Abstract
Microtubules play a central role in centering the nucleus or mitotic in eukaryotic cells. However, despite common use of microtubules for centring, physical mechanisms can vary greatly, and depend on cell size and cell type. In the small fission yeast cells, the nucleus can be centered by pushing forces that are generated when growing microtubules hit the cell boundary. This mechanism may not be possible in larger cells, because the compressive force that microtubules can sustain are limited by buckling, so maximal force decreases with microtubule length. In a well-studied intermediate sized cell, the C. elegans fertilized egg, centrosomes are centered by cortex-attached motors that pull on microtubules. This mechanism is widely assumed to be general for larger cells. However, re-evaluation of classic experiments in a very large cell, the fertilized amphibian egg, argues against such generality. In these large eggs, movement of asters away from a part of the cell boundary that they are touching cannot be mediated by cortical pulling, because the astral microtubules are too short to reach the opposite cell boundary. A century ago, Herlant and Brachet discovered that multiple asters within a single egg center relative to the cell boundary, but also relative to each other. Here, we summarize current understanding of microtubule organization during the first cell cycle in a fertilized Xenopus egg, discuss how microtubule asters move towards the center of this very large cell, and how multiple asters shape and position themselves relative to each other.
Introduction
Eukaryotic cells come in many shapes and sizes, but a common feature is that the interphase nucleus, and the mitotic spindle, are positioned in a specific location. This is usually the center of the cell, but off-center locations are common in specific biological circumstances, such as asymmetric division during embryogenesis. The mechanism for this centering is a fundamental question of cellular organization that has long puzzled cell biologists. Microtubules appear to play a key role, perhaps because they are one of the few cellular structures whose length scale approaches that of the whole cell, and also because their rapid polymerization dynamics allow them to explore the entire cytoplasmic space1. Microtubule-based force-generating mechanisms, that use polymerization dynamics and motor proteins, are conserved in all eukaryotes, yet microtubule-based centering mechanisms may not be. One reason for this is strong physical constraints on the length scales over which potential force-generating mechanisms can operate. Because of these constraints, fundamentally different centering mechanisms may operate in cells of different sizes. Recent research has addressed centering mechanisms mainly in rather small cells, exemplified by fissions yeast (length ~10μm) and medium-sized cells, exemplified by the C. elegans fertilized egg (length ~45 μm). Here, we discuss a much larger cell, the fertilized amphibian egg, whose much larger size (length ~1200 μm in Xenopus), we argue, demands novel mechanisms. These large-cell mechanisms are interesting in their own right, and they also illuminate aspects of cytoplasmic organization that may be generally relevant.
In the small fission yeast cell, the nucleus is centered by pushing forces that are generated when microtubules growing outwards from the nucleus encounter the cell boundary2. It is thought that this mechanism cannot work to center nuclei or spindles in larger cells because the maximal compressive force that microtubules can sustain drops in proportion to the square of their length. This 1/length2 argument holds for an elastic rod in liquid; the situation may be more complex in cytoplasm because embedding an elastic rod in an elastic gel increases the compressive force it can sustain3, and microtubule bundles can also sustain larger forces. Despite these potential caveats, both fundamental constraints from buckling, and direct experimental observation, support the view that centration of asters and nuclei in C. elegans eggs is driven by pulling forces on microtubules generated by minus end directed motors (presumably dynein) attached to the cell cortex 4–6. Pulling by cortical dynein also moves nuclei in small budding yeast cells7, though the strong asymmetry of those cells, associated with the budding cycle, make them not directly applicable to a discussion of centering. Dynein activity is also required for mitotic spindle and nucleus centering in mammalian tissue culture cells 8, 9. Because of that kind of data, and the elegance of the C. elegans work, pulling by minus end directed motors at the cortex seems to be accepted as the general mechanism of centering in larger cells10, 11. By reviewing older work, confirmed with new micrographs, we show that the cortical pulling model for aster centering cannot hold in a very large cell, the fertilized amphibian egg. We discuss alternative models, and their broader implications for cytoplasmic organization.
