Abstract
Practical relevance The feline haemotropic mycoplasmas (‘haemoplasmas’) are a group of bacteria that can induce haemolytic anaemia in cats. Mycoplasma haemofelis is the most pathogenic of the species; ‘Candidatus Mycoplasma haemominutum’ and ‘Candidatus Mycoplasma turicensis’ are less pathogenic. The natural route of transmission of feline haemoplasma infection has not been confirmed, but fleas are implicated. When disease results, common clinical signs are pallor, lethargy, anorexia, weight loss, depression, dehydration and pyrexia. Treatment with tetracyclines or fluoroquinolones is usually effective at resolving clinical disease, but clearance of infection may not result.

Global importance The feline haemoplasmas are found worldwide, although prevalence varies geographically.
Patient group Older male non-pedigree cats are believed to be at increased risk of haemoplasma infection, although younger cats are possibly more likely to show clinical disease associated with M haemofelis.
Clinical challenges The significance of feline haemoplasma infection is difficult to determine due to the existence of asymptomatic carrier cats and the variable pathogenicity of the haemoplasma species. Polymerase chain reaction (PCR) results should be interpreted in the light of the patient's clinical signs and haematological findings, infecting haemoplasma species and level of haemoplasma DNA present in the blood. Trial antibiotic treatment for haemoplasmosis may be warranted in suspected cases while awaiting PCR results.
Evidence base Aspects of feline haemoplasmosis, particularly risk factors, pathogenesis, diagnostic methods and treatment, have been the focus of much recent research. This article draws on the current evidence base with a view to helping clinicians diagnose and manage cases more effectively.

What are the haemotropic mycoplasmas?
The haemotropic mycoplasmas (‘haemoplasmas’) are mycoplasmal bacteria that infect erythrocytes, attaching to the red blood cell surface (Fig 1). These species, including Haemobartonella felis, were previously classified as rickettsial organisms due to their obligate parasitism, small size, erythrocyte tropism and suspected arthropod transmission. However, recent molecular sequencing and phylogeny data have shown these species to be more closely related to the family Mycoplasmaceae, a relationship that is further supported by certain phenotypic characteristics of the haemotropic mycoplasmas such as their small size and genomes, fastidious growth requirements and lack of a cell wall. This new classification led to the renaming of Haemobartonella species to reflect their mycoplasmal origin, with H felis being renamed Mycoplasma haemofelis. Molecular analysis also led to the discovery of two new feline species: ‘Candidatus Mycoplasma haemominutum’ and ‘Candidatus Mycoplasma turicensis’. One recent study from the USA suggested that cats could be further infected with an organism similar to one of the canine haemoplasmas called ‘Candidatus Mycoplasma haematoparvum’. 1 Thus, cats can be infected with a range of haemotropic mycoplasmas. A summary of the feline haemoplasma species is provided in the box on page 370.
FIG 1.
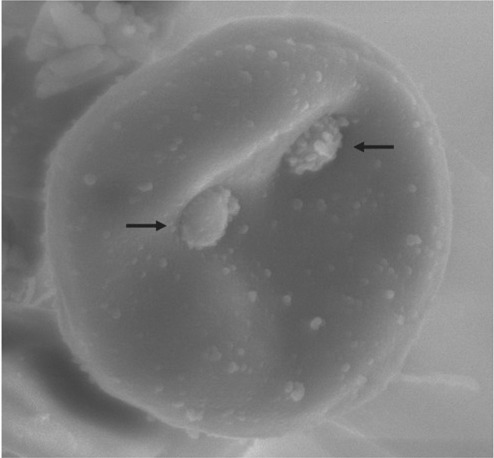
Scanning electron micrograph of a feline erythrocyte infected with Mycoplasma haemofelis. Two M haemofelis organisms (arrows) can be seen attached to the surface of the red blood cell
Feline haemoplasma species.
Mycoplasma haemofelis Often associated with haemolytic anaemia during acute infection.
‘Candidatus Mycoplasma haemominutum’ May result in a fall in red blood cell parameters, but anaemia is not normally induced unless concurrent disease is present.
‘Candidatus Mycoplasma turicensis’ May result in a fall in red blood cell parameters, but anaemia is not normally induced unless concurrent disease is present.
‘Candidatus Mycoplasma haematoparvum-like’ No data on pathogenesis is currently available.
Why Candidatus?
The assigning of a ‘Candidatus’ status to three of the feline haemoplasmas reflects their provisional classification in bacterial nomenclature. Characterisation (especially phenotypic) of these species has not been possible due to them being uncultivable in vitro. M haemofelis was not assigned this status, despite also being uncultivable in vitro, because it represents the new name for a species that previously existed (H felis), and bacterial nomenclature rules state that species cannot be assigned a ‘Candidatus’ status retrospectively.
Are the feline haemoplasmas pathogenic?
The feline haemoplasma species that are currently recognised vary in pathogenicity, with some isolates consistently inducing haemolytic anaemia whereas others result in few noticeable clinical signs (Fig 2). However, it is not only the infecting species or isolate that influences clinical signs — duration of infection and host factors, such as the presence of concurrent disease or retrovirus status, can also play a part. Age, too, may be important in that it has been suggested that younger cats are more susceptible to clinical haemoplasmosis.2,3 Other factors such as infecting organism dose or route of infection may also impact on outcome.
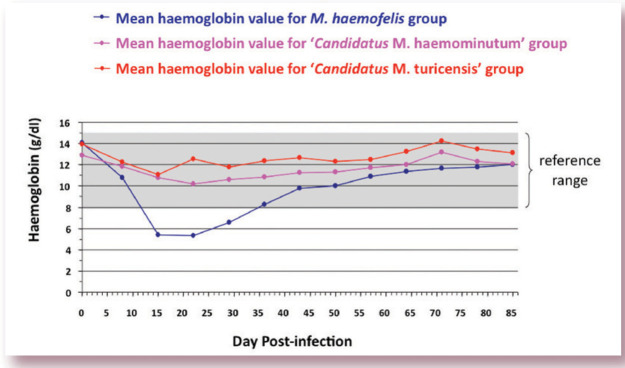
FIG 2.
Graph showing representative mean haemoglobin values for three groups of cats infected with either M haemofelis, ‘Candidatus M haemominutum’ or ‘Candidatus M turicensis’. The grey shaded area represents the reference range for haemoglobin (8–15 g/dl). As can be seen, only the cats infected with M haemofelis became anaemic, with the lowest haemoglobin values recorded between 2 and 3 weeks following infection. By contrast, cats infected with ‘Candidatus M haemominutum’ and ‘Candidatus M turicensis’ showed only a slight fall in haemoglobin values during the first 2–3 weeks of infection. Graph adapted from data collected for a previously published study 3
The influence of many of these factors has not yet been fully investigated or characterised. Unfortunately, therefore, generalised predictions of disease outcome following natural haemoplasma infection in an individual cat cannot be made. These factors must be considered when trying to determine the significance of haemoplasma infection in an individual cat.
Unfortunately, generalised predictions of disease outcome following natural haemoplasma infection in an individual cat cannot be made.
Mycoplasma haemofelis
While acute experimental M haemofelis infection often results in haemolytic anaemia, prevalence studies in naturally infected cats have not consistently demonstrated an association between anaemia and M haemofelis infection. Differing results could arise due to different populations of cats being sampled or different isolates of M haemofelis being involved in these studies, and/or reflect the acute versus chronic nature of M haemofelis infection (it has been hypothesised that acute M haemofelis infection is more likely to be associated with anaemia than chronic infection). 4 Nevertheless, of the known feline haemoplasma species, M haemofelis is regarded as being the most pathogenic and the one most likely to be associated with clinical disease.
‘Candidatus M haemominutum’
Experimental ‘Candidatus M haemominutum’ infection rarely results in significant clinical signs and anaemia is not usually induced, although a fall in red blood cell parameters can occur.3,5 Significant anaemia may arise following ‘Candidatus M haemominutum’ infection in retrovirus-infected cats, particularly those with feline leukaemia virus (FeLV) infection, in which ‘Candidatus M haemominutum’ may also play a role in inducing bone marrow disorders. 6 Studies in naturally infected cats have also suggested an influence on bone marrow disorders, with a blunted erythrocyte regenerative response having been recorded in cats infected with ‘Candidatus M haemominutum’. 1 Studies of naturally infected cats have usually failed to demonstrate associations between anaemia and ‘Candidatus M haemominutum’, although ‘Candidatus M haemominutum’-associated anaemia has been reported in a cat undergoing chemotherapy for lymphoma and a number of cases of apparent primary ‘Candidatus M haemominutum’-associated anaemia have been described.7,8 However, one US study found that ‘Candidatus M haemominutum’-infected cats were actually less likely to be anaemic than haemoplasma-negative cats. 1
The pathogenicity of ‘Candidatus M haemominutum’ infection is therefore difficult to summarise. It is likely that different ‘Candidatus M haemominutum’ isolates have varying pathogenicity and that the underlying health status of the cat plays a role in the outcome of infection.
