APOPTOSIS
The gastrointestinal tract is lined by a simple epithelium that undergoes constant renewal involving cell division, differentiation and cell death, in which HO-1 plays a major role in the regulation of the cell cycle/apoptosis, oxidative stress, inflammation, development of colon cancer[40]. Many studies have convincingly shown that HO-1 is a cytoprotective and antiapoptotic enzyme in gastrointestinal tumor cells exposed to diverse stimuli (Figure 1). It has been demonstrated that HO-1 is involved in the pathogenesis of human gastric cancer. Antiapoptotic effects of HO-1 in gastric cancer cells are independent of p53 status in a p38 MAPK- and ERK-mediated pathway with elevated caspase inhibitory protein-2 (c-IAP2) and decreased caspase-3 activity[41], in which nuclear factor-κB is implicated. The pathway of HO-1 was also investigated in HT29 human colon cancer cells by Park et al[42]. Another study also demonstrated that HO-1 induction resulted in resistance to apoptosis in a human colon cancer cell line, Caco-2, whose effects were independent of p38, but were mediated via Akt pathway[43]. In a similar study, Kim et al[44] reported that administration of Zerumbone (ZER) effectively suppressed mouse colon carcinogenesis through multiple modulation of growth, apoptosis and inflammation. Ohyama et al[45] examined the cytotoxicity of a crude extract from Vitex agnus-castus fruits (Vitex extract) in gastric signet ring carcinoma (KATO-III) cells. They found that cell apoptosis may be attributed to the inhibition of HO-1. It can be supposed that cytoprotective action of HO-1 can be mediated by the following factors: (a) decreased intracellular pro-oxidant levels; (b) increased bilirubin levels; and (c) elevated CO production[46]. On the contrary, flavonoids- (Vitex extract) induced apoptosis is caused through the induction of HO-1 in human colon carcinoma cell line, COLO 201[35]. The relationship between HO-1 and apoptosis remains to be clarified.
HO-1 AND TUMOR GROWTH AND METASTASIS
Apart from the cytoprotective action, HO-1 is commonly regarded as a potent proangiogenic enzyme. Angiogenesis is critical not only for tumor growth but also for metastasis. Thus, proangiogenic action of HO-1 may further support tumor progression[47]. Bussolati et al[48] reported that vascular endothelial growth factor (VEGF) induced prolonged HO-1 expression and activity in human endothelial cells and HO-1 inhibition abrogated VEGF-driven angiogenesis. Overexpression of HO-1 in pancreatic cancer cells[49] and melanoma cells[50] increased the occurrence of metastasis, while inhibition of HO activity completely inhibited the occurrence of metastasis[49]. In contrast, some authors have demonstrated that inhibition of the HO pathway by zinc deuteroporphyrin 2,4-bis glycol (ZnDPBG) in colon carcinoma had no effect on metastasis to the lung and even increases metastasis to the liver[51]. Furthermore, the rate of lymphatic tumor invasion was significantly lower in colorectal cancer samples expressing HO-1[34]. Thus, the mechanism of HO-1 in the metastatic potential of cancer cells is not recognized and it may depend on the type of cancer or other still not defined factors.
HO-1 AS A POTENTIAL THERAPEUTIC TARGET
Studies of the role of HO-1 seem to be important not only for better understanding of tumor growth regulation but also for clinical practice. HO-1 is often upregulated in gastrointestinal tumors[34], its expression is further increased in response to the different types of therapies such as photodynamic therapy[52,53] and pyrrolidine dithiocarbamate[54]. Since HO-1 may protect tumor cells against oxidative stress and can be regarded as an enzyme facilitating tumor progression, administration of HO-1 inhibitors might be effective for the treatment of gastrointestinal tumors. It has been demonstrated that pegylated zinc protoporphyrin, a potent HO inhibitor, administered in vitro induced apoptosis of human colon carcinoma SW480 cells and inhibited growth of murine colon carcinoma in vivo[55]. However, the growth, invasion and metastasis of tumors are a highly complex and multistep process, the mechanisms responsible for HO-1 in gastrointestinal tumors remain to be elucidated.