Microtubule organization during the first cell cycle in fertilized Xenopus eggs
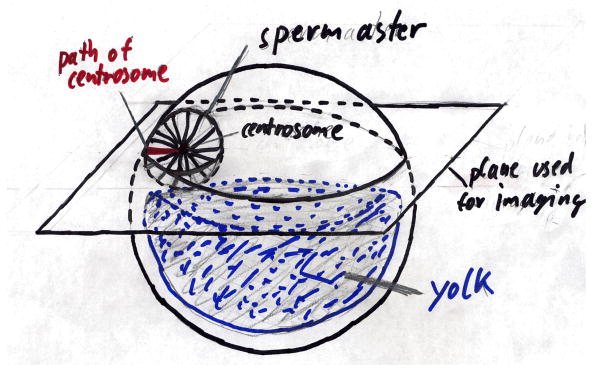
To set the stage for a discussion of centering mechanisms, we will first review microtubule organization during the first cell cycle in fertilized eggs of the clawed frog Xenopus laevis. Egg diameter in this species is ~ 1200 μm, which is ~ two orders of magnitude larger in length than typical cells in animal tissues12. Some amphibian have even larger eggs13. Time is normalized to fertilization (defined as 0) and first cleavage (defined as 1)14. Typical absolute values for the 0–1 interval is ~90 min at 23°C. The lower portion of a Xenopus egg is packed with large yolk granules, creating a density asymmetry that makes the egg orient under gravity15. The lowest part of the egg is called the vegetal pole, and the upper part the animal pole. In Xenopus, the animal half of the egg is brown-black due dark pigment in the cortex, while the vegetal half is white. Because of the packed yolk in the vegetal half of the egg, the distribution of free cytoplasm is not spherical, but looks more like a flattened hemisphere (Fig. 1). In discussing models for centering, we will neglect the vertical dimension, and concentrate on a horizontal plane through this hemisphere of cytoplasm, as shown in figure 1. All the following picture and cartoons depict positioning and movement in this plane. Asters can also move somewhat in the vertical plane, and we assume they do so by mechanisms similar to movement in the horizontal plane.
Figure 1.
Cartoon of a frog egg shortly after fertilization. The vegetal part (bottom) is heavily filled with yolk. The sperm enters randomly at the animal (top) part of the egg. The radial grow of the sperm aster leads to the movement of the centrosome towards the cell’s center. All microgrographs in this paper were taken in the plane shown.
Before fertilization, the egg is arrested in metaphase of meiosis II with a relatively small meiotic spindle attached to the cortex at the animal pole of the egg16. Sophisticated mechanisms ensure that only one sperm enters the egg, in the animal hemisphere17. Fertilization generates a wave of elevated Ca++ in the cytoplasm that triggers anaphase in the meiotic spindle followed by extrusion of half the maternal DNA into the second polar body. The sperm carries the male DNA and two centrioles, which form a microtubule organizing center that initiates outgrowth of a dense radial array of microtubules with their plus ends presumably oriented outwards18. This structure is called the sperm aster (Fig. 1 and 2A). The sperm aster’s diameter increases at a rate we estimate from images of embryos fixed at different times as ~30 μm/min. By ~0.5 the sperm aster grows to the point that its plus ends come close to the cortex all around the circle defined by the plane in figure 15. By this time, the centrosomes have moved towards the center of the cell (Fig. 2B)19, 20. The centering is not perfect; they tend to be closer to the site of sperm entry than the opposite side (Fig 2B and C), but it is clear they have moved a long way from where the sperm entered the egg, at least 300 μm in most cases. Below, we will discuss models for how this centering movement of the centrosomes might be driven.
Figure 2.
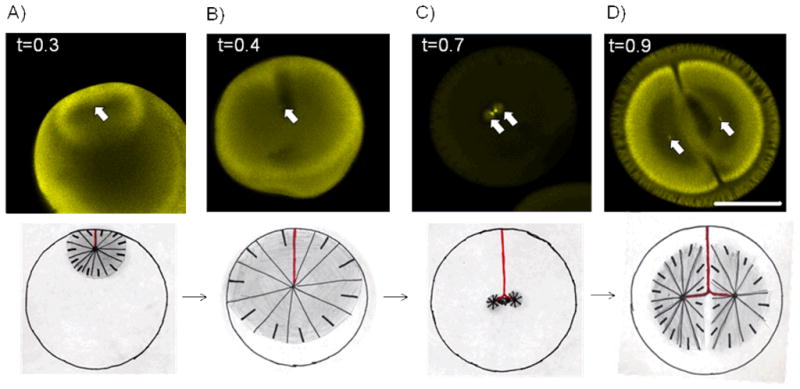
Overview of microtubule organization during the first cell cycle in X. laevis. Top: Immunostaining against tubulin, bar = 500 μm. Arrows indicate positions of centrosomes. Time (t) is normalized to first cleavage. Bottom: cartoon of corresponding time with the path of the centrosome in red and microtubules in black. A) and B) Growth of sperm aster moves centrosome towards the center of the cell. C) After sperm aster breaks down first mitotic spindle forms D) At telophase the astral microtubules grow out and the centrosomes are moved to the centers of the future daughter cells.
As soon a microtubules from the sperm aster reach the female nucleus, it starts to move towards the center of the aster, presumably pulled by dynein attached to the nuclear envelope21. In this way, the male and female pronuclei meet close to the centrosomes.