‘Candidatus M turicensis’
‘Candidatus M turicensis’ was first described in 2005, 9 where it was associated with haemolytic anaemia, although colleagues and I have reported a fall in red blood cell parameters without induction of anaemia with ‘Candidatus M turicensis’ infection. 3 In naturally infected cats, only those co-infected with ‘Candidatus M haemominutum’ or M haemofelis, and not ‘Candidatus M turicensis’-only infected cats, exhibited significantly lower packed cell volumes (PCVs) compared with haemoplasma-uninfected cats. 10 A significant number of ‘Candidatus M turicensis’-infected cats have concurrent diseases, such as neoplasia or feline immunodeficiency virus (FIV) infection, 4 suggesting that co-factors and immunosup-pression may be important in the pathogenesis of disease caused by this agent.
How common are the feline haemoplasmas?
In most prevalence studies ‘Candidatus M haemominutum’ has been the most common haemoplasma found, with fewer numbers of ‘Candidatus M turicensis’ and M haemofelis detected. However, high prevalences of the latter two species have occasionally been reported, such as in a South African study (‘Candidatus M turicensis’) 11 and a Canadian study (M haemofelis). 12 The only study that has documented ‘Candidatus M haematoparvum-like’ infection in cats was from the USA, and only a low prevalence (0.7%) of infection was found. 1 Interestingly, recent prevalence studies from the same group, 2 and a group in Italy, 13 failed to find any evidence of ‘Candidatus M haematoparvum-like’ infection in cats. The ranges of prevalence of feline haemoplasma species reported to date are summarised in Table 1.
TABLE 1.
Reported prevalences for the feline haemoplasmas
| Haemoplasma species | Reported prevalence∗ |
| ‘Candidatus M haemominutum’ | 10–32.1% |
| M haemofelis | 0.4–46.6% |
| ‘Candidatus M turicensis’ | 0.4–26% |
| ‘Candidatus M haematoparvum-like’ | 0–0.7% |
How are the feline haemoplasmas transmitted?
Social contact
Studies evaluating risk factors for haemoplasma infection have generally found that older male cats, with outdoor access, are more likely to be infected. The increased prevalence in male cats, together with reports that cat bite abscesses and outdoor roaming are risk factors, have led to the suggestion that horizontal transmission may occur via fighting. In line with this both ‘Candidatus M haemominutum’ and ‘Candidatus M turicensis’ DNA has been found in the saliva of infected cats.4,14 However, recent studies failed to show the successful transmission of ‘Candidatus M turicensis’ infection between cats by the oral or subcutaneous inoculation of ‘Candidatus M turicensis’-infected saliva, even though the same dose of ‘Candidatus M turicensis’ in blood led to successful transmission when inoculated subcutaneously. 15
More work in this field is required but it seems probable that haemoplasma transmission via social contact is unlikely but that aggressive interaction (eg, cat bites) may result in transmission if the recipient cat is exposed to infectious blood rather than just saliva.
Arthropod vector
The cat flea Ctenocephalides felis has been incriminated in the transmission of haemoplasmosis between cats although only transient transmission of M haemofelis to a cat via the haematophagous activity of Ct felis was demonstrated. 16 Studies have found evidence of feline haemoplasma infection in fleas collected from cats,12,17–19 and in some ticks.20,21 Additionally, in one US study, 22 community-sourced cats that were housed indoors without a history of ticks or fleas were less likely to have haemoplasma infections than those that had access outdoors or had a history of ticks or fleas. The clustered geographic distribution of haemoplasma infection in US and Swiss studies is also supportive of arthropod-borne transmission.1,4 However, despite this supportive evidence, direct evidence for the role of vectors in natural/field haemoplasma transmission between cats is lacking. We have certainly seen haemoplasma transmission between cats in the absence of vectors so, even if fleas or ticks have the ability to act as vectors for haemoplasmosis in cats, other means of haemoplasma transmission must exist.
It seems probable that haemoplasma transmission via social contact is unlikely but that aggressive interaction (eg, cat bites) may result in transmission if the recipient cat is exposed to infectious blood rather than just saliva.
Even if fleas or ticks have the ability to act as vectors for haemoplasmosis in cats, other means of transmission must exist.
Blood transfusions
Transmission via contaminated blood transfusions has been reported, 4 and the use of freshly collected blood from a haemoplasma-infected blood donor for transfusion would very likely result in transmission of infection to the recipient cat. The risk of haemoplasma transmission when using stored blood for transfusions depends on the viability of haemoplasmas in stored blood.
One study evaluated this by looking at the survival of haemoplasma organisms in blood collected into citrate-phosphate-dextrose-adenine (CPDA) anticoagulant. 23 Haemoplasma survival was assessed by the ability to transmit M haemofelis or ‘Candidatus M haemominutum’ infection to naive cats via the intravenous inoculation of infected blood that had been stored in CPDA anticoagulant at 4°C for 1 h, 1 week or 1 month. M haemofelis was only successfully transmitted to the naive cat given the blood that had been stored for 1 h; there was evidence of subsequent in vivo amplification of M haemofelis organisms in the recipient cat's blood, as organism numbers increased during the 3-week post-inoculation monitoring period. Some evidence for ‘Candidatus M haemominutum’ transmission was also found using the blood stored for 1 h, although organism numbers in the recipient naive cat did not increase post-inoculation. ‘Candidatus M haemominutum’-infected blood that had been stored for 1 week resulted in a single positive polymerase chain reaction (PCR) result in the naive cat, in only one of two PCR assays used, so successful persistent transmission was not seen. But this work suggested that ‘Candidatus M haemominutum’ may be able to survive for up to a week in CPDA anticoagulant.
Interestingly, and in contrast to these results, experimental studies at the University of Bristol have found that the viability of haemoplasma organisms in blood collected into EDTA or heparin anticoagulants is very short-lived (less than 1 h), as inoculations with blood stored for longer periods have failed. Survival of haemoplasma organisms outside of the host therefore needs more investigation. This is hard to research because of the current absence of a haemoplasma in vitro culture system, meaning that only in vivo inoculations can prove organism viability.
Other modes of transmission
Other possible modes of haemoplasma transmission include vertical transmission from queen to kittens during pregnancy, at birth or via lactation. Although not reported, transmission may also be possible via the use of multi-use vials between cats, or the inappropriate use of the same equipment (eg, surgical instruments) on different cats without adequate cleaning/sterilisation, particularly if blood contamination was significant and only a short time elapsed between consecutive procedures.
How do haemoplasmas induce anaemia?
Most of the haemolysis associated with haemoplasma infection is believed to be extravascular in nature, occurring in the spleen and liver especially, but also in the lungs and bone marrow. Intravascular haemolysis has also been reported, as has increased osmotic fragility of haemoplasma-infected red blood cells. 9
Positive Coombs' tests and autoagglutination, indicating the presence of erythrocyte-bound antibodies, have been demonstrated in anaemic M haemofelis-infected cats. 3 Such erythrocyte-bound antibodies could be responsible for immune-mediated destruction of red blood cells. In a study describing the nature of positive Coombs' tests in M haemofelis-infected cats, 3 erythrocyte-bound antibodies reactive at 4°C (both IgM and IgG) appeared a few days earlier than those reactive at 37°C (primarily IgG). Despite this, in most cats the erythrocyte-bound antibodies appeared only after anaemia had started to develop. The absence of erythrocyte-bound antibodies at the onset of development of anaemia could reflect a problem with the sensitivity of detection of erythrocyte-bound antibodies; however, an alternative explanation is that erythrocyte-bound antibodies appear as a result of haemoplasma-induced haemolysis, rather than initiating the haemolysis. Indeed we have documented disappearance of these antibodies with antibiotic and supportive treatment alone, without the need for specific glucocorticoid treatment, questioning the key role of such antibodies in haemoplasma-induced anaemia.
What are the clinical signs of haemoplasmosis?
As mentioned, the clinical signs of haemoplasma infection depend on factors such as the haemoplasma species involved, stage of infection and whether concurrent diseases or infections are present. Common signs exhibited by acutely ill cats include pallor (Fig 3), lethargy, anorexia, weight loss, depression and dehydration. Intermittent pyrexia is often seen, particularly in the acute stage disease, as is splenomegaly, which may reflect extramedullary haematopoiesis. Jaundice is uncommon unless severe acute haemolysis occurs.
FIG 3.
