Abstract
Inflammatory myofibroblastic tumor (IMT) is an uncommon benign neoplasm with locally aggressive behavior but malignant change is rare. We report an unusual case of pelvic-abdominal inflammatory myofibroblastic tumor with malignant transformation in a 14-year-old boy presenting with abdominal pain and 9 kg body weight loss in one month. Computed tomography revealed a huge pelvi-abdominal mass (30 cm), possibly originating from the pelvic extraperitoneal space, protruding into the abdomen leading to upward displacement of the bowel loops, downward displacement of the urinary bladder, massive central necrosis, a well-enhanced peripheral solid component with prominent peritumoral vascularity. Subsequent examination confirmed the computed tomographic findings. Histopathologic examination revealed proliferative epitheloid and spindle cells, inflammatory cell infiltration and high mitotic counts. Immunohistochemistry was strongly positive for anaplastic lymphoma kinase and revealed a high proliferative index (ki-67 = 40%). DNA sequencing and electronic microscopy further confirmed the primitive fibroblastic cell phenotype of the tumor and a final diagnosis of inflammatory myofibroblastic tumor with malignant transformation was established. Rapid tumor recurrence was noted 20 d after radical tumor resection. To our knowledge, this is the largest documented case of IMT in a pediatric patient and the first report of IMT with malignant transformation originating from the pelvic extraperitoneal space.
Keywords: Inflammatory myofibroblastic tumor, Malignant transformation, Pediatric patient, Pelvis, Extraperitoneal space, Computed tomography
INTRODUCTION
Inflammatory myofibroblastic tumor (IMT) is an uncommon benign neoplasm with locally aggressive behavior and a tendency to recur[1-10]. An IMT is commonly found in the lung as an asymptomatic solitary lung mass detected on chest radiography[1-3]. Extrapulmonary IMT can occur in nearly every site in the body but is most commonly found in the mesentery and omentum, orbit, and gastrointestinal and genitourinary tracts[1-4]. IMT of the retroperitoneum is unusual[3-6]. On rare occasions, IMT may exhibit malignant transformation or may evolve into lymphoreticular malignancy[2,3,7]. Herein, we describe an extremely unusual case of IMT with malignant transformation originating from the pelvic extraperitoneal space in a pediatric patient.
CASE REPORT
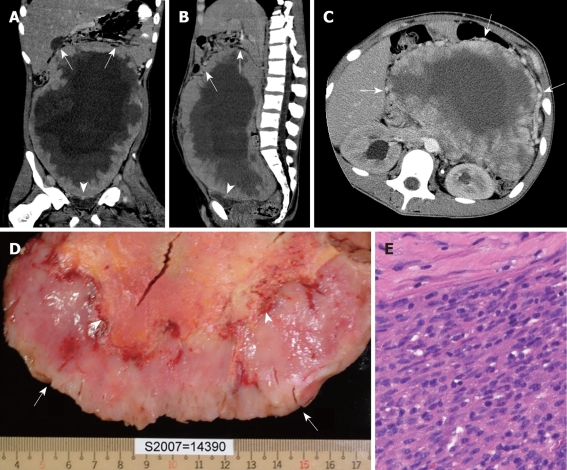
A previously healthy 14-year-old boy presented with abdominal pain and fullness with body weight loss of 9 kg in one month. A physical examination revealed slightly pale conjunctiva, a 23 cm × 33 cm fixed pelvi-abdominal mass and engorged superficial veins on the abdominal wall. Laboratory investigations showed that hemoglobin was 11.8 g/dL and the leukocyte count was 13.3 × 109/L with eosinophilia (eosinophil count 17.8%), and an elevated C-reactive protein level (77.7 mg/L). Computed tomography (CT) showed a huge pelvi-abdominal mass (30 cm), possibly originating from the pelvic extraperitoneal space, protruding into the abdomen with upward displacement of the bowel loops, downward displacement of the urinary bladder, massive central necrosis, and a well-enhanced peripheral solid component with prominent peritumoral vascularity (Figure 1A-C). Based on such CT features, the surgeons were notified for cautious surgical dissection of the extraperitoneal space as well as the possibility of massive bleeding and the need for blood transfusions during the operation. The initial impression included mesenteric fibromatosis, IMT, gastro-intestinal stromal tumor or mesenteric sarcoma. During surgery, CT findings were confirmed and prominent peritumoral vascularity was encountered resulting in a blood loss of 3900 mL during radical tumor resection and necessitated transfusion