Abstract
OBJECTIVES
To describe cross-sectional and longitudinal associations with dehydroepiandrosterone sulfate (DHEAS) and change in DHEAS with age.
DESIGN
Longitudinal cohort study.
SETTING
Pittsburgh, Pennsylvania.
PARTICIPANTS
Cardiovascular Health Study All Stars study participants assessed in 2005/06 (N =989, mean age 85.2, 63.5% women, 16.5% African American).
MEASUREMENTS
Health characteristics were assessed in 2005/06 according to DHEAS level, mean DHEAS and DHEAS change across age categories were tested, and linear and logistic regression was used to identify factors present in 1996/97 associated with continuous and categorical DHEAS change.
RESULTS
Mean ± standard deviation DHEAS was 0.555 ± 0.414 μg/mL in 1996/97 and 0.482 ± 0.449 μg/mL in 2005/06 for women and 0.845 ± 0.520 μg/mL in 1996/97 and 0.658 ± 0.516 μg/mL in 2005/06 for men. In 2005/06, DHEAS was lower in women and subjects with cardiovascular disease (CVD) and chronic pulmonary disease and higher for African Americans and subjects with hypertension and high cholesterol. Mean DHEAS change was greater in men (− 0.200 μg/mL) than in women (− 0.078 μg/mL) (P<.001). Each 1-year increase in age attenuated the effect of male sex by 0.01 μg/mL (P =.009), abolishing the sex difference in DHEAS change by age 79. Presence of CVD before the study period was associated with greater absolute DHEAS change (β = − 0.04 μg/mL, P =.04) and with the fourth quartile of DHEAS change versus the first to third quartiles (odds ratio =1.46, 95% confidence interval =1.03–2.05).
CONCLUSION
DHEAS change continues into very old age, is not homogenous, is affected by sex, and is associated with prevalent CVD. Future studies should investigate factors that might accelerate DHEAS decline.
Keywords: dehydroepiandrosterone sulfate, cardiovascular disease, gender, aging
Dehydroepiandrosterone (DHEA) and its sulfated ester (DHEAS) are the most-abundant sex steroid hormones that the adrenal glands produce. Because 99% of DHEA is sulfated before it leaves the adrenals, DHEAS represents the majority of circulating hormone in the body.1 DHEAS peaks at age 20 and declines rapidly after age 25.2 By age 80, DHEAS levels are 10% to 20% of those in younger individuals.3 This strong correlation suggests that DHEAS could be an etiological or nonetiological biomarker of aging.
Previous studies have investigated the outcomes that DHEAS predicts and sought to define its association with mortality independent of age. Data supportive of this are conflicting.4 Earlier studies reported no association with mortality in women, whereas later studies indicated that higher DHEAS is associated with lower all-cause mortality and cardiovascular-specific mortality in men.4 More-recent reports indicate that low DHEAS is associated with greater mortality in men and women.5–7 One study found that low and high DHEAS each confer greater mortality in disabled older women (U-shaped association) and that the slope and variability of DHEAS change, rather than baseline DHEAS level, is associated with greater mortality.8,9 These reports suggest that a disordered neuro-endocrine axis or altered homeostasis increases mortality rather than a unidirectional shift in hormone levels.
Despite these previous findings, the cause of DHEAS decline remains unknown. Identifying predictors of DHEAS change would help clarify whether DHEAS reflects normal aging, certain pathophysiological processes, or extreme longevity and thus help redefine DHEAS as a potential biomarker of aging. Perhaps DHEAS decline is a consequence rather than a predictor of age-related decline. It was hypothesized that specific age-related factors would be associated with greater or lesser DHEAS decline. To define predictors and correlates of DHEAS change, DHEAS change was measured in the Cardiovascular Health Study All Stars (CHS-All Stars) cohort.