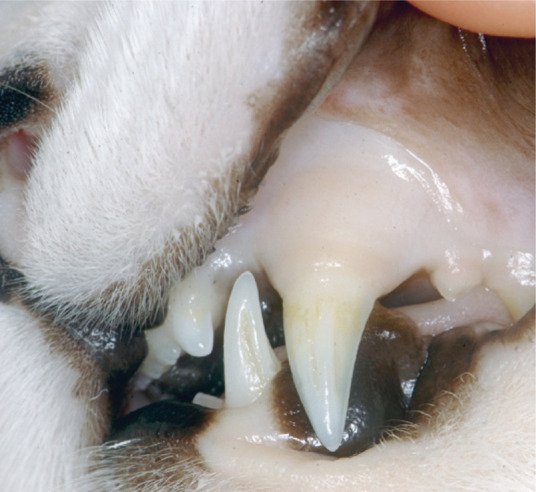
Pale mucous membranes of an anaemic cat with acute M haemofelis infection. Clinical jaundice, due to acute severe haemolysis, is only occasionally seen in cats with haemoplasmosis
Carrier Cats.
Cats that recover from haemoplasma infection may remain chronically infected. In our experience, long-term carrier status appears to be especially common following ‘Candidatus M haemominutum’ infection, although suspected clearance of this infection has been reported with and without antibiotic treatment. 4 M. haemofelis-infected cats may spontaneously clear infection from peripheral blood after infection without antibiotic treatment, and such clearance has also been reported with ‘CandidatusM. turicensis’ infection. It is clear that variation exists in the long-term host-organism interaction, making long-term predictions of outcome impossible. In any haemoplasma-infected carrier cat the potential for reactivation of infection exists, which can result in clinical disease, 24 although this seems to be quite rare once haemoplasma infection has become established beyond acute infection.
How is haemoplasmosis diagnosed?
Blood smear examination
Diagnosis of haemoplasma infection used to rely on cytological examination of a Romanowsky-stained blood smear, with organisms appearing on the surface of erythrocytes as rounded bodies singly (Fig 4), in pairs, or occasionally in chains. Diff-Quik or filtered Giemsa stains can be used. However, cytological diagnosis has poor sensitivity and specificity. Sensitivity is a particular issue, with figures of only 0–37.5% reported in various studies.25–27 Specificity is usually higher, with values of 84–98% reported,25,26 although these figures are based on board-certified clinical pathologists examining and interpreting blood smears.
FIG 4.
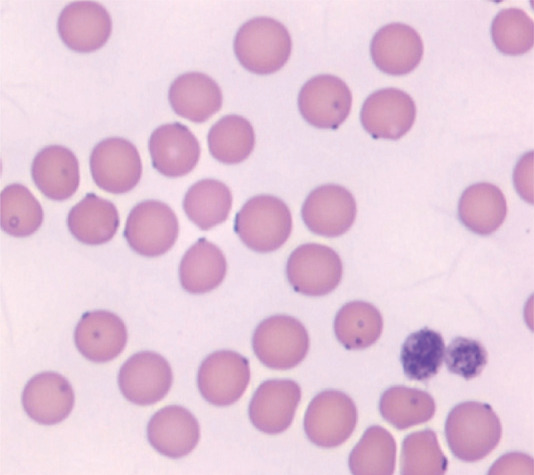
Romanowsky-stained blood smear from a cat infected with ‘Candidatus M haemominutum’. Organisms are seen as dark purple-blue dots on the surface of red blood cells. Although the organisms are clearly visible here, cytological confirmation of haemoplasma infection is very insensitive What are the clinical sign of haemoplasmosis?
If reliable blood smear interpretation is available, the cytological detection of organisms during acute infection can be useful as a bench-side and immediate diagnostic test. However, in the author's experience, many cases diagnosed as being haemoplasma-infected on the basis of blood smear interpretation in practice have been false positives, with stain precipitate, Howell-Jolly bodies and artefacts due to slow blood smear drying being the most common reasons for error. Additionally cytology cannot differentiate between haemoplasma species.
Laboratory features of pathogenic haemoplasmosis.
Haematology
When anaemia is induced by haemoplasma infection, it is typically regenerative (or pre-regenerative if sampling occurs very soon after the onset of anaemia), macrocytic, and normo- or hypochromic. However, significant reticulocytosis is not always present, even when pre-regenerative cases are excluded, possibly due to concurrent retroviral infections or other disease processes. Release of sequestered erythrocytes from the spleen may also result in a marked rise in red blood cell count without an accompanying reticulocytosis. As mentioned on page 372, positive Coombs' tests and autoagglutination may also occur.
Biochemistry
Serum biochemistry may reveal hyperproteinaemia due to dehydration or an acute phase response, and increased liver enzyme levels may arise from hepatic hypoxic damage. Hyperbilirubinaemia can result from the haemolysis. 3
Polymerase chain reaction
Polymerase chain reaction amplifies specific lengths of DNA so that potentially tiny amounts of DNA in samples are detectable. When designed properly, PCR has the potential to be extremely sensitive and specific for haemoplasma diagnosis. 28 Sensitivity and specificity should always be available from laboratories offering PCR assays commercially, so that their reliability can be evaluated objectively. Additionally laboratories undertaking PCR should always use priate negative and positive controls, as well as internal amplification controls, to monitor for contamination or problems with the assay.
Polymerase chain reaction is known to be more sensitive than cytology for haemoplasma detection.24,25,27,29–31 All feline haemoplasma PCR assays described so far are based on amplification of segments of the haemoplasma 16S rRNA gene. Most conventional, nonquantitative PCR (cPCR) assays detect and distinguish both ‘Candidatus M haemominutum’ and M haemofelis,29,30,32 but do not amplify or differentiate ‘Candidatus M turicensis’, although a recent study has described a separate specific cPCR for ‘Candidatus M turicensis’. 33 Real-time quantitative PCR (qPCR) assays 34 have an additional level of specificity compared with cPCR, through the use of fluorogenic probes in addition to primers. Quantitative PCR assays exist that amplify and differentiate all three feline haemoplasma species. Additionally some available assays are duplexed to concurrently amplify an internal control, such as the feline 28S rRNA gene, to ensure that any negative PCR results have not arisen due to problems with DNA extraction, quality or PCR inhibition. 34 Quantitative PCRs also allow quantification of the amount of haemoplasma DNA present in the patient's blood, which may help determine the significance of haemoplasma infection and/or allow monitoring of the response to treatment (see later). Quantitative PCR furthermore is a lot more rapid than cPCR and has a reduced risk of contamination compared with cPCR, since the tubes containing the PCR product are never opened during analysis.
Sampling for haemoplasma PCR.
Only a small amount of blood is required for PCR. Most diagnostic laboratories request 1 ml of anti-coagulated blood, although in reality PCR is usually performed using DNA extracted from smaller volumes of blood (0.1 or 0.2 ml). EDTA-anticoagulated blood is usually required since heparin inhibits PCR. DNA is quite stable within blood samples so rapid or cold shipping of samples is not required. Blood sampling for PCR should preferably be undertaken before a cat undergoes any antibiotic treatment.
What is the typical course of haemoplasma infection?
Quantative PCR assays have allowed quantification of haemoplasma copy (organism) numbers in the blood of infected cats.3–5,35,36 Previously, accurate quantification of haemoplasma infection in vivo had not been possible due to the uncultivable status of the haemoplasmas. Cats experimentally infected with haemoplasma species initially show a rapid increase in copy numbers, with peak numbers typically being reached around 2–4 weeks after infection (Fig 5). However, M haemofelis copy numbers can fluctuate greatly, even within the initial post-infection period. Some M haemofelis-infected cats continue to show very large changes in M haemofelis copy numbers for several months following experimental infection (Fig 6), which should be borne in mind when interpreting qPCR results. These fluctuations can be as large and as rapid as a four-log difference over 2 or 3 days, or a seven-log difference over 12 days. In contrast, ‘Candidatus M haemominutum’- and ‘Candidatus M turicensis’-infected cats show less or little fluctuation in copy numbers.
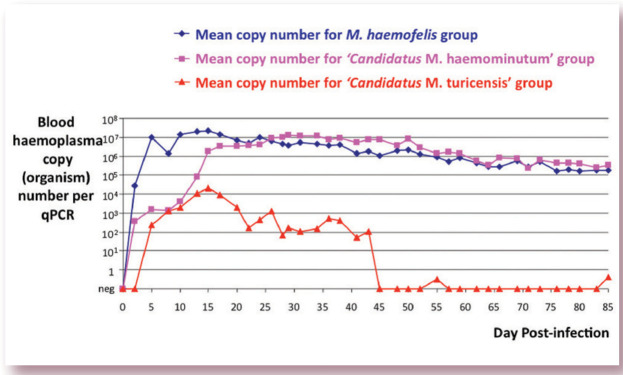
FIG 5.