of packed red blood cells (6 units) and fresh-frozen plasma (4 units). The gross specimen was a slightly lobulated mass with an uneven peripheral solid component and massive central necrosis (Figure 1D). Histopathologic examination revealed predominantly epitheloid and spindle cells, prominent staghorn vascular channels, inflammatory cell infiltration and high mitotic counts (14/10-high-power-field) (Figure 1E). Immunohistochemistry was strongly positive for anaplastic lymphoma kinase (ALK) and revealed a high proliferative index (ki-67 = 40%) but was negative for desmin, HHF-35, Fli-1, CD31, CD34, CD117, S-100, chromogranin A, synaptophysin, and P-53. Real-time polymerization chain reaction assay revealed strong expression of TPM3-ALK fusion transcript and DNA sequencing revealed ALK fusion gene breakpoint at TPM3 Exon 8 to ALK Exon 21. Electron microscopy of the spindle cells revealed lack of subplasmalemal thin actin filament and microtendon in fibronexa on the cell surface confirming primitive fibroblastic cell phenotype. A final diagnosis of IMT with malignant transformation was established. The patient recovered uneventfully and was discharged 10 d after surgery. Unfortunately, a recurrent palpable mass was noted over the lower abdomen 20 d after discharge. He refused further treatment and died 6 mo later.
Figure 1.
Malignant inflammatory myofibroblastic tumor in a 14-year-old with a history of abdominal pain and body weight loss. A, B: Coronal (A) and sagittal (B) computed tomography (CT) images reveal a huge pelvi-abdominal mass with an uneven peripheral solid component and massive central necrosis, with upward displacement of the bowel loops (arrows) and downward compression on the urinary bladder (arrowheads), highly suggestive of an extraperitoneal tumor originating from the perivesical space; C: Axial CT image at the level of the kidney clearly demonstrates prominent vessels around the tumor (arrows); D: The cut-surface of the gross specimen reveals an uneven peripheral solid component (arrows) and massive central necrosis (arrowheads); E: Photomicrograph shows proliferation of spindle tumor cells in the peripheral solid part (HE stain, original magnification × 200).
DISCUSSION
IMT has been reported as a quasineoplastic lesion histopathologically characterized by proliferation of myofibroblastic spindle cells and inflammatory cells. Most cases of IMT are found in the lung, orbit, mesentery and omentum, and gastrointestinal and genitourinary tracts[1-4]. IMT arising from uncommon locations including the liver, spleen, head and neck, central nervous system, heart, appendix and superficial soft tissue has also been documented[2-8]. Excluding urinary tract lesions, retroperitoneal IMT is unusual and only 10 cases have been described[3-6]. To our knowledge, this is the first reported case of IMT in the pelvic extraperitoneal perivesical space.
The etiology of IMT is contentious and whether it is a post-inflammatory process or a true neoplasm remains controversial[2-8]. Proponents for post-inflammatory or immunologic processes advocate the association of IMT and infectious agents such as mycobacteria, corynebacteria, the Epstein-Barr virus and human herpes virus, as well prior histories of surgery, trauma, and steroid usage[2-5]. In contrast, proponents for neoplasm emphasize the aggressive behavior, cytogenetic clonality, involvement of specific chromosome region 2p23 and the possibility of distant metastasis[5-8]. In this particular case, there was no definite evidence of related infection, a traumatic episode or prior steroid usage. On the other hand, histopathologic and immunohistochemical features such as high mitotic counts, positive ALK and a high proliferative index as well as the rapid recurrence after initial radical resection were supportive of the neoplastic nature of IMT.