METHODS
Study Population
CHS-All Stars was designed to study the determinants of functional aging in participants of the CHS. CHS is a four-center, longitudinal, observational, community-based study of the onset, progression, and course of cardiovascular disease (CVD) in 5,888 older men and women.10,11 The CHS cohort was aged 65 and older at enrollment in 1989/90 and was supplemented with added minority participant recruitment in 1992/93. Participants and eligible household members were identified from a random sample of Medicare enrollees at each field center. To be eligible, participants had to be aged 65 and older, not have cancer under active treatment, not be wheelchair- or bed-bound in the home, and not be planning to move out of the area within 3 years.
In 2005/06, 1,677 CHS participants were re-recruited and evaluated for the CHS-All Stars study. In-person examination including DHEAS levels were performed for 989 of these participants. To measure DHEAS change, DHEAS was assessed in these 989 individuals using blood samples from 1996/97. In 1996/97, the sample was 63.5% female and 16.5% African American, and the mean ± standard deviation age was 76.3 ± 3.6. In 2005/06, the mean age was 85.2 ± 3.6. All CHS-related institutional review boards approved all procedures relating to CHS and CHS-All Stars.
Measurement of DHEAS
Plasma DHEAS was measured in samples drawn 9 years apart and analyzed as pairs in 2007. A competitive immunoassay kit (Alpco Diagnostics, Windham, NH) with interassay coefficients of variation of 3.8% to 7.2% was used.
Measurement of Health Characteristics
From the baseline CHS examination (1989/90) until 1998/99, study participants completed up to 10 annual clinic visits and semiannual phone calls, after which phone follow-up was conducted semiannually until 2005/06. Information collected at clinic visits included demographics, vital signs, anthropometric factors, medical history and behaviors, physical function, and psychosocial interviews.10 In 2005/06, CHS-All Stars examinations were conducted that included measurement of physical and cognitive function, pulse, blood pressure, anthropometric factors, and medical history. Blood was collected and stored at both time points, and analysis of fasting insulin and glucose occurred centrally.12 Body mass index (BMI) was calculated as the weight (kg)/height (m)2.
Disease present in 1996/97 was tabulated for each participant. Hypertension was defined as definite if systolic blood pressure was 140 mmHg or higher, diastolic pressure was 90 mmHg or higher, or the subject was using antihypertensive medication. Diabetes mellitus was classified using the American Diabetes Association criteria as not present, impaired fasting glucose, or diabetes mellitus.13 Cerebrovascular disease (CBVD) included stroke or transient ischemic attack. Coronary heart disease (CHD) was present if one of the following was reported and confirmed: myocardial infarction, angina pectoris, or history of angioplasty or bypass surgery. Cardiovascular disease (CVD) was present if CBVD or CHD was present. An expert panel adjudicated CVD outcomes using medical records and medication information, and prevalent CVD status was updated at each examination.14 Chronic pulmonary disease (asthma, bronchitis, or emphysema), arthritis, cancer, and kidney disease were assessed according to self-report of physician diagnosis. Depression was defined as a score greater than 10 on a modified 10-item Center for Epidemiologic Studies Depression Scale (CES-D).15,16
Statistical Analysis
Overall and sex-specific DHEAS quartiles were calculated for measurements from 2005/06. Using these quartiles we conducted a cross-sectional analysis of 2005/06 health characteristics. We tested for differences in means of continuous characteristics with analysis of variance and assessed the distribution of categorical characteristics with the χ2-test.
To assess the degree of linear relationship between age and DHEAS in 2005/06, the Pearson correlation coefficient was calculated independently for women (n =628) and men (n =361). Differences in mean DHEAS and mean DHEAS change across age and gender categories were tested using the two-sample t-test. DHEAS change was calculated as 2005/06 DHEAS minus 1996/97 DHEAS. Two outlier change values (2.23 and 4.53 μg/mL) were excluded from regression analysis because of high leverage.
It was hypothesized that health characteristics present at the first DHEAS measurement (1996/97) would be related to subsequent absolute DHEAS change, and this was evaluated using multivariable linear regression. Independent variables included demographics, smoking history, BMI, total cholesterol, and presence of disease. Based on preliminary findings of a significant interaction between age and sex (Figure 2), an age–sex interaction was additionally adjusted for in all linear models. Multivariable logistic regression was used to investigate predictors of categorical DHEAS change, which was calculated using sex-specific quartiles. Results show odds of falling into the fourth quartile versus the first to third quartiles of DHEAS change. The full model included all sociodemographic and health characteristics measured at baseline as part of this analysis. The final models included only those covariates found to be significant in the full model (P<.05). A significance level of .05 and SAS 9.2 (SAS Institute, Inc., Cary, NC) were used for all analyses.