As the first mitosis is initiated, both nuclear envelopes, and the sperm aster, disassemble, and a mitotic spindle assembles (Fig. 2C)22. In smaller cells, such as the C. elegans egg, the mitotic spindle finds the center of the cell using long astral microtubules9, but in the frog egg is clear that the sperm aster is responsible for moving both the centrosomes and the DNA to approximately the cell’s center, and the spindle then forms in that spot. The metaphase spindle probably could not center itself in the frog egg, because its astral microtubules are much shorter than the radius of the egg (Figure 2C)22, 23.
At anaphase, the sister chromatids separate, and the astral microtubules of the spindle start to grow out rapidly; again we estimate an elongation rate of ~15μm/min based on fixed images. Anaphase chromosome movement presumably starts with a conventional, kinetochore-based anaphase-A. Anaphase-B movement in these large egg cells is atypical, presumably to allow a large segregation distance when spindles are small relative to the egg. The sister DNA masses move apart rapidly over a distance much larger than the metaphase spindle length, the reach a position ~half way between the center of the egg and its periphery (figure 2D). This requires that the DNA masses move ~250 μm in ~25 min. Approximately half this movement occurs while the DNA is still condensed, and half after the nuclear envelope has reformed23. The origin of the forces that drive and direct this large anaphase-telophase segregation movement are unclear. The centrosomes are positioned a few tens of microns ahead of the moving nuclei, and appear to be pulling them, but it is far from clear why the centrosomes move apart in a straight line that is parallel to the spindle axis. Because these movements can be viewed as asters moving towards the center of a volume of cytoplasm, we suspect they may be driven by the same forces that cause centering of the sperm aster, which we discuss below.
The paired asters, we here call telophase-asters, originate in the centrosomes of the anaphase mitotic spindle and not only move the sister nuclei apart but are also responsible for determining the cleavage plane. It is believed that the site of cleavage furrow ingression is specified by a line along the cortex, normal to the direction of chromosome segregation, where the two antiparallel arrays of microtubules from the pair of asters come together the cortex 24.
Besides their role in cell division and chromosome separation, microtubules are also involved in determining the future dorso-ventral axis of the embryo. In this paper we would like to concentrate on microtubules involved in centering. Detailed descriptions of microtubules involved in setting up dorso-ventral axes are presented elsewhere25–27.
An interesting aspect of the organization of both the sperm aster (Fig. 2A and B), and the subsequent telophase asters (Fig. 2D) is that they appear hollow in tubulin immunofluorescence images28, as if many of the microtubules in the periphery of the aster do not have their minus ends located near the centrosome. We do not think this hollow aster image is an artifact of fixation or stain penetration, because higher microtubules density close to the outline of the asters can not only be seen near the egg’s surface but also deep inside (Fig. 2D and 4 B). Also, we think it physically impossible that all the microtubules with plus ends at the periphery of the aster could have minus ends close to the centrosome, because of physical packing constraints. If all microtubules were continuous from center to periphery, their density in a plane tangential to the aster would have to scale as 1/radius2 as the plane moved outwards from the center, and as 1/radius in a plane that cut through the center of the aster. Our immunofluorescence images are completely inconsistent with this relationship, since the asters get brighter towards the outside not dimmer. We presume a subset of the astral microtubules are nucleated at centrosomes and run continuously out to the periphery, since the centrosome stays in the center of the aster as the aster moves and expands, implying the centrosome is physically connected to the aster periphery. But we believe that the majority of microtubules in these asters must have a different origin. Perhaps they are nucleated from the sides of existing microtubules, pointing in the same direction, for example. Microtubules are nucleated in the absence of centrosomes in egg meiotic spindles, and in this case too it may be important that new microtubules point in the same direction as the majority of microtubules near them, to preserve the gradient of polarity in each half spindle29. We suspect that both situations require a biochemical mechanism that nucleates new microtubules in the vicinity of old ones, and pointing in the same direction.
Figure 4.