The sizes of reported IMT ranged from 0.4 cm to 40 cm[1-10]. An intraabominal IMT is usually larger and the largest reported IMT (40 cm) occurred in the liver of a 29 year-old man[9]. Our patient had the largest reported IMT among pediatric patients. The clinical presentation varies depending on the anatomical location of the lesion[1-10]. As demonstrated in our patient, patients with abdominal IMT usually present with abdominal pain, palpable mass, and body weight loss. Occasionally, features of inflammation such as fever, an elevated erythrocyte sedimentation rate and a markedly elevated leukocyte count (> 80 × 109/L) are encountered[10].
The CT appearance of abdominal IMT is variable. The mass may be iso- or hypodense to muscle on unenhanced scans with variable enhancement patterns including homogeneous, heterogeneous, peripheral and delayed central filling patterns[1,4-10]. As seen in our patient, central necrosis may occur in a large abdominal IMT and the differential diagnosis of a large abdominal mass with central necrosis encompasses giant neurilemmoma, gastrointestinal stromal tumor and fibromatosis[11-13]. A giant abdominal neurogenic tumor in a patient without von Recklinghausen’s disease was unusual and the lack of direct involvement of the gastrointestinal tract rendered gastrointestinal stromal tumor less likely[11,12]. CT demonstration of prominent peritumoral vascularity was suggestive of prominent regional inflammatory changes, supporting the diagnosis of IMT rather than fibromatosis[13]. In this particular case, coronal and sagittal reconstructed CT images clearly depicted the mass effect with upward displacement of the bowel loops and downward displacement of the urinary bladder, implying that the tumor might be extraperitoneally located. This information was helpful for preoperative planning, advocating cautious dissection of the extraperioneal spaces during surgery. In addition, surgeons were forewarned of the possibility of massive bleeding during tumor resection and the necessity for blood transfusion. Although CT may offer suggestive features of this unusual tumor, the definite diagnosis relies on detailed histopathologic and immunohistochemical evaluations[2-10]. ALK is an oncoprotein which essentially serves as a surrogate marker of chromosome rearrangement of 2p23 in IMT. Real-time polymerization chain reaction assay revealed strong expression of TPM3-ALK fusion transcript in our patient and in addition, DNA sequencing revealed ALK fusion gene breakpoint at TPM3 Exon 8 to ALK Exon 21 allowing further confirmation of IMT[14]. Chemotherapy, radiotherapy, and even immunomodulation have not been reported consistently effective against this aggressive tumor[7,8]. Radical resection remains the treatment of choice for patients with IMT and is adequate in most circumscribed tumors. However, mesenteric or retroperitoneal origin of the tumor, large tumor (> 8 cm), multinodular or ill-defined tumor morphology and incomplete tumor resection of IMT are factors associated with higher recurrence rates (17%-37%)[2-6,10]. In this particular patient, despite radical tumor resection being accomplished, rapid tumor recurrence was noted in 20 d and fatality in 6 mo, further confirming the highly malignant nature of the mass. In retrospect, the presence of abundant peritumoral vascularity demonstrated on CT was suggestive of high metabolic demand of the tumor which might be a clue to potential malignant change.
In summary, this report documents the first known case of pelvic extraperitoneal IMT with malignant transformation in a pediatric patient. In light of this case, IMT should be considered in the differential diagnosis of pelvi-abdominal mass with large central necrosis and the presence of prominent peritumoral vascularity may also be a clue of high metabolic demand and even malignant transformation.