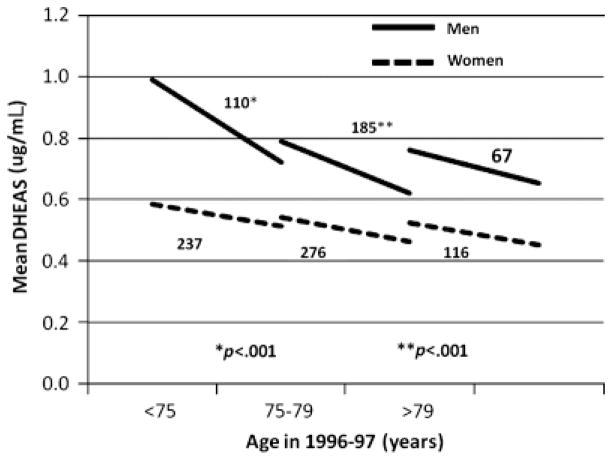
Figure 2.
Dehydroepiandrosterone sulfate change in men and women from 1996/97 to 2005/06 according to age category.
RESULTS
Cross-Sectional Analysis
Mean DHEAS was 0.555 ± 0.414 μg/mL in 1996/97 and 0.482 ± 0.449 μg/mL in 2005/06 for women and was 0.845 ± 0.520 μg/mL in 1996/97 and 0.658 ± 0.516 μg/mL in 2005/06 for men. DHEAS was correlated with age in 2005/06 in women (correlation coefficient (r) = − 0.09, P =.02) and men (r = − 0.12, P =.02). In 2005/06, DHEAS was lower for women and subjects with CVD, CHD, and chronic pulmonary disease and higher for African Americans and subjects with hypertension and high total cholesterol (Table 1).
Table 1.
Cross-Sectional Analysis of 2005/06 Health Characteristics According to Dehydroepiandrosterone Sulfate (DHEAS) Quartiles
| Health Characteristic | DHEAS Quartile* | P-Value† | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Age, mean ± SD | 85.9 ± 3.6 | 85.4 ± 3.7 | 85.0 ± 3.6 | 84.7 ± 3.3 | .001 |
| Male, n (%)‡ | 55 (22.3) | 88 (35.5) | 97 (39.1) | 122 (49.2) | <.001 |
| African American, n (%) | 31 (12.6) | 25 (10.2) | 48 (19.5) | 58 (23.6) | <.001 |
| Ever smoked, n (%) | 122 (49.4) | 116 (47.0) | 122 (49.4) | 129 (52.0) | .74 |
| Currently married, n (%) | 113 (45.8) | 101 (40.7) | 97 (39.3) | 97 (39.1) | .40 |
| Hypertension, n (%) | 161 (66.3) | 156 (63.7) | 179 (72.5) | 186 (75.0) | .02 |
| Diabetes mellitus, n (%) | 46 (18.6) | 40 (16.1) | 52 (21.1) | 46 (18.5) | .58 |
| Cardiovascular disease, n (%) | 111 (44.9) | 96 (38.7) | 87 (35.2) | 81 (32.5) | .03 |
| Coronary heart disease, n (%) | 83 (33.6) | 76 (30.7) | 61 (24.7) | 54 (21.7) | .01 |
| Cerebrovascular disease, n (%) | 51 (20.7) | 38 (15.3) | 38 (15.4) | 36 (14.5) | .23 |