Graph showing representative mean haemoplasma copy numbers over time for three groups of cats infected with either M haemofelis, ‘Candidatus M haemominutum’ or ‘Candidatus M turicensis’. Haemoplasma copy numbers were measured by qPCR and the values shown represent those present per 5 μl of blood, assuming perfectly efficient DNA extraction and PCR. As can be seen, cats infected with ‘Candidatus M turicensis’ have significantly lower blood copy numbers than cats infected with the other two species, and the ‘Candidatus M turicensis’-infected cats in this study become qPCR negative as early as 45 days after infection. Cats infected with M haemofelis and ‘Candidatus M turicensis’ tend to reach peak copy numbers around 2 weeks following infection, whereas copy numbers in cats infected with ‘Candidatus M haemominutum’ tend to peak a little later, at around 4 weeks following infection. Since the lines shown in this graph represent the mean haemoplasma copy numbers recorded for each group of cats, the marked fluctuation in copy numbers often seen in individual M haemofelis-infected cats is not visible here, but is represented in Fig 6. Graph adapted from data collected for a previously published study 3
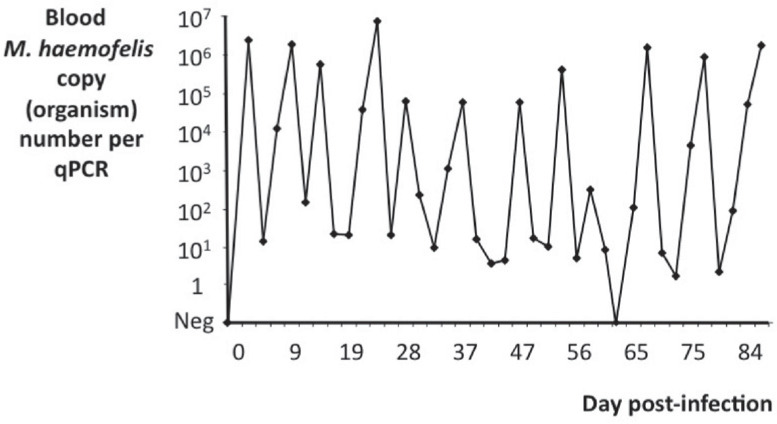
FIG 6.
Graph showing blood haemoplasma copy numbers over time in an individual cat infected with M haemofelis. Marked fluctuations are apparent, with several log-fold differences in copy numbers apparent over the course of a few days. In our experience, up to a third of cats infected with M haemofelis show these marked fluctuations in copy numbers, which have been interpreted as cycling of haemoplasma organisms in the blood. Graph adapted from data collected for a previously published study 35
The reason for the marked fluctuations in blood M haemofelis copy numbers over time seen in some cats has been a focus of recent research. Possible causes include sequestration and subsequent release of organisms from organs such as the spleen, liver or lungs, 37 cyclical antigenic variation used by some haemotropic and mycoplasmal species to evade the host immune response,38,39 or the rapid removal and subsequent rapid multiplication of organisms remaining in the blood. One recent study failed to show any evidence of tissue sequestration of M haemofelis organisms, 36 so this is unlikely to be the cause. The marked increase in M haemofelis copy numbers seen in the blood immediately after initial infection confirms that rapid multiplication of organisms is possible in infected cats, so it seems likely that such rapid multiplication (rather than organism release from sequestration sites) may be responsible for the marked rapid increases in M haemofelis copy numbers seen in infected cats during M haemofelis cycling. The marked rapid decreases in M haemofelis copy numbers could arise due to rapid effective clearance of organisms from the blood and their subsequent destruction.
Up to 10% of healthy cats at any time are infected with feline haemoplasmas, emphasising that a positive PCR result does not always correlate with the presence of clinical haemoplasmal disease.
Haemoplasma infection has been detected as early as one day post-infection by qPCR in the blood of experimental cats. Following recovery from acute clinical disease, cats can remain chronically infected. In some cases this chronic carrier status is lifelong, whereas in others anti biotic therapy or spontaneous elimination of the organism results in apparent clearance of infection. Cats usually remain PCR positive during chronic carrier status. Up to 10% of healthy cats at any time are infected with feline haemoplasmas,12,25 emphasising that a positive PCR result does not always correlate with the presence of clinical haemoplasmal disease. This must be considered when interpreting haemoplasma PCR results.
When to treat?
A couple of issues need to be considered when deciding if haemoplasmosis treatment is appropriate in an individual case:
First given that asymptomatic carrier cats exist, it must be borne in mind that the presence of infection is not always accompanied by associated clinical signs;
Secondly, there is no known antibiotic treatment regimen that reliably eliminates haemoplasma infection, so treatment may not result in haemoplasma clearance, even though the clinical signs of anaemia will usually improve.
If haemoplasma infection is diagnosed in a cat showing clinical signs then treatment should be instigated, ideally to clear infection but certainly to induce a clinical response. Treatment is also indicated in cases with concurrent infections or diseases that could exacerbate the potential for disease due to haemoplasma infection (eg, in an immunosuppressed FIV-infected cat). In cases in which treatment is instigated, monitoring can be performed so that treatment efficacy can be assessed.
What are the recommended treatment approaches?
Antibiotics
Tetracycline and fluoroquinolone antibiotics are effective at reducing haemoplasma organism numbers in the blood, although consistent predictable elimination of haemoplasma infection has not been shown in any study.5,24,29,35,40,41 However, antibiotics usually do improve clinical signs and haematological abnormalities associated with haemoplasma infection, even if elimination of haemoplasma infection does not always result.
The box on page 376 discusses the choice of antibiotics for feline haemoplasmosis, recommended dosages, treatment considerations and factors that may affect treatment efficacy.
Supportive care
We have found that cats clinically ill with haemoplasmosis are often profoundly dehydrated, so correction of this with intravenous fluid therapy is important. The degree of anaemia in haemoplasmosis cases may be masked by the dehydration causing haemoconcentration, so haematological parameters must be monitored after correction of dehydration. Encouraging food intake (eg, offering tempting warmed food, hand feeding) is important in inappetent cats. More aggressive nutritional support may be required if anorexia is prolonged but in our experience anorexia is usually short-lived once appropriate treatment for haemoplasmosis has been given.
Although cats are often able to tolerate anaemia quite well, if the anaemia is severe (PCV <12%) and/or has developed acutely and is accompanied by significant associated clinical signs, a blood transfusion, or treatment with an oxygen carrying haemoglobin compound (eg, Oxyglobin; OPK Biotech) if a blood donor or blood products are not available, may be required. If a blood transfusion is given, this should only be performed after blood typing (and cross-matching, as advised by some) both the donor and recipient. Oxyglobin has a long shelf-life and is stable at room temperature, so can be easily stocked by practices that deal with feline anaemia cases, although it is unlicensed for use in cats. It is a potent colloid so care with its use must be taken, particularly in cases prone to circulatory overload; for example, cats with cardiac disease (occult hypertrophic cardio-myopathy), renal disease or respiratory disease. We have used a dose of 5–10 ml/kg in cats, administered intravenously at a rate of 0.5–2 ml/kg/h. The degree of anaemia can be monitored using haemoglobin values.
Which antibiotic?
Tetracyclines
Tetracyclines, such as oxytetracycline at a dosage of 22 mg/kg q8h PO, 28 are usually effective in the treatment of haemoplasmosis. Doxycycline (10 mg/kg q24h PO) is usually the preferred tetracycline for the treatment of feline hemoplasmosis, with prolonged treatment courses (up to 8 weeks) recommended to increase the chance of eliminating infection. 41 Evidence exists for doxycycline efficacy against all three feline haemoplasma species, although controlled studies have only been performed for M haemofelis.40–42 Anecdotally, it has been reported that some cats vomit when given doxycycline once daily at 10 mg/kg, and that twice daily dosing of 5 mg/kg is better tolerated. Some doxycycline formulations have also been associated with oesophagitis and oesophageal strictures in cats.43,44 Such side effects are believed to be related to the high acidity of some doxycycline preparations when dissolved (particularly the doxycycline hydrate form, used in a commercial tablet preparation in the UK), which induces irritation of the oesophageal and gastric mucosa. Such doxycycline dosing must therefore always be followed by a small amount of food or syringing of water to encourage complete swallowing of tablets into the stomach. Other formulations, such as doxycycline monohydrate, are far less acidic and take longer to dissolve in the neutral environment of the oesophagus, and so are associated with fewer side effects. Doxycycline monohydrate solutions are licensed for use in cats in various countries including Australia, New Zealand and South Africa.