Footnotes
Peer reviewer: Andrew Seng Boon Chua, MD, Department of Gastroenterology, Gastro Centre Ipoh, 1, lorong Rani, 31, lebuhraya Tmn Ipoh, Ipoh Garden South, IPOH 30350, Malaysia
S- Editor Wang YR L- Editor O'Neill M E- Editor Lin YP
References
- 1.Narla LD, Newman B, Spottswood SS, Narla S, Kolli R. Inflammatory pseudotumor. Radiographics. 2003;23:719–729. doi: 10.1148/rg.233025073. [DOI] [PubMed] [Google Scholar]
- 2.Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859–872. doi: 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Coffin CM, Humphrey PA, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor: a clinical and pathological survey. Semin Diagn Pathol. 1998;15:85–101. [PubMed] [Google Scholar]
- 4.Ramachandra S, Hollowood K, Bisceglia M, Fletcher CD. Inflammatory pseudotumour of soft tissues: a clinicopathological and immunohistochemical analysis of 18 cases. Histopathology. 1995;27:313–323. doi: 10.1111/j.1365-2559.1995.tb01521.x. [DOI] [PubMed] [Google Scholar]
- 5.Mali VP, Tan HC, Loh D, Prabhakaran K. Inflammatory tumour of the retroperitoneum--a case report. Ann Acad Med Singapore. 2005;34:632–635. [PubMed] [Google Scholar]
- 6.Attili SV, Chandra CR, Hemant DK, Bapsy PP, RamaRao C, Anupama G. Retroperitoneal inflammatory myofibroblastic tumor. World J Surg Oncol. 2005;3:66. doi: 10.1186/1477-7819-3-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dishop MK, Warner BW, Dehner LP, Kriss VM, Greenwood MF, Geil JD, Moscow JA. Successful treatment of inflammatory myofibroblastic tumor with malignant transformation by surgical resection and chemotherapy. J Pediatr Hematol Oncol. 2003;25:153–158. doi: 10.1097/00043426-200302000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet JP, Basset T, Dijoux D. Abdominal inflammatory myofibroblastic tumors in children: report of an appendiceal case and review of the literature. J Pediatr Surg. 1996;31:1311–1314. doi: 10.1016/s0022-3468(96)90262-6. [DOI] [PubMed] [Google Scholar]
- 9.Chen KT. Inflammatory pseudotumor of the liver. Hum Pathol. 1984;15:694–696. doi: 10.1016/s0046-8177(84)80298-1. [DOI] [PubMed] [Google Scholar]
- 10.Karnak I, Senocak ME, Ciftci AO, Cağlar M, Bingöl-Koloğlu M, Tanyel FC, Büyükpamukçu N. Inflammatory myofibroblastic tumor in children: diagnosis and treatment. J Pediatr Surg. 2001;36:908–912. doi: 10.1053/jpsu.2001.23970. [DOI] [PubMed] [Google Scholar]
- 11.Bastounis E, Asimacopoulos PJ, Pikoulis E, Leppäniemi AK, Aggouras D, Papakonstadinou K, Papalambros E. Benign retroperitoneal neural sheath tumors in patients without von Recklinghausen's disease. Scand J Urol Nephrol. 1997;31:129–136. doi: 10.3109/00365599709070317. [DOI] [PubMed] [Google Scholar]
- 12.Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics. 2003;23:283–304, 456; quiz 532. doi: 10.1148/rg.232025146. [DOI] [PubMed] [Google Scholar]
- 13.Ko SF, Lin JW, Ng SH, Huang CC, Wan YL, Huang HY, Sheen-Chen SM. Spontaneous isolated mesenteric fibromatosis: sonographic and computed tomographic findings with pathologic correlation. Ultrasound Med Biol. 2006;32:1141–1149. doi: 10.1016/j.ultrasmedbio.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Cook JR, Dehner LP, Collins MH, Ma Z, Morris SW, Coffin CM, Hill DA. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immunohistochemical study. Am J Surg Pathol. 2001;25:1364–1371. doi: 10.1097/00000478-200111000-00003. [DOI] [PubMed] [Google Scholar]