| Chronic pulmonary disease, n (%) | 84 (34.0) | 61 (24.6) | 54 (21.9) | 56 (22.5) | .01 |
| Kidney disease, n (%) | 19 (7.7) | 14 (5.7) | 20 (8.1) | 12 (4.8) | .39 |
| Cancer, n (%) | 115 (46.6) | 104 (41.9) | 90 (36.4) | 96 (38.6) | .11 |
| Depression, n (%) | 53 (21.5) | 52 (21.0) | 63 (25.6) | 47 (19.0) | .34 |
| Arthritis, n (%) | 209 (84.6) | 191 (77.0) | 192 (77.7) | 191 (76.7) | .10 |
| Total cholesterol, mg/dL, mean ± SD | 174.2 ± 40.4 | 180.4 ± 40.0 | 185.2 ± 36.4 | 185.5 ± 41.2 | .004 |
| Body mass index, kg/m2, mean ± SD | 26.5 ± 4.7 | 26.8 ± 4.8 | 26.9 ± 4.8 | 26.2 ± 4.1 | .31 |
| Fasting glucose, mg/dL, median (interquartile range)§ | 94 ± 16 | 94 ± 15 | 96 ± 14 | 96 ± 17 | .14 |
Sex-specific quartiles (Q) for comparison (μg/mL) are women, Q1 =0.0100–0.2185, Q2 =0.2185–0.3731, Q3 =0.3731–0.6230, Q4 =0.6230–4.564; men, Q1 =0.0100–0.3258, Q2 =0.3258–0.5213, Q3 =0.5213–0.8376, Q4 =0.8376–5.313.
P-value for analysis of variance, chi-square test, or Fisher exact test.
Overall quartiles for comparison are (μg/mL) are Q1 =0.0100–0.2510, Q2 =0.2510–0.4311, Q3 =0.4311–0.7100, Q4 =0.7100–5.313.
Wilcoxon rank sum test used for comparison.
SD =standard deviation.
Distribution of DHEAS Change over 9 Years
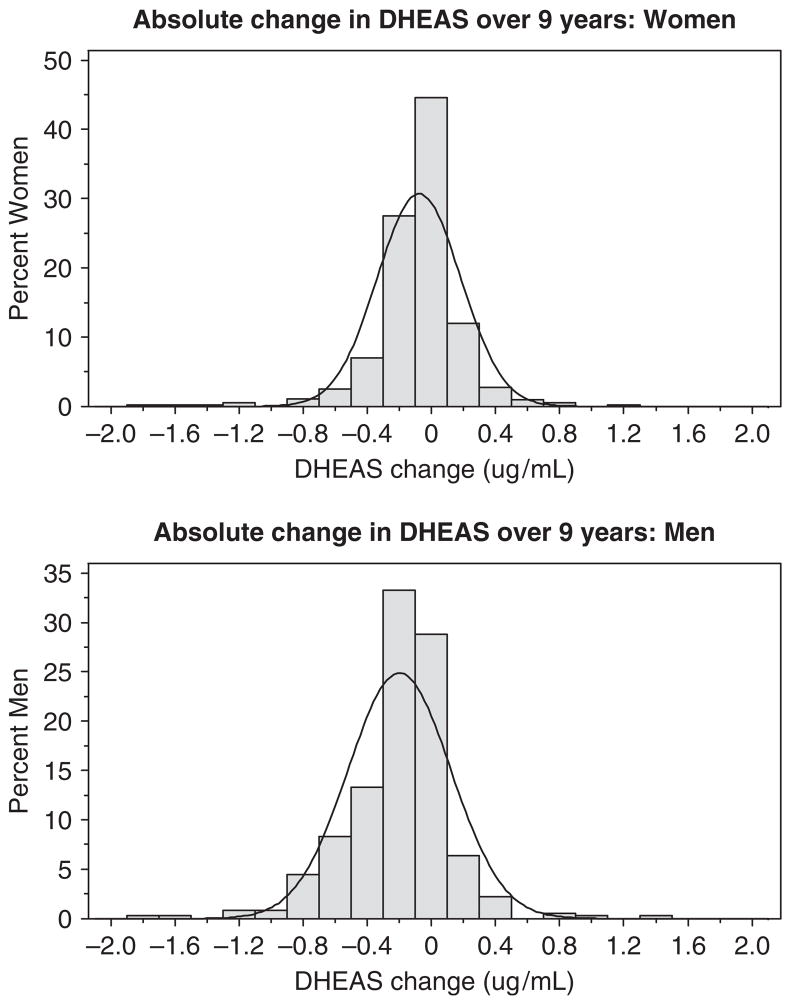
The spectrum of DHEAS change was not normally distributed; a substantial number of participants changed minimally (Figure 1). Overall, mean DHEAS change in men (− 0.200 ± 0.320 μg/mL) was greater than in women (− 0.078 ± 0.260 μg/mL) (P<.001). In subjects younger than 75, mean change was three times as great in men as in women (− 0.250 vs − 0.080 μg/mL, P<.001) (Figure 2). Mean change was again greater in men (− 0.201 vs − 0.071 μg/mL, P<.001) in subjects aged 75 to 79, but similar to women in subjects aged 80 and older.