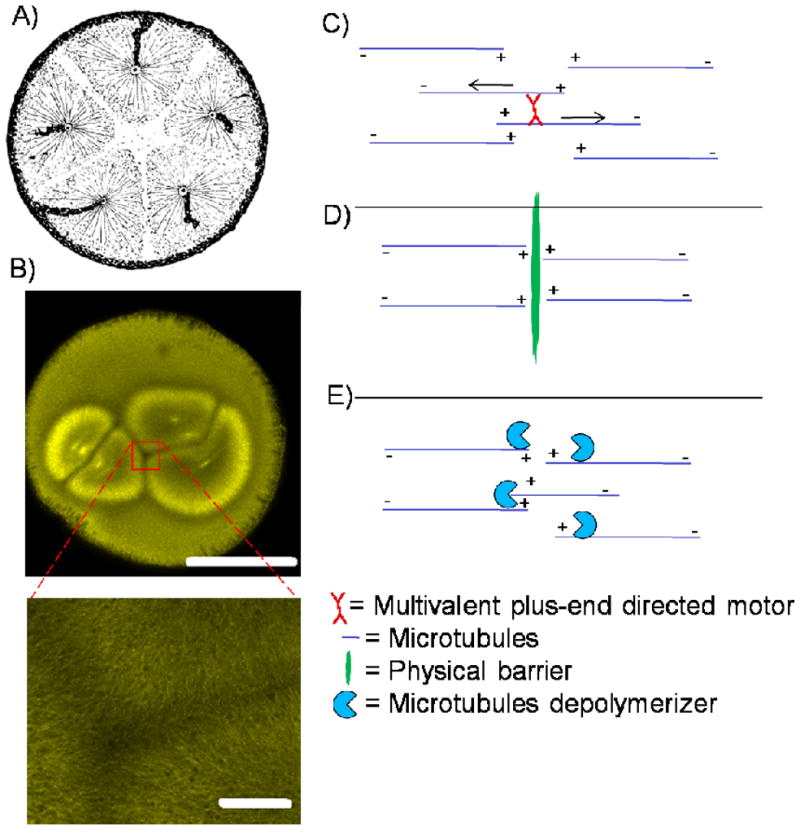
How do asters notice each other? A) Sperm asters in polyspermic embryo space each other apart creating microtubule-sparse regions between them. Reprint by Herlant and Brachet (1910), kind permission of Springer Link.37 B) Immunostaining of telophase in a di-spermic embryo with magnification of region between asters. Bars are 500 μm and 50 μm. C to E: Models that could explain how asters could notice each other: C) Multivalent plus-end directed motors push asters apart. D) A physical barrier is created between asters. E) Orientation-dependent microtubule depolymerizer chews up microtubules that enter with opposing polarity.
How might asters center?
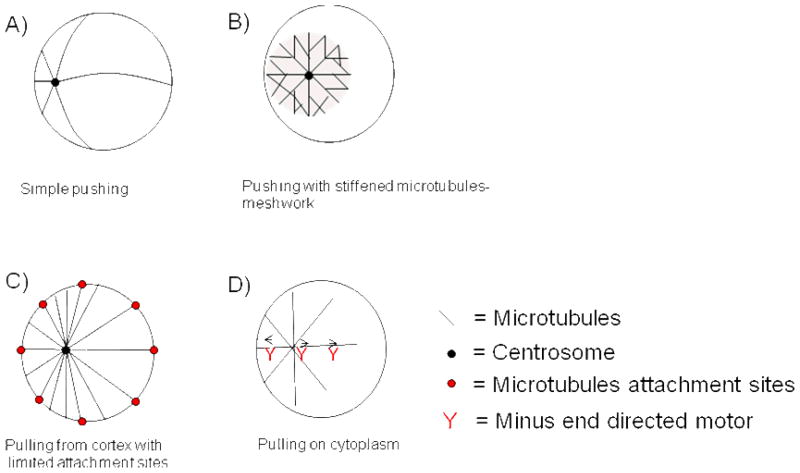
As discussed above, both the sperm aster and the telophase asters move towards the center of a volume of cytoplasm that is not occupied by microtubules. In the case of the sperm aster, it alone occupies that volume, and it moves to the center, while in telophase two asters move apart from each other, to a position half way between the periphery and the other aster. We will now evaluate four potential models to account for the forces that drive and direct these centralizing movements: A) Simple microtubule pushing B) Pushing with a stiffened microtubule meshwork C) Pulling from the cortex with limited attachment sites. D) Pulling on the cytoplasm.
A) Simple microtubule pushing
In this model, individual astral microtubules nucleated from the centrosome push against the cortex or yolk granules in the cytoplasm 30. The pushing force is generated by plus end polymerization against a barrier, which is believed to be the main mechanism for centering the interphase nucleus in the fission yeast S. pombe2. The compressive force a microtubule can sustain before it buckles, called the Euler force (Fe) is given by30:
| 1) |
Where R is the radius of the rod, L isits length and C is a material-dependent constant. Centering can be achieved by one of two mechanisms: either the microtubule only push off against one side because that side is closer, or they push off against both sides, but the force is less on the long side because microtubules have a greater tendency to buckle on the long side (Fig. 3A). The estimated force required to move an intermediate sized aster with 200 μm radius through the cytoplasm at observed rates was ~100 pN31. For comparison, the maximal force that can be transmitted by a single microtubule 200 μm long is ~8 fN31. One therefore would need ~ 12,000 microtubules pointing approximately in the right direction to overcome the viscous drag on the aster. This is thought to be unlikely31. This model also predicts that the speed of aster movement decreases as it moves away from the cortex where the sperm entered the egg. This is because the aster-size increases (and therefore its drag) but the compressive force that the pushing microtubules can sustain decreases (because their length increases). This is inconsistent with observations that sperm and telophase aster velocity is approximately constant throughout their movement 14, 19, 23. By these arguments, the simple microtubule pushing model is probably unrealistic
Figure 3.