Fluoroquinolones
Fluoroquinolones are also often an effective treatment for haemoplasmosis. Enrofloxacin (5 mg/kg q24h PO) has been successfully used to treat M haemofelis infection in controlled studies. However, diffuse retinal degeneration and acute blindness have been reported following enrofloxacin treatment in cats. Although this is said to be rare, great caution must be exercised with its use in cats, and other fluoroquinolones should be used in preference whenever possible. Marbofloxacin has also been effective at reducing M haemofelis and ‘Candidatus M haemominutum’ organism numbers in the blood of treated cats in controlled studies, using the recommended UK dosage of 2 mg/kg q24h PO, although the fall in ‘Candidatus M haemominutum’ organism numbers was less pronounced than that of M haemofelis.5,35 A study using marbofloxacin at the recommended US dose of 2.75 mg/kg q24h PO found evidence of improved haematological parameters in treated M haemofelis-infected cats compared with untreated controls. 45 To the author's knowledge, no reports of ocular abnormalities in cats following marbo floxacin treatment have been published, and the US study included regular fundic examinations of marbofloxacin-treated cats and found no evidence of adverse effects. 45 The efficacy of 14 days of prado-floxacin treatment of experimental M haemofelis infection has recently been reported, 42 where it was found to be effective (at the standard 5 mg/kg q24h PO dose, as well as a higher dose of 10 mg/kg q24h PO) at improving clinical signs and haematological values, and lowering M haemofelis copy numbers in the blood.
Imidocarb
Although imidocarb dipropionate has been efficacious in some field haemoplasma cases, a controlled study failed to show any beneficial effect of this antiprotozoal agent on clinical signs or haematological values in M haemofelis-infected cats.46,47 However, it may be worth considering imidocarb treatment in chronic haemoplasma cases that appear to be refractory to tetracyclines and/or fluoroquinolones, as some cases have shown a clinical improvement with treatment. Recommended dosages are 5 mg/kg IM every 2 weeks for two to four injections. The injectable nature of this drug may be an advantage for some owners.
Factors affecting haemoplasmosis antibiotic efficacy.
It is possible that different isolates, as well as different haemoplasma species, respond to antibiotic treatments differently. Other factors may contribute to antibiotic resistance. A recent study found that one isolate of the porcine haemoplasma Mycoplasma suis was able to penetrate porcine erythrocytes and that its intracellular location was associated with marked resistance to antibiotic therapy. 48 Additionally the route of administration of antibiotics may play a role in the efficacy of treatment, and this should be investigated in the future. It may be that, for example, initial intravenous anti biotic administration is advantageous for the treatment of acute haemoplasmosis, to increase and/or hasten maximal antibiotic blood levels. In our experience, although antibiotics may not always induce infection clearance, a clinical response I to antibiotics is usually seen. However, some cases do appear to be clinically refractory to standard anti biotic treatments. Occasionally dual therapy (typically doxycycline and marbofloxacin) has been tried, with variable success, but it may be I worth considering introducing an additional agent if monotherapy with doxycycline or a fluoro quinolone is inadequate. The use of corticosteroids might also be considered in refractory cases with severe immune-mediated haemolysis (see corticosteroids section, page 377), although we have very rarely needed to use them in this context.
Corticosteroids
It has been reported the anaemia induced by haemoplasma infection is in part immune-mediated and so corticosteroids have been recommended as adjunct therapy. 49 However, the value of corticosteroids in the treatment of haemoplasmosis has not been proven and, as mentioned earlier, anaemic Coombs'-positive cats infected with M haemofelis respond to antibiotic treatment alone,3,50 which calls into question the need for immunosuppressive treatment. Indeed in some cases concurrent corticosteroid therapy has been associated with a delay in obtaining apparent haemoplasma clearance from the blood. It has been shown that a ‘Candidatus M turicensis’ infected cat that received methylprednisolone acetate prior to infection developed a more severe anaemia than an immunocompetent cat, 9 so corticosteroids have the potential to exacerbate disease too.
Are haemoplasmas zoonotic?
Previous reports have documented haemoplasmosis in humans using cytology, although our attempts to successfully amplify haemoplasma DNA from samples recruited from some of these studies have failed (unpublished observations), suggesting that previous reports of human haemoplasmosis based solely on cytological diagnosis should be viewed with caution. More recently, however, PCR has been used to show evidence of haemoplasma infection in humans. PCR has documented the presence of an organism very similar to M suis in people in China, although clinical haemoplasmosis was not common.51,52 In another study, a M haemofelis-like organism was detected in an immunosuppressed HIV-positive patient who had concurrent bartonellosis and a history of cat scratches. 53 However, the contribution of the M haemofelis-like infection to the man's clinical signs was not discussed. These reports certainly suggest the existence of zoonotic haemoplasma infections, which will need to be further investigated to clearly define the epidemiology of human haemoplasmosis.
We would recommended only considering corticosteroid treatment in cases that are deteriorating despite administration of an appropriate antibiotic and in which immunemediated haemolytic anaemia (IMHA) is thought to be a major component of the disease, or in cases in which haemoplasma infection is not strongly believed to be the underlying cause of disease.
A goal of any treatment protocol for haemoplasmosis should be to eliminate infection.
How is the treatment response monitored?
Since the haemoplasmas have not been grown in vitro to date, qPCR offers a reliable alternative means of assessing the response to antibiotics. Cats can become PCR negative during anti biotic treatment (but it may take a number of days or even weeks for the haemoplasma levels to fall to below detection limits),5,35 and may become PCR positive again when antibiotic treatment is stopped. 50 Ideally blood sampling for PCR should precede the start of antibiotic treatment, although a positive result with high organism numbers would indicate that the therapy is not optimally effective.
Blood sampling for qPCR should be repeated a week or two after starting antibiotic therapy to ensure therapy is effective at lowering haemoplasma copy numbers. If efficacy is seen, therapy should be continued for up to 8 weeks, ideally with documentation of negative PCR results at completion of treatment. A goal of any treatment protocol for haemoplasmosis should be to eliminate infection, although proving infection has been eliminated is difficult without performing PCR on the whole host! Repeatedly negative results on blood samples tested with a sensitive PCR assay (monthly is suggested, two to three times) are probably most reliable to indicate elimination. If negative results do not result from treatment (ie, elimination is not possible), control of clinical signs and a reduction in copy numbers in the blood nevertheless indicates treatment efficacy. However, recrudescence of disease remains possible as long as these cases remain haemoplasma positive.
Considerations in interpreting PCR results.
Interpret PCR results in the light of the patient's clinical signs, haematological parameters, the haemoplasma species detected, and the amount of haemoplasma DNA present in the blood as determined by qPCR.
A positive PCR result does not equate with the haemoplasma being the cause of the cat's clinical signs; a PCR assay detects infection rather than confirming the cause of the cat's anaemia.
Be aware that M haemofelis organism loads in the blood of some cats can spontaneously fluctuate quite markedly, with increases and decreases occurring quickly over the course of a day or two. Hence, do not always assume decreases are indicative of effective antibiotic therapy or spontaneous elimination of the organism.
Asymptomatic carrier cats exist for all haemoplasma species but this status seems to be especially long term with ‘Candidatus M haemominutum’.
Samples for PCR should ideally be collected before antibiotic treatment is started, although documentation of a high blood organism load during treatment may be useful in indicating treatment failure.
Elimination of infection is very difficult to prove but repeatedly negative PCR results (monthly is suggested, two to three times) on blood samples are supportive of elimination.
Discordant results (ie, positive and negative results on the same sample) can occur if the number of haemoplasma organisms in the blood is very low and close to the sensitivity detection limit of the PCR assay. This is because when such low haemoplasma levels are present, the aliquot of sample used in the PCR may not contain the single copy of haemoplasma DNA that allows successful amplification and the subsequent positive PCR result.
Case notes.
A 2-year-old male domestic shorthair cat presented with a 1 week history of progressive lethargy and anorexia. He was fully vaccinated and wormed and had access outdoors. On clinical examination he was slightly dull and had pale mucous membranes. He was tachycardic (heart rate 220 beats per minute) with a left-sided grade II/VI systolic heart murmur, tachypnoeic (respiratory rate 60 breaths per minute) and pyrexic (39.6°C/103.4°F). Abdominal palpation was unremarkable but skin tenting was evident, indicating dehydration.
Initial diagnostic testing and management
-
Blood smear examination A freshly collected blood sample was rapidly dried and stained with Diff-Quik. Microscopy examination revealed variation in erythrocyte size (anisocytosis), bluish colouring to some of the erythrocytes (polychromasia), the presence of some nucleated erythrocytes and agglutination of erythrocytes. Additionally small blue cellular bodies were seen on the surface of some erythrocytes. Platelets were present in adequate numbers.