Figure 1.
Distribution of dehydroepiandrosterone sulfate change over 9 years in women and men.
Associations with Absolute DHEAS Change in Linear Models
In the full model, male sex, African-American race, and CVD were significantly associated with absolute DHEAS change (Table 2). Thus, these covariates were retained in the final model, but in the final model, only male sex and CVD were significantly associated with absolute DHEAS change. The age–sex interaction term coefficient in the final model (0.01 μg/mL, P =.009) illustrated that, for each 1-year increase in age, the effect of male sex on DHEAS change was slightly attenuated. BMI and cholesterol were not associated with DHEAS change.
Table 2.
Linear Association Between Health Characteristics and Dehydroepiandrosterone Sulfate (DHEAS) Change (μg/mL)
| Characteristic | βchar | P-Value |
|---|---|---|
| Full model* | ||
| Demographics | ||
| Male | − 0.13 | <.001 |
| African American | 0.05 | .04 |
| Ever smoked | 0.01 | .52 |
| Currently married | 0.01 | .57 |
| Previous disease† | ||
| Hypertension | − 0.02 | .49 |
| Diabetes mellitus | − 0.07 | .23 |
| CVD | − 0.05 | .04 |
| CHD | − 0.03 | .24 |
| CBVD | − 0.07 | .19 |
| Chronic pulmonary disease | − 0.01 | .59 |
| Kidney disease | 0.00 | .98 |
| Cancer | 0.02 | .47 |
| Depression | 0.03 | .36 |
| Arthritis | − 0.02 | .35 |
| Final model‡ | ||
| Male | − 0.13 | <.001 |
| African American | 0.03 | .18 |
| CVD | − 0.04 | .04 |
β adjusted for all factors in the full model and additionally mean age, body mass index, cholesterol, and age–sex interaction. Cardiovascular disease (CVD) or coronary heart disease (CHD)/cerebrovascular disease (CBVD) was added separately.
Previous disease includes disease present before the first measurement of DHEAS in 1996/97. The reference category is absence of that disease before 1996/97.
β adjusted for all factors in the final model and additionally mean age and age–sex interaction. The final model includes only factors found to be significant in the full model.
Associations with Categorical DHEAS Change in Logistic Models
Only CVD was significantly associated with greater categorical DHEAS change (Table 3).
Table 3.
Odds of Greater Dehydroepiandrosterone Sulfate (DHEAS) Change Given Presence of Health Characteristic
| Characteristic | Odds Ratio (95% Confidence Interval)* |
|---|---|
| Full model† | |
| Demographics | |
| African American | 0.67 (0.43–1.05) |
| Ever smoked | 0.88 (0.65–1.20) |
| Currently married | 0.90 (0.65–1.24) |
| Previous disease‡ | |
| Hypertension | 1.27 (0.92–1.76) |
| Diabetes mellitus | 1.44 (0.87–2.37) |
| CVD | 1.43 (1.01–2.05) |
| CHD | 1.31 (0.89–1.93) |
| CBVD | 1.49 (0.76–2.92) |
| Chronic pulmonary disease | 0.94 (0.64–1.39) |
| Kidney disease | 1.34 (0.66–2.74) |
| Cancer | 0.94 (0.63–1.41) |
| Depression | 0.80 (0.50–1.26) |
| Arthritis | 1.15 (0.80–1.65) |
| Final model§ | |
| CVD | 1.46 (1.03–2.05) |
Odds of quartile (Q)4 vs Q1 to Q3 of DHEAS change. Sex-specific quartiles (μg/mL) are women, Q1–Q3 =0.0440 to − 1.7946, Q4 = − 1.7947 to − 0.1804; Men, Q1–Q3 = − 0.0367 to − 1.8297, Q4 = − 1.8298 to − 0.3374.