Models on how asters could find the center of a very large cell. A) In the simple pushing model the force that can be transmitted via microtubules is inversely proportional to the square of their length.30 B) In the pushing with stiffened microtubules meshwork model the aster is stiffened via bundling, crosslinking or embedding in an elastic gel 3. Microtubules can transfer relevant pushing forces over long distance. C) In the pulling on the cortex with limited attachment sites model minus end directed motors are saturated with microtubules. The sum of forces on the centrosome points towards the cell’s center.6 D) In the pulling on the cytoplasm model minus-end directed motors are attached to a component of the cytoplasm (e.g. yolk or cytoskeleton). The longer a microtubule, the more motors are pulling which leads to centering.31, 34
B) Pushing with a stiffened microtubule meshwork
despite the arguments in A), we think pushing models cannot be ruled out yet. It seems feasible that a stiffened microtubules meshwork might be able to transfer large pushing forces over long distances.
If rods are bundled together so tightly that sliding between them is blocked, the effective diameter of the new rod is proportional to the square root of the number of microtubules (n) used. From formula 1 it follows that the Euler force Fe is then proportional to n2. For differently connected microtubules, like in a meshwork, the Euler buckling formula no longer applies. When microtubles are embedded in an elastic network, e.g. of actin filaments, they no longer buckle as a single curve when compressed, but rather in a series of bends whose spacing depends on the properties of the network. This allows even a single microtubule to bear a much larger compressive load 3.
We do not know the structure of the sperm aster, and whether the microtubules within it are bundled, crosslinked, or embedded in a network of actin or intermeditate filaments. The hollow appearance of asters in immunofluorescence, discussed above, is inconsistent with the single microtubule pushing models in A), and perhaps consistent with a physical picture of asters as a cross-linked stiffened meshwork. We could imagine that a stiff aster blows up like a balloon inside the sphere of cytoplasm (Fig. 3C). Soon after fertilization the sperm aster only touches one side of the cell boundary, so it naturally centers as it expands. As soon as the aster fills up the whole cell, forces are approximately equal, until the aster breaks down. In telophase one could therefore imagine two balloons inside one cell that blow up and center and repel each other at the same time.
We posit this model to encourage discussion. We note that a very stiff aster should be spherical, but this is not what we observe, especially in telophase where the two asters seem to delineate a plane where they meet at the cell center (Fig. 2D).
C) Pulling from the cortex with limited attachment sites
pulling on microtubules by cortical dynein is a well established mechanism for moving asters in C. elegans and budding yeast4, 5, 7. A problem with naive cortical pulling models is that they tend to decenter asters, not center them. As more microtubules are likely to hit the closer boundary than the one that is further away. A model where all microtubules that touch the cortex generate pulling forces causes movement of the aster towards the boundary11.
Grill and Hyman proposed an elegant solution, that only a limited number of motors or anchoring sites exist on the cortex 6. In this model, cortical motors are saturated with microtubules, so more net pulling force is generated on the side that faces away from the center (Fig. 3B). Laser cutting experiments suggest that this model is a good description for the early cells (~ 45 μm) in C. elegans5 and evidence from variance of pulling forces was consistent with limited motor numbers in that system32. Presumably persuaded by the C. elegans and yeast data, most students of the centering problem now seem to consider cortical pulling model the general solution, ad fission yeast cells the small length scale exception10, 11. In our opinion, however, cortical pulling models cannot explain sperm and telophase aster movement in amphibian eggs, because the asters start moving well before microtubule reach the opposite cortex (e.g. Fig. 2A and D). In Xenopus, unlike in C. elegans, the sperm centrosome and its aster start moving towards the cell center shortly after fertilization, well before microtubles reach the opposite cortex in immunofluorescence views (Fig. 2A and B). It is very hard to imagine that any aster microtubules can extend ~ 1200 μm within less than 10 minutes. All the microtubules we visualize by (admittedly crude) immunofluorescence imaging appear to grow out as a fairly homogeneous front at the surface of the aster. We see no evidence for a subset of faster and longer microtubules, and know of no mechanism by which a subset could grow much faster than the bulk population. Our estimate of aster expansion rate in the egg (~30μm/min increase of aster diameter => ~15μm/min microtubules growth rate) is fairly similar to the measured single microtubule plus-end growth rate in interphase egg extracts (~15μm/min33).