CONCLUSION
HO-1 system may play an important role in different pathophysiological conditions, in which pharmacologic modulation of HO-1 system may represent an effective and cooperative strategy to facilitate tumor growth and metastasis, although the exact effects can depend on the type of disease. Therefore, down-regulating the HO-1 system by pharmacological or genetic means will be a new therapeutic approach in the management of gastrointestinal tumors. A comprehensive understanding of the underlying mechanisms for the observed effects of HO-1 and its products will be necessary before their use can be evaluated in clinical applications for the prevention and/or treatment of human diseases such as gastrointestinal tumors.
Footnotes
Peer reviewers: Toru Hiyama, MD, PhD, Health Service Center, Hiroshima University, 1-7-1 Kagamiyama, Higashihiroshima 739-8521, Japan; Yasuhiro Matsumura, MD, PhD, National Cancer Center Research Institute East, 6-5-1 Kashiwanoha, Kashiwa, Chiba 277-8577, Japan
S- Editor Tian L L- Editor Ma JY E- Editor Lin YP
References
- 1.Maines MD. The heme oxygenase system: past, present, and future. Antioxid Redox Signal. 2004;6:797–801. doi: 10.1089/ars.2004.6.797. [DOI] [PubMed] [Google Scholar]
- 2.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 3.McCoubrey WK Jr, Huang TJ, Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem. 1997;247:725–732. doi: 10.1111/j.1432-1033.1997.00725.x. [DOI] [PubMed] [Google Scholar]
- 4.Miura N, Shinohara Y. Cytotoxic effect and apoptosis induction by silver nanoparticles in HeLa cells. Biochem Biophys Res Commun. 2009;390:733–737. doi: 10.1016/j.bbrc.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 5.Xue H, Guo H, Li YC, Hao ZM. Heme oxygenase-1 induction by hemin protects liver cells from ischemia/reperfusion injury in cirrhotic rats. World J Gastroenterol. 2007;13:5384–5390. doi: 10.3748/wjg.v13.i40.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umeda K, Takahashi T, Inoue K, Shimizu H, Maeda S, Morimatsu H, Omori E, Akagi R, Katayama H, Morita K. Prevention of hemorrhagic shock-induced intestinal tissue injury by glutamine via heme oxygenase-1 induction. Shock. 2009;31:40–49. doi: 10.1097/SHK.0b013e318177823a. [DOI] [PubMed] [Google Scholar]
- 7.Tsuji T, Kato A, Yasuda H, Miyaji T, Luo J, Sakao Y, Ito H, Fujigaki Y, Hishida A. The dimethylthiourea-induced attenuation of cisplatin nephrotoxicity is associated with the augmented induction of heat shock proteins. Toxicol Appl Pharmacol. 2009;234:202–208. doi: 10.1016/j.taap.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 8.Chang AY, Chan JY, Cheng HL, Tsai CY, Chan SH. Hypoxia-inducible factor 1/heme oxygenase 1 cascade as upstream signals in the prolife role of heat shock protein 70 at rostral ventrolateral medulla during experimental brain stem death. Shock. 2009;32:651–658. doi: 10.1097/SHK.0b013e3181a71027. [DOI] [PubMed] [Google Scholar]
- 9.Reeve VE, Allanson M, Cho JL, Arun SJ, Domanski D. Interdependence between heme oxygenase-1 induction and estrogen-receptor-beta signaling mediates photoimmune protection by UVA radiation in mice. J Invest Dermatol. 2009;129:2702–2710. doi: 10.1038/jid.2009.121. [DOI] [PubMed] [Google Scholar]
- 10.Schulz-Geske S, Erdmann K, Wong RJ, Stevenson DK, Schröder H, Grosser N. Molecular mechanism and functional consequences of lansoprazole-mediated heme oxygenase-1 induction. World J Gastroenterol. 2009;15:4392–4401. doi: 10.3748/wjg.15.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 12.Duckers HJ, Boehm M, True AL, Yet SF, San H, Park JL, Clinton Webb R, Lee ME, Nabel GJ, Nabel EG. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med. 2001;7:693–698. doi: 10.1038/89068. [DOI] [PubMed] [Google Scholar]