Adjusted for all factors in the full model and additionally mean age, body mass index, and cholesterol. Cardiovascular disease (CVD) or coronary heart disease (CHD)/cerebrovascular disease (CBVD) was added separately.
Previous disease includes disease present before the first measurement of DHEAS in 1996/97. The reference category is absence of that disease before 1996/97.
Additionally adjusted for mean age. The final model includes only factors found to be significant in the full model.
DISCUSSION
Although most participants in this very old cohort declined minimally, DHEAS decline was nonetheless noted in a substantial number of individuals. DHEAS was negatively correlated with age into the ninth decade of life, similar to reports in younger individuals.2–8 Cross-sectionally, lower DHEAS was associated with certain health characteristics. On average, men began at a higher DHEAS level and changed more, although the effect of sex on DHEAS change was attenuated by the age of 79. Finally, CVD was found to be associated with greater DHEAS change. Collectively, these observations indicate that DHEAS continues to change in very old individuals and that change is associated with sex and prevalent CVD.
To determine which factors might augment or ameliorate DHEAS decline, a large number of health characteristics prevalent at the start of the study period were examined. Only CVD present before the study period was associated with greater DHEAS change. Pathological research previously elucidated that the zona reticularis, which is most responsible for DHEA production, is highly susceptibility to vascular damage.17 Given these findings, it might be that DHEAS reflects underlying vascular disease manifesting as endocrine dysfunction. This might explain why some studies have found an association between low DHEAS and incident CVD; the individuals with low DHEAS might have had subclinical CVD preceding DHEAS measurement that later precipitated clinical CVD. Furthermore, this hypothesized inverse correlation between DHEAS level and subclinical CVD might explain why low DHEAS is associated with all-cause and CVD-specific mortality; in this case, low DHEAS is a surrogate for greater vascular disease, which leads to death. These speculations lead to the hypothesis that DHEAS might function as a nonetiological biomarker of aging by sensitively reflecting cumulative vascular disease burden that begins far before middle age but rarely causes vascular obstruction before age 50. These are speculations only, and these results are correlative rather than causal, necessitating that future research examine the effect of subclinical vascular disease on subsequent DHEAS change.
Although previous CVD was associated with greater decline in DHEAS, and in 2005/06, subjects in the lowest quartile of DHEAS also had the highest incidence of CVD, subjects in the lowest quartile of DHEAS had lower mean cholesterol and blood pressure. These are seemingly contradictory associations and warranted further investigation. Post hoc analysis confirmed that prevalent CVD at baseline was associated with greater use of statins, any lipid medications, or total number of medications (all P<.001) but was not associated with mean cholesterol or hypertension. Therefore, it may be that subjects who were diagnosed with CVD at baseline received more-aggressive control of blood pressure and cholesterol, which probably accounted for the observed associations.
Previous studies have noted that, on average, men have higher DHEAS than woman.4 The data from the current study indicate that this general trend continues into very old age. Additionally, the current study showed that DHEAS change is associated with sex, DHEAS changes to a greater extent in men than in women, and age modifies these sex-specific rates of change. These data suggest that DHEAS change might naturally differ according to sex or that risk factors for greater DHEAS change, such as cardiovascular disease in this study, have a higher prevalence or act more strongly in men. It was also found that, in very old age, DHEAS levels in men and women become similar. Convergence of DHEAS levels in very old age suggests that a physiological minimum of DHEAS might exist, which has not be discussed previously. This minimum could be sex-specific or not. To definitively illustrate how DHEAS changes in men and women, future studies should measure DHEAS in the same individuals as they pass through middle, old, and very old age.