Another experiment that strongly argues against pulling from the cortex was performed by Herlant and Brachet ~ 100 years ago. A frog egg was fertilized with multiple sperms. Each sperm triggered the growth of its own sperm aster. The asters centered relative to the cellular boundary but also relative to each other (Fig. 4A). Therefore the asters had to move away from the cortex that they were touching towards cortex they were not touching. This behavior is inconsistent with pulling from the cortex as the main or only centering mechanism. We will discuss how asters notice each other further below.
D) Pulling on the cytoplasm
In this model, a molecular motor that is able to move towards the microtubules minus-end (presumably dynein) is distributed throughout the cytoplasm and attached to something e.g. ER, yolk, or other cytoskeletal polymers (Figure 3D)31, 34. The longer the microtubules, the more motors it engages, leading to a length-dependent pulling force, and therefore an attractively simple centering mechanism. This is similar to the mechanism proposed by Hays and Salmon for centering chromosomes in metaphase spindles in insect spermatocytes35. In C. elegans, yolk particles are transported to the center of the aster in a dynein-dependent way36. The drag forces generated by this movement could generate the required force. But cytoskeletal components such as intermediate filaments or actin also seem plausible as motor-anchoring elements. How could such elements be elastic enough to be pulled on but at the same time allow the sperm aster to move through it? Perhaps dynamic structures could fulfill both requirements. A beautiful experiment performed in Sand Dollar embryos 34 is consistent with this model, and inconsistent with A to C above: Hamaguchi and Hiramoto incubated the fertilized egg in the microtubule depolymerizing drug colcemid, which is readily converted into an inactive derivative, lumicolcemid, by 360nm light. Illuminating parts of the embryo with UV light generated defined regions in which microtubule growth was allowed. The sperm aster moved to the center of these UV-treated regions, and followed the UV-treated regions when it was moved. This worked independent of whether or not the region contained any cell cortex. This model, which we currently favor, focuses attention on the question of the physical nature of the egg cytoplasm, which must somehow be solid enough to sustain pulling forces, but liquid enough to allow aster movement. A potential clue to how this is possible is the nature of the trail left in the cytoplasm as the sperm aster moves through it. This trail is easily visualized in light micrographs as a region of disturbed cytoplasm that persists for many minutes. Perhaps centrosomes can somehow melt the cytoplasm in their immediate vicinity, which then re-solidifies as the move on, leaving behind a long-lasting imprint of their passage.
How do asters interact with each other?
The behavior of multiple asters from a poly-spermic fertilization is interesting in its own right (Fig. 4A) 37, 38, and we think the way asters interact in this situation may also be relevant to normal telophase asters, where a pair of asters from the two spindle poles interact at the presumptive cleavage plane (Fig. 2D). To allow comparison of these situations, we show an image of telophase asters at the end of the first mitosis in a dispermic embryo (Fig. 4B). Likely similarity between the normal interaction of paired asters during telophase, and the abnormal interaction due to polyspermy, is evident from the similarity of all the aster boundaries. Microtubules from the different asters do not seem to inter-penetrate, rather they delineate a plane between interacting asters (which appears as a line in confocal micrographs) where the microtubule density is lower. Asters appear to treat this aster-aster boundary in a manner similar to the aster-cortex boundary, in that their centers move away from both kind of boundaries, to center in the space delineated by the combination of cell boundaries and aster-aster boundaries. Aster trajectories can be visualized in figure 4A by the trail that is left in the egg cytoplasm along the path followed by each centrosome towards the center of its own territory. This centering movement leads to rather precise spacing of asters in the polyspermic condition (Fig. 4A), and, we argue, directs the movement of the telophase asters away from each other in a straight line during normal telophase. The telophase aster-aster boundary is also of interest because initiates the cleavage furrow where it touches the cortex, as discussed above. A century has passed since the regular spacing of polyspermic asters was described, and to our knowledge, the underlying mechanism is not understood. Based on modern understanding of microtubule organization, we propose three classes of model for discussion that are not mutually exclusive. The first two are related to mechanisms that have been discussed for formation of midzone and phragmoplast microtubule arrays during, respectively, animal and plant cytokinesis. However we note that midzones and phragmoplasts are probably based on anti-parallel bundles at the midline, while the aster-aster interaction is characterized by a lower density at the midline.
Multivalent, plus-end directed motors could push microtubules of opposing polarity apart (Fig. 4C). This function is analogous to the proposed function of the MKLP1-RACGAP1 complex in animal cytokinesis39, and it may be worth looking for those proteins, or others plus end directed cytokinesis mostors, between asters.