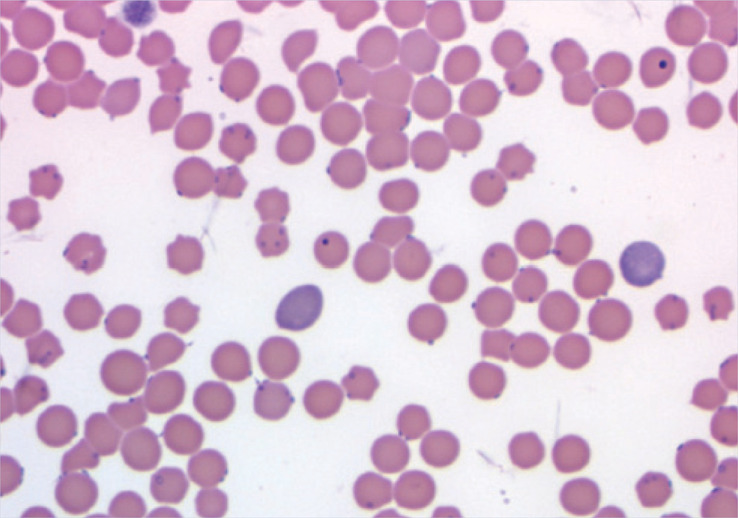 Blood smear showing polychromasia, anisocytosis and the presence of small blue cellular bodies on the surface of some erythrocytes
Blood smear showing polychromasia, anisocytosis and the presence of small blue cellular bodies on the surface of some erythrocytes -
PCV An in-house estimation performed using a small sample of EDTA anticoagulated blood revealed a PCV of 16% (reference range 25–45%), consistent with moderate anaemia.
To correct the dehydration present, the cat was immediately given intravenous fluid therapy (2 ml/kg/h maintenance requirement + 4 ml/kg/h to correct the estimated 10% dehydration for 24 h [total 6 ml/kg/h] of lactated Ringer's solution, an isotonic replacement fluid). He was placed in a quiet area of the hospital and regularly monitored while other diagnostics were performed to elucidate the cause of his anaemia.
Slide agglutination test Four drops of normal (0.9%) saline and one drop of whole EDTA blood were placed on a microscope slide, which was gently rocked to encourage mixing. Within a few minutes distinct red speckles had developed, consistent with gross macroscopic agglutination (sticking together of red blood cells in disorganised clumps) due to the presence of warm agglutinating erythrocyte-bound antibodies. The agglutination was confirmed by examination of the slide under the microscope after adding a cover slip. The saline in the slide agglutination test should disperse any rouleaux formation (organised stacking of overlapping red blood cells in columns) in the cat, which grossly can mimic agglutination. Some recommend using up to 10 drops of saline to one drop of blood in the slide agglutination test, but a ratio of 1:4 is usually adequate.
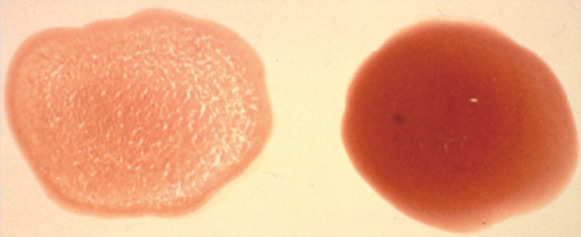
Positive slide agglutination test. The red speckles in the sample of saline and blood on the left indicate the presence of erythrocyte-bound antibodies. A negative control on the right is shown for comparison
Major differential diagnoses to consider in an anaemic cat
-
Regenerative anaemia due to blood loss
Acute blood loss will result in anaemia but hypovolaemic shock is usually the initial concern:
— Trauma (eg, road traffic accident);
— Coagulopathy (liver disease, rodenticide toxicity, haemophilia);
— Thrombocytopenia;
— Systemic amyloidosis can result in spontaneous hepatic rupture and acute abdominal haemorrhage in young to middle-aged cats. Siamese and related cats appear to be predisposed;
— Gastroduodenal ulceration and bleeding can arise due to neoplasia (mast cell tumours, gastrinoma, lymphoma), NSAID toxicity or inflammatory bowel disease;
— ‘Menrath’ ulcers (ie, bleeding lesions in the mouth of cats associated with erosion of palatine vessels).
Chronic blood loss is occasionally seen in cats:
— Severe ectoparasitism (fleas and lice) in kittens;
— Gastroduodenal ulceration and bleeding with a more chronic course due to neoplasia, NSAID toxicity or inflammatory bowel disease;
— Systemic amyloidosis can also result in a more chronic course of intermittent hepatic bleeding;
— Chronic bleeding from the urinary tract due to neoplasia or severe prolonged inflammation.
-
Regenerative anaemia due to haemolysis
— Primary IMHA (recent reports suggest it is more common than previously believed);
— IMHA secondary to infectious agents such as FeLV, haemoplasmas and feline infectious peritonitis (FIP) virus, drugs (eg, methimazole, trimethoprim-sulphonamides), neoplasia (eg, lymphoma, myeloproliferative disorders) and haemolytic blood transfusion reactions;
— Infections causing haemolysis (FeLV infection, haemoplasmosis, Babesia species, cytauxzoonosis, FIV infection);
— Oxidant injury causing Heinz body haemolytic anaemia (due to exposure to chemicals or toxins [onions] and some disease states [diabetic ketoacidosis, hyperthyroidism, lymphoma]);
— Hypophosphataemia (<0.35 mmol/l) can result in haemolysis and has been associated with diseases such as diabetes mellitus and hepatic lipidosis;
— Inherited red blood cell defects such as osmotic fragility and pyruvate kinase deficiency in Abyssinians and Somalis;
— Microangiopathic haemolytic anaemia due to damage to the vascular endothelium.
-
Non-regenerative anaemia
Primary bone marrow disorders often result in moderate to severe anaemia:
— Pure red cell aplasia;
— Aplastic anaemia;
— Myelodysplastic syndromes;
— Myelophthisis;
— Myeloproliferative diseases.
Systemic causes of bone marrow suppression are often associated with milder anaemia:
— Anaemia of inflammatory (chronic) disease (very common in cats);
— Chronic renal failure;
— FeLV- or FIV-associated non-regenerative anaemia.
Further laboratory assessment
In this case, evidence for regeneration (polychromasia and anisocytosis) was seen on the blood smear, pointing to haemolysis or blood loss as being the most likely cause, although confirmation of the regenerative nature of the anaemia (as well as the degree of regeneration) required enumeration of reticulocytes. No evidence of blood loss was present, and the positive slide agglutination test indicated the presence of erythrocyte-bound antibodies in the cat, suggesting IMHA to be present. The cat had not been given any medications before presentation. The presence of small blue cellular bodies on the surface of some erythrocytes made haemoplasmosis a major differential diagnosis. The pyrexia at presentation was also consistent with haemoplasmosis.
In view of these findings the cat was immediately given oral doxycycline, followed by syringed water to ensure complete swallowing of the tablet, and the response to treatment was monitored.
Routine haematology, performed a few hours after the start of intravenous fluid therapy, indicated the presence of a severe macrocytic anaemia and evidence of a degenerative neutrophilic left shift (see table). The macrocytosis was excessive and, although this could reflect regeneration, other possible causes of macrocytosis include agglutination of red blood cells precluding accurate estimation of red blood cell size, a myelodysplastic syndrome or FeLV infection (the latter two causes usually being non-regenerative). The haematocrit was lower than the PCV measured previously, possibly due to partial correction of dehydration as a result of fluid therapy.
A serum biochemistry profile revealed markedly elevated ALT (672 iu/l; reference range 15–60), mildly elevated bilirubin (25 μmol/l; reference range <10), and a normal serum protein concentration. No other significant abnormalities were found. The elevated ALT was thought to reflect hepatic hypoxia as a result of the anaemia while hyperbilirubinaemia was consistent with ongoing haemolysis.
In-house FeLV and FIV ELISAs were negative.
A reticulocyte count was also requested and revealed 380 × 10 9 /l aggregate reticulocytes, consistent with substantial regeneration.
Haematology results
| Parameter | Value | Reference range |
| Haemoglobin | 4.4 g/dl | 8–14 |
| RBC | 1.57 × 10 12 /l | 5.5–10 |
| Haematocrit | 13 l/l | 25–45 |
| MCV | 82.7 fl | 39–55 |
| MCHC | 33.8 % | 30–36 |
| Total WBC | 12.9 × 10 9 /l | 7–20 |
| PMNs | 6.45 × 10 9 /l | 2.5–12.8 |
| Band PMNs | 1.032 × 10 9 /l | 0.0–0.0 |
| Lymphocytes | 4.644 × 10 9 /l | 1.5–7.0 |
| Monocytes | 0.774 × 10 9 /l | 0.07–0.85 |
| Eosinophils | 0.0 × 10 9 /l | 0.0–1.0 |
| Platelets | 224∗ × 10 9 /l | 300–500 |
Abnormal values shown in bold
NB platelet clumps visible on the smear
Blood was submitted for haemoplasma PCR testing but results were not immediately available.
Presumptive diagnosis and treatment
At this stage a diagnosis of IMHA was made, believed to be secondary to haemoplasma infection.