- 13.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 14.Brouard S, Berberat PO, Tobiasch E, Seldon MP, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-kappa B to protect endothelial cells from tumor necrosis factor-alpha-mediated apoptosis. J Biol Chem. 2002;277:17950–17961. doi: 10.1074/jbc.M108317200. [DOI] [PubMed] [Google Scholar]
- 15.Petrache I, Otterbein LE, Alam J, Wiegand GW, Choi AM. Heme oxygenase-1 inhibits TNF-alpha-induced apoptosis in cultured fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2000;278:L312–L319. doi: 10.1152/ajplung.2000.278.2.L312. [DOI] [PubMed] [Google Scholar]
- 16.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 17.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 18.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chin BY, Otterbein LE. Carbon monoxide is a poison... to microbes! CO as a bactericidal molecule. Curr Opin Pharmacol. 2009;9:490–500. doi: 10.1016/j.coph.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 21.Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic Biol Med. 2005;39:1–25. doi: 10.1016/j.freeradbiomed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Gruttadauria S, di Francesco F, Vizzini GB, Luca A, Spada M, Cintorino D, Li Petri S, Pietrosi G, Pagano D, Gridelli B. Early graft dysfunction following adult-to-adult living-related liver transplantation: predictive factors and outcomes. World J Gastroenterol. 2009;15:4556–4560. doi: 10.3748/wjg.15.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fondevila C, Shen XD, Tsuchiyashi S, Yamashita K, Csizmadia E, Lassman C, Busuttil RW, Kupiec-Weglinski JW, Bach FH. Biliverdin therapy protects rat livers from ischemia and reperfusion injury. Hepatology. 2004;40:1333–1341. doi: 10.1002/hep.20480. [DOI] [PubMed] [Google Scholar]
- 24.Nakao A, Otterbein LE, Overhaus M, Sarady JK, Tsung A, Kimizuka K, Nalesnik MA, Kaizu T, Uchiyama T, Liu F, et al. Biliverdin protects the functional integrity of a transplanted syngeneic small bowel. Gastroenterology. 2004;127:595–606. doi: 10.1053/j.gastro.2004.05.059. [DOI] [PubMed] [Google Scholar]
- 25.Zhang M, Li B, Yan LN, Yin F, Wen TF, Zeng Y, Zhao JC, Ma YK. Development of a survival evaluation model for liver transplant recipients with hepatocellular carcinoma secondary to hepatitis B. World J Gastroenterol. 2008;14:1280–1285. doi: 10.3748/wjg.14.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willis D, Moore AR, Frederick R, Willoughby DA. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat Med. 1996;2:87–90. doi: 10.1038/nm0196-87. [DOI] [PubMed] [Google Scholar]
- 27.Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, Tysoe SA, Wolosker H, Barañano DE, Doré S, et al. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol. 1999;1:152–157. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- 28.Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992;267:18148–18153. [PubMed] [Google Scholar]
- 29.Berberat PO, Katori M, Kaczmarek E, Anselmo D, Lassman C, Ke B, Shen X, Busuttil RW, Yamashita K, Csizmadia E, et al. Heavy chain ferritin acts as an antiapoptotic gene that protects livers from ischemia reperfusion injury. FASEB J. 2003;17:1724–1726. doi: 10.1096/fj.03-0229fje. [DOI] [PubMed] [Google Scholar]