A physical barrier is assembled between two neighboring asters or within an aster (Fig. 4D). For example, plus end-directed transport might lead to accumulation of vesicles between asters. This proposal is related to the telophase disc model for midzone organization during cytokinesis40. Consistent with it, a poorly defined physical structure of some kind, called the diastema, has been observed between telophase asters in eggs41, 42. Dense accumulation of membranous organelles at plus ends of telophase microtubules was noted in a recent study of monopolar cytokinesis43. Concievable, a dense wall of vesicles could act as a physical barrier to microtubule polymerization, and direct aster movement as we discussed for the cortex in centring models B and D above.
Factors attached to astral microtubules (or to vesicles they accumulate) could depolymerize microtubules of opposite polarity, leading to the microtubule-free zone between asters (Fig. 4E). This mechanism would not generate the kind of physical barrier required for aster centering by pushing models A and B above, but it would suffice to control microtubule length in such as way as to allow centering by model D. Support for this model might come from localization of known depolymerization factors such as Kinesin-8 and -13 depolymerases44.
Conclusion
Studying how very large cells find their centers is interesting in its own right, and it also provides an original perspective on a universal problem. Because of their large sizes, amphibian eggs were among the first model organisms for cell division a century ago and earlier. These early studies have been largely forgotten in the modern obsession with molecular details and the difficulty of real-time imaging makes amphibian eggs challenging for modern methods. Nevertheless, it should be feasible to distinguish pushing versus pulling models for aster centering, for example using localized perturbation of microtubules dynamics8. Elucidating how asters sense, and repel, each other at their common boundaries may be more difficult. We suggest above some molecular candidates whose localization and function could be tested, and reconstitution of this phenomenon might be feasible in egg extracts. Physical extremes, in this case a very large cytoplasm, are always interesting in biology. Amphibian eggs, while challenging to work with, may provide insights that were missed in smaller, more transparent cells.
Materials and Methods
Immunostaining and handling of embryos was performed as described 23. Dispermic embryo was obtained accidently.
Acknowledgments
We would like to thank Stefan Grill and Yifat Merbl for reading the manuscript, Will Ludington, Horatiu Fantana, Frank Julicher, Jagesh Shah, Andrew Murray, Marc Kirschner, Cell division group Woods Hole and people in the Mitchison lab for helpful suggestions and discussion, Nikon Imaging Center (HMS) and Angela DePace for help with imaging. DJN was supported by the Life Sciences Research Foundation, sponsored by Novartis. This work was supported by the National Institutes of Health (NIH) grant GM39565
References
- 1.Mitchison TJ, Kirschner MW. Properties of the kinetochore in vitro. II. Microtubule capture and ATP-dependent translocation. J Cell Biol. 1985;101:766–77. doi: 10.1083/jcb.101.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tran PT, Marsh L, Doye V, Inoue S, Chang F. A mechanism for nuclear positioning in fission yeast based on microtubule pushing. J Cell Biol. 2001;153:397–411. doi: 10.1083/jcb.153.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brangwynne CP, MacKintosh FC, Kumar S, Geisse NA, Talbot J, Mahadevan L, Parker KK, Ingber DE, Weitz DA. Microtubules can bear enhanced compressive loads in living cells because of lateral reinforcement. J Cell Biol. 2006;173:733–41. doi: 10.1083/jcb.200601060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grill SW, Gonczy P, Stelzer EH, Hyman AA. Polarity controls forces governing asymmetric spindle positioning in the Caenorhabditis elegans embryo. Nature. 2001;409:630–3. doi: 10.1038/35054572. [DOI] [PubMed] [Google Scholar]
- 5.Grill SW, Howard J, Schaffer E, Stelzer EH, Hyman AA. The distribution of active force generators controls mitotic spindle position. Science. 2003;301:518–21. doi: 10.1126/science.1086560. [DOI] [PubMed] [Google Scholar]