Although previous studies have not chiefly examined DHEAS change, the data from the current study agree with previous observations.2–8 The current study confirmed that serum DHEAS is lower in women than in men and decreases with age. The range of DHEAS levels in this cohort, roughly 0 to 3 μg/mL, matches those documented in other studies of people of similar age. This is important because defining DHEAS in this population and identifying a possible physiological minimum of DHEAS are necessary if DHEAS might be used as an aging biomarker across the lifespan. Like us, a previous study found that DHEAS was unrelated to BMI, education, and diabetes mellitus.8 Biological data support the finding that CVD augments DHEAS decline.17 Ultimately, though, because previous studies have not examined a large number of adults with a mean age similar to that in the current study, it is difficult to compare these results directly with those previously published.
Several factors strengthen these results. First, a large sample of adults was analyzed that, on average, was older than those in previous studies.4–9 Second, there were substantial numbers of women and men, enabling sex-specific associations to be identified, including an important age–sex interaction. Third, subjects came from a large biracial community-based cohort, aiding generalizability. Fourth, chronic disease and risk factors were well characterized. Fifth, the models were robust; post hoc analysis indicated that adjustment for baseline DHEAS or mean DHEAS (a measurement with less error than baseline DHEAS) in models of DHEAS change did not affect estimates appreciably and did not alter significance. Not adjusting for baseline is also justified in change models in which the dependent variable undergoes change before the baseline measurement, as in the current study, because baseline adjustment can unduly inflate coefficient estimates.18
These analyses have several limitations. First, those who participated in the CHS-All Stars examination were somewhat younger and healthier than those who did not, and this was more apparent in those who completed an in-person examination and blood draw than those who did not.19 This would tend to bias associations between prior risk factors and DHEAS levels toward the null. The findings can be generalized to other long-lived survivors who have experienced significant declines in function during the 9-year period of observation. Additionally, the authors plan to continue to follow the cohort and to determine whether baseline DHEAS or DHEAS change is associated with mortality in the oldest old. Second, CHS-All Stars subjects by definition survived to the second study date (2005/06), and therefore the analysis is of a survival cohort. These factors could limit generalizability to the larger age-matched population with characteristically worse health and survival. Nevertheless, these limitations are inherent in studying DHEAS in the oldest old because these individuals have, by definition, outlived their birth-cohort’s life expectancy. Subsequently, it would be prudent to examine the predictors of DHEAS change identified here in younger populations, which would require repeated measurements of DHEAS in these populations.
Although it remains unclear whether DHEAS is a robust biomarker of aging, novel associations between DHEAS change, sex, and CVD have been identified. These associations help explain what DHEAS levels reflect in aging humans. From a causal perspective, because DHEAS is at the intersection of endocrine and vascular physiology, metabolism, and immune function, all of which have known mechanistic influences on aging, it is sensible to know which factors influence DHEAS change. The most informative DHEAS research requires large general cohorts with multiple measurements of a broad spectrum of molecular factors in these pathways. Provided adequate power and measurement of covariates, this would allow researchers and clinicians to identify possibly small, although clinically meaningful, associations with DHEAS and DHEAS change.
Acknowledgments
Sponsor’s Role: The funding institutes had no role in the design, methods, subject recruitment, data collection, analysis, and preparation of manuscript or in the decision to submit the manuscript for publication.
Footnotes
The work from which this manuscript was derived appeared as an abstract at the 2009 American Geriatrics Society Annual Meeting, Chicago, Illinois, April 29–May 2, where it won the Presidential Poster Award in the epidemiology category.
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Study concept and design: ARC, AMA, JR, ABN. Acquisition of subjects and/or data: ARC, JR, ABN. Laboratory analysis of samples: MC. Analysis and interpretation of data: JLS, RMB, ARC, AMA, JR, MC, ABN. Preparation of manuscript: JLS, RMB, ARC, AMA, JR, MC, ABN.