- 30.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 31.Lee J, Lee SK, Lee BU, Lee HJ, Cho NP, Yoon JH, Choi HR, Lee SK, Kim EC. Upregulation of heme oxygenase-1 in oral epithelial dysplasias. Int J Oral Maxillofac Surg. 2008;37:287–292. doi: 10.1016/j.ijom.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 32.Nuhn P, Künzli BM, Hennig R, Mitkus T, Ramanauskas T, Nobiling R, Meuer SC, Friess H, Berberat PO. Heme oxygenase-1 and its metabolites affect pancreatic tumor growth in vivo. Mol Cancer. 2009;8:37. doi: 10.1186/1476-4598-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sass G, Leukel P, Schmitz V, Raskopf E, Ocker M, Neureiter D, Meissnitzer M, Tasika E, Tannapfel A, Tiegs G. Inhibition of heme oxygenase 1 expression by small interfering RNA decreases orthotopic tumor growth in livers of mice. Int J Cancer. 2008;123:1269–1277. doi: 10.1002/ijc.23695. [DOI] [PubMed] [Google Scholar]
- 34.Becker JC, Fukui H, Imai Y, Sekikawa A, Kimura T, Yamagishi H, Yoshitake N, Pohle T, Domschke W, Fujimori T. Colonic expression of heme oxygenase-1 is associated with a better long-term survival in patients with colorectal cancer. Scand J Gastroenterol. 2007;42:852–858. doi: 10.1080/00365520701192383. [DOI] [PubMed] [Google Scholar]
- 35.Imai M, Kikuchi H, Denda T, Ohyama K, Hirobe C, Toyoda H. Cytotoxic effects of flavonoids against a human colon cancer derived cell line, COLO 201: a potential natural anti-cancer substance. Cancer Lett. 2009;276:74–80. doi: 10.1016/j.canlet.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 36.Kocanova S, Buytaert E, Matroule JY, Piette J, Golab J, de Witte P, Agostinis P. Induction of heme-oxygenase 1 requires the p38MAPK and PI3K pathways and suppresses apoptotic cell death following hypericin-mediated photodynamic therapy. Apoptosis. 2007;12:731–741. doi: 10.1007/s10495-006-0016-x. [DOI] [PubMed] [Google Scholar]
- 37.Lo SS, Lin SC, Wu CW, Chen JH, Yeh WI, Chung MY, Lui WY. Heme oxygenase-1 gene promoter polymorphism is associated with risk of gastric adenocarcinoma and lymphovascular tumor invasion. Ann Surg Oncol. 2007;14:2250–2256. doi: 10.1245/s10434-006-9290-7. [DOI] [PubMed] [Google Scholar]
- 38.Vashist YK, Uzunoglu G, Cataldegirmen G, Kalinin V, Schurr P, Koenig AM, Thieltges S, Zehler O, Schneider C, Izbicki JR, et al. Haeme oxygenase-1 promoter polymorphism is an independent prognostic marker of gastrointestinal stromal tumour. Histopathology. 2009;54:303–308. doi: 10.1111/j.1365-2559.2009.03221.x. [DOI] [PubMed] [Google Scholar]
- 39.Sawa T, Mounawar M, Tatemichi M, Gilibert I, Katoh T, Ohshima H. Increased risk of gastric cancer in Japanese subjects is associated with microsatellite polymorphisms in the heme oxygenase-1 and the inducible nitric oxide synthase gene promoters. Cancer Lett. 2008;269:78–84. doi: 10.1016/j.canlet.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 40.Oates PS, West AR. Heme in intestinal epithelial cell turnover, differentiation, detoxification, inflammation, carcinogenesis, absorption and motility. World J Gastroenterol. 2006;12:4281–4295. doi: 10.3748/wjg.v12.i27.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu ZM, Chen GG, Ng EK, Leung WK, Sung JJ, Chung SC. Upregulation of heme oxygenase-1 and p21 confers resistance to apoptosis in human gastric cancer cells. Oncogene. 2004;23:503–513. doi: 10.1038/sj.onc.1207173. [DOI] [PubMed] [Google Scholar]
- 42.Park EJ, Lim JH, Nam SI, Park JW, Kwon TK. Rottlerin induces heme oxygenase-1 (HO-1) up-regulation through reactive oxygen species (ROS) dependent and PKC delta-independent pathway in human colon cancer HT29 cells. Biochimie. 2010;92:110–115. doi: 10.1016/j.biochi.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Busserolles J, Megías J, Terencio MC, Alcaraz MJ. Heme oxygenase-1 inhibits apoptosis in Caco-2 cells via activation of Akt pathway. Int J Biochem Cell Biol. 2006;38:1510–1517. doi: 10.1016/j.biocel.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Kim M, Miyamoto S, Yasui Y, Oyama T, Murakami A, Tanaka T. Zerumbone, a tropical ginger sesquiterpene, inhibits colon and lung carcinogenesis in mice. Int J Cancer. 2009;124:264–271. doi: 10.1002/ijc.23923. [DOI] [PubMed] [Google Scholar]