Doxycycline treatment for potential haemoplasma infection had already been started and was continued while the patient's response to therapy was assessed. Immediate treatment with corticosteroids was considered, due to the diagnosis of IMHA, but was withheld as the patient did not deteriorate, and responded well (within 24 h) to initial treatment with antibiotics and fluid therapy (as do most cases of acute haemoplasmosis).
Had the patient not improved, and in the absence of confirmed haemoplasmosis, trial treatment with 2 mg/kg/day of prednisolone would have been justified in case the IMHA was primary in nature.
A blood transfusion was not required in this case, primarily because the cat's clinical status was not severe enough to warrant this, and his PCV remained above 13% and rose quickly following treatment. If a blood transfusion had been required, blood typing of the donor and recipient, and/or crossmatching, would have been necessary, although this may have been difficult to interpret due to the autoagglutination present.
The patient was given tempting foods by hand and his PCV and hydration status were monitored closely over the next few days. His dehydration was corrected within 24 h and he began eating voluntarily, enabling us to reduce and stop intravenous fluid therapy within 3 days. His PCV rose steadily after starting doxycycline therapy, reaching 16% after 2 days of treatment and 19% after 4 days of treatment.
The cat was discharged 5 days after presentation, on continuing doxycycline treatment. Flea treatment was also recommended in view of fleas being potential vectors of haemoplasmas.
PCR confirmation and monitoring
PCR analysis revealed the presence of large numbers of M haemofelis in the blood, confirming the diagnosis of M haemofelis-associated IMHA.
At a recheck, a week after presentation, the cat's PCV was 23%. Repeat PCR analysis 2 weeks after starting doxycycline therapy showed only low levels of M haemofelis in the blood. Therapy was continued for a total of 8 weeks, after which a negative PCR result was obtained.
Acknowledgements
Most of the author's recent work on feline haemoplasmas has been funded by the Wellcome Trust (Grant no 077718), and Merial and Pfizer Animal Health are thanked for contributing to the funding of aspects of the author's feline haemoplasma research. The author would like to acknowledge many collaborators in contributing to her published haemoplasma work. In particular, Emily Barker, Richard Birtles, Michael Day, Tim Gruffydd-Jones, Dave Harbour, Chris Helps, Regina Hofmann-Lehmann, Michael Lappin, Remo Lobetti, Richard Malik, Hal Neimark, Iain Peters, Xavier Roura, Sue Shaw and Barbara Willi are thanked.
KEY POINTS.
Three main feline haemoplasma species exist: Mycoplasma haemofelis, ‘Candidatus Mycoplasma haemominutum’ and ‘Candidatus Mycoplasma turicensis’.
M haemofelis is the most pathogenic species; ‘Candidatus M haemominutum’ and ‘Candidatus M turicensis’ are less pathogenic, unless concurrent disease or immunosuppression is present.
‘Candidatus M haemominutum’ infection is more common than ‘Candidatus M turicensis’ and M haemofelis infections.
Clinical signs include pallor, lethargy, anorexia, weight loss, depression, dehydration and pyrexia.
The natural route of feline haemoplasma transmission between cats has not been confirmed, but fleas are implicated.
Asymptomatic haemoplasma carrier cats exist, so documentation of the presence of infection in an anaemic cat does not necessarily mean that the infection is the cause of the cat's anaemia.
Reliable diagnosis is based on polymerase chain reaction (PCR) assays performed on blood samples.
Since PCR results are unlikely to be available immediately, trial treatment for haemoplasma-associated haemolytic anaemia with antibiotics (tetracyclines or fluoroquinolones) is warranted in suspected cases. A rapid response (often within 24 h) to antibiotic treatment and other supportive care is usually seen in cases with haemoplasmosis.
Corticosteroids are not usually required for the treatment of haemoplasmosis. However, if the patient is deteriorating despite starting antibiotic treatment, and/or has no other supportive evidence for haemoplasmosis (pyrexia, history of outdoor access and/or fleas), and has erythrocyte-bound antibodies confirmed (positive slide agglutination test or Coombs' test), and there is no other obvious cause of haemolysis present, treatment for primary immune-mediated haemolytic anaemia is warranted and prednisolone can be tried.
References
- 1.Sykes JE, Drazenovich NL, Ball LM, Leutenegger CM. Use of conventional and real-time polymerase chain reaction to determine the epidemiology of hemoplasma infections in anemic and nonanemic cats. J Vet Intern Med 2007; 21: 685–93. [DOI] [PubMed] [Google Scholar]
- 2.Sykes JE, Terry JC, Lindsay LL, Owens SD. Prevalences of various hemoplasma species among cats in the United States with possible hemoplasmosis. J Am Vet Med Assoc 2008; 232: 372–79. [DOI] [PubMed] [Google Scholar]
- 3.Tasker S, Peters IR, Papasouliotis K, et al. Description of outcomes of experimental infection with feline haemoplasmas: copy numbers, haematology, Coombs' testing and blood glucose concentrations. Vet Microbiol 2009; 139: 323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willi B, Boretti FS, Baumgartner C, et al. Prevalence, risk factor analysis, and follow-up of infections caused by three feline hemoplasma species in cats in Switzerland. J Clin Microbiol 2006; 44: 961–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tasker S, Caney SMA, Day MJ, et al. Effect of chronic FIV infection, and efficacy of marbofloxacin treatment, on ‘Candidatus Mycoplasma haemominutum’ infection. Microbes Infect 2006; 8: 653–61. [DOI] [PubMed] [Google Scholar]
- 6.George JW, Rideout BA, Griffey SM, Pedersen NC. Effect of preexisting FeLV infection or FeLV and feline immunodeficiency virus coinfection on pathogenicity of the small variant of Haemobartonella felis in cats. Am J Vet Res 2002; 63: 1172–78. [DOI] [PubMed] [Google Scholar]
- 7.Hornok S, Meli ML, Gonczi E, et al. First molecular identification of ‘Candidatus mycoplasma haemominutum’ from a cat with fatal haemolytic anaemia in Hungary. Acta Vet Hung 2008; 56: 441–50. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds CA, Lappin MR. ‘Candidatus Mycoplasma haemominutum’ infections in 21 client-owned cats. J Am Anim Hosp Assoc 2007; 43: 249–57. [DOI] [PubMed] [Google Scholar]
- 9.Willi B, Boretti FS, Cattori V, et al. Identification, molecular characterisation and experimental transmission of a new hemoplasma isolate from a cat with hemolytic anaemia in Switzerland. J Clin Microbiol 2005; 43: 2581–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willi B, Tasker S, Boretti FS, et al. Phylogenetic and risk factor analysis for ‘Candidatus Mycoplasma turicensis’ in United Kingdom, Australian and South African pet cats. J Clin Microbiol 2006; 44: 4430–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lobetti RG, Tasker S. Diagnosis of feline haemoplasma infection using a real-time PCR. J South African Vet Assoc 2004; 75: 94–99. [DOI] [PubMed] [Google Scholar]
- 12.Kamrani A, Parreira VR, Greenwood J, Prescott JF. The prevalence of Bartonella, hemoplasma, and Rickettsia felis infections in domestic cats and in cat fleas in Ontario. Can J Vet Res 2008; 72: 411–19. [PMC free article] [PubMed] [Google Scholar]
- 13.Gentilini F, Novacco M, Turba ME, Willi B, Bacci ML, HofmannLehmann R. Use of combined conventional and real-time PCR to determine the epidemiology of feline haemoplasma infections in northern Italy. J Feline Med Surg 2009; 11: 277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean RS, Helps CR, Gruffydd Jones TJ, Tasker S. Use of real-time PCR to detect Mycoplasma haemofelis and ‘Candidatus Mycoplasma haemominutum’ in the saliva and salivary glands of haemoplasma-infected cats. J Feline Med Surg 2008; 10: 413–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Museux K, Boretti FS, Willi B, et al. In vivo transmission studies of ‘Candidatus Mycoplasma turicensis’ in the domestic cat. Vet Res 2009; 40: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woods JE, Brewer MM, Hawley JR, Wisnewski N, Lappin MR. Evaluation of experimental transmission of ‘Candidatus Mycoplasma haemominutum’ and Mycoplasma haemofelis by Ctenocephalides felis to cats. Am J Vet Res 2005; 66: 1008–12. [DOI] [PubMed] [Google Scholar]