Financial Disclosure: The research reported in this article was supported by National Institute on Aging (NIA) Grant AG-023629. CHS was supported by Contracts N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, and N01-HC-45133 and Grant U01 HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by NIA Grants R01 AG-15928, R01 AG-20098, and AG-027058; NHLBI Grant R01 HL-075366; and the University of Pittsburgh Claude D. Pepper Older Americans Independence Center P30-AG-024827. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
References
- 1.Longcope C. Metabolism of dehydroepiandrosterone. Ann N Y Acad Sci. 1995;774:143–148. doi: 10.1111/j.1749-6632.1995.tb17378.x. [DOI] [PubMed] [Google Scholar]
- 2.Orentreich N, Brind JL, Rizer RL, et al. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59:551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- 3.Vermeulen A. Dehydroepiandrosterone sulfate and aging. Ann N Y Acad Sci. 1995;774:121–127. doi: 10.1111/j.1749-6632.1995.tb17376.x. [DOI] [PubMed] [Google Scholar]
- 4.Tchernof A, Labrie F. Dehydroepiandrosterone, obesity and cardiovascular disease risk: A review of human studies. Eur J Endocrinol. 2004;151:1–14. doi: 10.1530/eje.0.1510001. [DOI] [PubMed] [Google Scholar]
- 5.Maggio M, Lauretani F, Ceda GP, et al. Relationship between low levels of anabolic hormones and 6-year mortality in older men. Arch Intern Med. 2007;167:2249–2254. doi: 10.1001/archinte.167.20.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glei DA, Goldman N. Dehydroepiandrosterone sulfate (DHEAS) and risk for mortality among older Taiwanese. Ann Epidemiol. 2006;16:510–515. doi: 10.1016/j.annepidem.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Enomoto M, Adachi H, Fukami A, et al. Serum dehydroepiandrosterone sulfate levels predict longevity in men: 27-year follow-up study in a community-based cohort (Tanushimaru Study) J Am Geriatr Soc. 2008;56:994–998. doi: 10.1111/j.1532-5415.2008.01692.x. [DOI] [PubMed] [Google Scholar]
- 8.Cappola AR, Xue Q-L, Walston JD, et al. DHEAS levels and mortality in disabled older women: The Women’s Health and Aging Study I. J Gerontol A Biol Med Sci. 2006;61A:957–962. doi: 10.1093/gerona/61.9.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cappola AR, O’Meara ES, Guo W, et al. Trajectories of dehydroepiandrosterone sulfate predict mortality in older adults: The Cardiovascular Health Study. J Gerontol A Biol Med Sci. 2009;64A:1268–1274. doi: 10.1093/gerona/glp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 11.Tell GS, Fried LP, Hermanson B, et al. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 12.Cushman M, Cornell ES, Howard PR, et al. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–270. [PubMed] [Google Scholar]
- 13.American Diabetes Association. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 14.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 15.Orme J, Reis J, Herz E. Factorial and indiscriminate validity of the Center for Epidemiological Studies Depression (CES-D) scale. J Clin Psychol. 1986;42:28–33. doi: 10.1002/1097-4679(198601)42:1<28::aid-jclp2270420104>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 16.Schulz R, Beach SR, Ives DG, et al. Association between depression and mortality in older adults: The Cardiovascular Health Study. Arch Intern Med. 2000;160:1761–1768. doi: 10.1001/archinte.160.12.1761. [DOI] [PubMed] [Google Scholar]
- 17.Angeli A, Masera RG, Magri F, et al. The adrenal cortex in physiological and pathological aging: Issues of clinical relevance. J Endocrinol Invest. 1999;22:13–18. [PubMed] [Google Scholar]
- 18.Glymour MM, Weuve J, Berkman LF, et al. When is baseline adjustment useful in analysis of change? An example with education and cognitive change. Am J Epidemiol. 2005;162:267–278. doi: 10.1093/aje/kwi187. [DOI] [PubMed] [Google Scholar]
- 19.Newman AB, Arnold AM, Sachs MC, et al. Long-term function in an older cohort — The Cardiovascular Health Study All Stars study. J Am Geriatr Soc. 2009;57:432–440. doi: 10.1111/j.1532-5415.2008.02152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]