- 45.Ohyama K, Akaike T, Hirobe C, Yamakawa T. Cytotoxicity and apoptotic inducibility of Vitex agnus-castus fruit extract in cultured human normal and cancer cells and effect on growth. Biol Pharm Bull. 2003;26:10–18. doi: 10.1248/bpb.26.10. [DOI] [PubMed] [Google Scholar]
- 46.Fang J, Akaike T, Maeda H. Antiapoptotic role of heme oxygenase (HO) and the potential of HO as a target in anticancer treatment. Apoptosis. 2004;9:27–35. doi: 10.1023/B:APPT.0000012119.83734.4e. [DOI] [PubMed] [Google Scholar]
- 47.Jozkowicz A, Was H, Dulak J. Heme oxygenase-1 in tumors: is it a false friend? Antioxid Redox Signal. 2007;9:2099–2117. doi: 10.1089/ars.2007.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bussolati B, Ahmed A, Pemberton H, Landis RC, Di Carlo F, Haskard DO, Mason JC. Bifunctional role for VEGF-induced heme oxygenase-1 in vivo: induction of angiogenesis and inhibition of leukocytic infiltration. Blood. 2004;103:761–766. doi: 10.1182/blood-2003-06-1974. [DOI] [PubMed] [Google Scholar]
- 49.Sunamura M, Duda DG, Ghattas MH, Lozonschi L, Motoi F, Yamauchi J, Matsuno S, Shibahara S, Abraham NG. Heme oxygenase-1 accelerates tumor angiogenesis of human pancreatic cancer. Angiogenesis. 2003;6:15–24. doi: 10.1023/a:1025803600840. [DOI] [PubMed] [Google Scholar]
- 50.Was H, Cichon T, Smolarczyk R, Rudnicka D, Stopa M, Chevalier C, Leger JJ, Lackowska B, Grochot A, Bojkowska K, et al. Overexpression of heme oxygenase-1 in murine melanoma: increased proliferation and viability of tumor cells, decreased survival of mice. Am J Pathol. 2006;169:2181–2198. doi: 10.2353/ajpath.2006.051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishikawa T, Yoshida N, Higashihara H, Inoue M, Uchiyama K, Takagi T, Handa O, Kokura S, Naito Y, Okanoue T, et al. Different effects of constitutive nitric oxide synthase and heme oxygenase on pulmonary or liver metastasis of colon cancer in mice. Clin Exp Metastasis. 2003;20:445–450. doi: 10.1023/a:1025448403124. [DOI] [PubMed] [Google Scholar]
- 52.Nowis D, Legat M, Grzela T, Niderla J, Wilczek E, Wilczynski GM, Głodkowska E, Mrówka P, Issat T, Dulak J, et al. Heme oxygenase-1 protects tumor cells against photodynamic therapy-mediated cytotoxicity. Oncogene. 2006;25:3365–3374. doi: 10.1038/sj.onc.1209378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jalili A, Makowski M, Switaj T, Nowis D, Wilczynski GM, Wilczek E, Chorazy-Massalska M, Radzikowska A, Maslinski W, Biały L, et al. Effective photoimmunotherapy of murine colon carcinoma induced by the combination of photodynamic therapy and dendritic cells. Clin Cancer Res. 2004;10:4498–4508. doi: 10.1158/1078-0432.CCR-04-0367. [DOI] [PubMed] [Google Scholar]
- 54.Hellmuth M, Wetzler C, Nold M, Chang JH, Frank S, Pfeilschifter J, Mühl H. Expression of interleukin-8, heme oxygenase-1 and vascular endothelial growth factor in DLD-1 colon carcinoma cells exposed to pyrrolidine dithiocarbamate. Carcinogenesis. 2002;23:1273–1279. doi: 10.1093/carcin/23.8.1273. [DOI] [PubMed] [Google Scholar]
- 55.Fang J, Sawa T, Akaike T, Greish K, Maeda H. Enhancement of chemotherapeutic response of tumor cells by a heme oxygenase inhibitor, pegylated zinc protoporphyrin. Int J Cancer. 2004;109:1–8. doi: 10.1002/ijc.11644. [DOI] [PubMed] [Google Scholar]