- 17.Shaw SE, Kenny MJ, Tasker S, Birtles RJ. Pathogen carriage by the cat flea Ctenocephalides felis (Bouché) in the United Kingdom. Vet Microbiol 2004; 102: 183–88. [DOI] [PubMed] [Google Scholar]
- 18.Lappin MR, Griffin B, Brunt J, et al. Prevalence of Bartonella species, haemoplasma species, Ehrlichia species, Anaplasma phagocytophilum, and Neorickettsia risticii DNA in the blood of cats and their fleas in the United States. J Feline Med Surg 2006; 8: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornok S, Meli ML, Perreten A, et al. Molecular investigation of hard ticks (Acari: Ixodidae) and fleas (Siphonaptera: Pulicidae) as potential vectors of rickettsial and mycoplasmal agents. Vet Microbiol 2010; 140: 98–104. [DOI] [PubMed] [Google Scholar]
- 20.Willi B, Boretti FS, Meli ML, et al. Real-time PCR investigation of potential vectors, reservoirs and shedding patterns of feline hemotropic mycoplasmas. Appl Environ Microbiol 2007; 73: 3798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taroura S, Shimada Y, Sakata Y, et al. Detection of DNA of ‘Candidatus Mycoplasma haemominutum’ and Spiroplasma sp. in unfed ticks collected from vegetation in Japan. J Vet Med Sci 2005; 67: 1277–79. [DOI] [PubMed] [Google Scholar]
- 22.Hackett TB, Jensen WA, Lehman TL, et al. Prevalence of DNA of Mycoplasma haemofelis, ‘Candidatus Mycoplasma haemominutum’, Anaplasma phagocytophilum, and species of Bartonella, Neorickettsia, and Ehrlichia in cats used as blood donors in the United States. J Am Vet Med Assoc 2006; 229: 700–5. [DOI] [PubMed] [Google Scholar]
- 23.Gary AT, Richmond HL, Tasker S, Hackett TB, Lappin MR. Survival of Mycoplasma haemofelis and ‘Candidatus Mycoplasma haemominutum’ in blood of cats used for transfusions. J Feline Med Surg 2006; 8: 321–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foley JE, Harrus S, Poland A, Chomel B, Pedersen NC. Molecular, clinical, and pathologic comparison of two distinct strains of Haemobartonella felis in domestic cats. Am J Vet Res 1998; 59: 1581–88. [PubMed] [Google Scholar]
- 25.Tasker S, Binns SH, Day MJ, et al. Use of a PCR assay to assess prevalence and risk factors for Mycoplasma haemofelis and ‘Candidatus Mycoplasma haemominutum’ in cats in the United Kingdom. Vet Rec 2003; 152: 193–98. [DOI] [PubMed] [Google Scholar]
- 26.Bauer N, Balzer HJ, Thure S, Moritz A. Prevalence of feline haemotropic mycoplasmas in convenience samples of cats in Germany. J Feline Med Surg 2008; 10: 252–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westfall DS, Jensen WA, Reagan WJ, Radecki SV, Lappin MR. Inoculation of two genotypes of Haemobartonella felis (California and Ohio variants) to induce infection in cats and the response to treatment with azithromycin. Am J Vet Res 2001; 62: 687–91. [DOI] [PubMed] [Google Scholar]
- 28.Tasker S, Lappin MR. Haemobartonella felis: recent developments in diagnosis and treatment. J Feline Med Surg 2002; 4: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berent LM, Messick JB, Cooper SK. Detection of Haemobartonella felis in cats with experimentally induced acute and chronic infections, using a polymerase chain reaction assay. Am J Vet Res 1998; 59: 1215–20. [PubMed] [Google Scholar]
- 30.Jensen WA, Lappin MR, Kamkar S, Reagen WJ. Use of a polymerase chain reaction assay to detect and differentiate two strains of Haemobartonella felis infection in naturally infected cats. Am J Vet Res 2001; 62: 604–8. [DOI] [PubMed] [Google Scholar]
- 31.Tasker S, Helps CR, Day MJ, Gruffydd-Jones TJ, Harbour DA. Use of real-time PCR to detect and quantify Mycoplasma haemofelis and ‘Candidatus Mycoplasma haemominutum’ DNA. J Clin Microbiol 2003; 41: 439–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Criado-Fornelio A, Martinez-Marcos A, Buling-Sarana A, Barba-Carretero JC. Presence of Mycoplasma haemofelis, Mycoplasma haemominutum and piroplasmids in cats from southern Europe: a molecular study. Vet Microbiol 2003; 93: 307–17. [DOI] [PubMed] [Google Scholar]
- 33.Santos AP, Messick JB, Biondo AW, et al. Design, optimization, and application of a conventional PCR assay with an internal control for detection of ‘Candidatus Mycoplasma turicensis’ 16S rDNA in domestic cats from Brazil. Vet Clin Pathol 2009; 38: 443–52. [DOI] [PubMed] [Google Scholar]
- 34.Peters IR, Helps CR, Willi B, Hofmann-Lehmann R, Tasker S. The prevalence of three species of feline haemoplasmas in samples submitted to a diagnostics service as determined by three novel real-time duplex PCR assays. Vet Microbiol 2008; 126: 142–50. [DOI] [PubMed] [Google Scholar]
- 35.Tasker S, Caney SMA, Day MJ, et al. Effect of chronic FIV infection, and efficacy of marbofloxacin treatment, on Mycoplasma haemofelis infection. Vet Microbiol 2006; 117: 169–79. [DOI] [PubMed] [Google Scholar]
- 36.Tasker S, Peters IR, Day MJ, et al. Distribution of Mycoplasma haemofelis in blood and tissues following experimental infection Microb Pathog 2009; 47: 334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maede Y. Studies on feline haemobartonellosis. V. Role of the spleen in cats infected with Haemobartonella felis. Jap J Vet Sci 1978; 40: 141–46. [DOI] [PubMed] [Google Scholar]
- 38.Alleman AR, Palmer GH, McGuire TC, McElwain TF, Perryman LE, Barbet AF. Anaplasma marginale major surface protein 3 is encoded by a polymorphic, multigene family. Infect Immun 1997; 65: 156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev 1998; 62: 1094–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dowers KL, Olver C, Radecki S V, Lappin MR. Use of enrofloxacin for treatment of large-form Haemobartonella felis in experimentally infected cats. J Am Vet Med Assoc 2002; 221: 250–53. [DOI] [PubMed] [Google Scholar]
- 41.Tasker S, Helps CR, Day MJ, Harbour DA, Gruffydd-Jones TJ, Lappin M. Use of a Taqman PCR to determine the response of Mycoplasma haemofelis infection to antibiotic treatment. J Microbiol Methods 2004; 56: 63–71. [DOI] [PubMed] [Google Scholar]
- 42.Dowers KL, Tasker S, Radecki SV, Lappin MR. Use of prado-floxacin to treat experimentally induced Mycoplasma hemofelis infection in cats. Am J Vet Res 2009; 70: 105–11. [DOI] [PubMed] [Google Scholar]
- 43.German AJ, Cannon MJ, Dye C, et al. Oesophageal strictures in cats associated with doxycycline therapy. J Feline Med Surg 2005; 7: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGrotty YL, Knottenbelt CM. Oesophageal stricture in a cat due to oral administration of tetracyclines. J Small Anim Pract 2002; 43: 221–23. [DOI] [PubMed] [Google Scholar]
- 45.Ishak AM, Dowers KL, Cavanaugh MT, et al. Marbofloxacin for the treatment of experimentally induced Mycoplasma haemofelis infection in cats. J Vet Intern Med 2008; 22: 288–92. [DOI] [PubMed] [Google Scholar]
- 46.Lappin MR, Brewer M, Radecki S. Effects of imidocarb dipropionate in cats with chronic haemobartonellosis. Vet Therapeutics 2002; 2: 144–49. [PubMed] [Google Scholar]
- 47.Woods JE, Brewer M, Radecki SV, Lappin MR. Treatment of Mycoplasma haemofelis infected cats with imidocarb dipropionate. J Vet Intern Med 2004; 18: 436. [Google Scholar]
- 48.Groebel K, Hoelzle K, Wittenbrink MM, Ziegler U, Hoelzle LE. Unraveling a paradigm: Mycoplasma suis invades porcine erythrocytes. Infect Immun 2009; 77: 576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.VanSteenhouse JL, Millard JR, Taboada J. Feline haemobartonellosis. Compend Contin Educ Pract Vet 1993; 15: 535–45. [Google Scholar]
- 50.Tasker S. Feline haemoplasmas — detection, infection, dynamics and distribution. PhD Thesis, University of Bristol, 2002. [Google Scholar]
- 51.Yuan C, Liang A, Yu F, et al. Eperythrozoon infection identified in an unknown aetiology anaemic patient. Ann Microbiol 2007; 57: 467–69. [Google Scholar]
- 52.Yuan CL, Liang AB, Yao CB, et al. Prevalence of Mycoplasma suis (Eperythrozoon suis) infection in swine and swine-farm workers in Shanghai, China. Am J Vet Res 2009; 70: 890–94. [DOI] [PubMed] [Google Scholar]
- 53.Santos AP, Santos RP, Biondo AW, et al. Hemoplasma infection in HIV-positive patient, Brazil. Emerg Infect Dis 2008; 14: 1922–94. [DOI] [PMC free article] [PubMed] [Google Scholar]