- 6.Grill SW, Hyman AA. Spindle positioning by cortical pulling forces. Dev Cell. 2005;8:461–5. doi: 10.1016/j.devcel.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Adames NR, Cooper JA. Microtubule interactions with the cell cortex causing nuclear movements in Saccharomyces cerevisiae. J Cell Biol. 2000;149:863–74. doi: 10.1083/jcb.149.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burakov A, Nadezhdina E, Slepchenko B, Rodionov V. Centrosome positioning in interphase cells. J Cell Biol. 2003;162:963–9. doi: 10.1083/jcb.200305082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connell CB, Wang YL. Mammalian spindle orientation and position respond to changes in cell shape in a dynein-dependent fashion. Mol Biol Cell. 2000;11:1765–74. doi: 10.1091/mbc.11.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallee RB, Stehman SA. How dynein helps the cell find its center: a servomechanical model. Trends Cell Biol. 2005;15:288–94. doi: 10.1016/j.tcb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Dogterom M, Kerssemakers JW, Romet-Lemonne G, Janson ME. Force generation by dynamic microtubules. Curr Opin Cell Biol. 2005;17:67–74. doi: 10.1016/j.ceb.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Montorzi M, Burgos MH, Falchuk KH. Xenopus laevis embryo development: arrest of epidermal cell differentiation by the chelating agent 1,10-phenanthroline. Mol Reprod Dev. 2000;55:75–82. doi: 10.1002/(SICI)1098-2795(200001)55:1<75::AID-MRD10>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 13.Elinson RP, Beckham Y. Development in frogs with large eggs and the origin of amniotes. Zoology. 2002;105:105–17. doi: 10.1078/0944-2006-00060. [DOI] [PubMed] [Google Scholar]
- 14.Ubbels GA, Hara K, Koster CH, Kirschner MW. Evidence for a functional role of the cytoskeleton in determination of the dorsoventral axis in Xenopus laevis eggs. J Embryol Exp Morphol. 1983;77:15–37. [PubMed] [Google Scholar]
- 15.Hertwig O. Ueber den Werth der ersten Furchungszellen f ur die Organbildung des Embryo. Experimentelle Studien am Frosch- und Tritonei. Arch mikr Anat. 1893;xlii:662–807. [Google Scholar]
- 16.Weber KL, Sokac AM, Berg JS, Cheney RE, Bement WM. A microtubule-binding myosin required for nuclear anchoring and spindle assembly. Nature. 2004;431:325–9. doi: 10.1038/nature02834. [DOI] [PubMed] [Google Scholar]
- 17.Wong JL, Wessel GM. Defending the zygote: search for the ancestral animal block to polyspermy. Curr Top Dev Biol. 2006;72:1–151. doi: 10.1016/S0070-2153(05)72001-9. [DOI] [PubMed] [Google Scholar]
- 18.Felix MA, Antony C, Wright M, Maro B. Centrosome assembly in vitro: role of gamma-tubulin recruitment in Xenopus sperm aster formation. J Cell Biol. 1994;124:19–31. doi: 10.1083/jcb.124.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewartsavage J, Grey RD. THE TEMPORAL AND SPATIAL RELATIONSHIPS BETWEEN CORTICAL CONTRACTION, SPERM TRAIL FORMATION, AND PRONUCLEAR MIGRATION IN FERTILIZED XENOPUS EGGS. Wilhelm Rouxs Archives of Developmental Biology. 1982;191:241–5. doi: 10.1007/BF00848411. [DOI] [PubMed] [Google Scholar]
- 20.Hausen P, Riebesell M. The early development of Xenopus laevis: an atlas of the histology. Springer-Verlag; 1991. [Google Scholar]
- 21.Reinsch S, Karsenti E. Movement of nuclei along microtubules in Xenopus egg extracts. Curr Biol. 1997;7:211–4. doi: 10.1016/s0960-9822(97)70092-7. [DOI] [PubMed] [Google Scholar]
- 22.Cha B, Cassimeris L, Gard DL. XMAP230 is required for normal spindle assembly in vivo and in vitro. J Cell Sci. 1999;112 (Pt 23):4337–46. doi: 10.1242/jcs.112.23.4337. [DOI] [PubMed] [Google Scholar]
- 23.Wuhr M, Chen Y, Dumont S, Groen AC, Needleman DJ, Salic A, Mitchison TJ. Evidence for an upper limit to mitotic spindle length. Curr Biol. 2008;18:1256–61. doi: 10.1016/j.cub.2008.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rappaport R. Experiments concerning the cleavage stimulus in sand dollar eggs. J Exp Zool. 1961;148:81–9. doi: 10.1002/jez.1401480107. [DOI] [PubMed] [Google Scholar]
- 25.Houliston E, Elinson RP. Patterns of microtubule polymerization relating to cortical rotation in Xenopus laevis eggs. Development. 1991;112:107–17. doi: 10.1242/dev.112.1.107. [DOI] [PubMed] [Google Scholar]
- 26.Weaver C, Kimelman D. Move it or lose it: axis specification in Xenopus. Development. 2004;131:3491–9. doi: 10.1242/dev.01284. [DOI] [PubMed] [Google Scholar]
- 27.Houliston E, Elinson RP. Microtubules and cytoplasmic reorganization in the frog egg. Curr Top Dev Biol. 1992;26:53–70. doi: 10.1016/s0070-2153(08)60440-8. [DOI] [PubMed] [Google Scholar]
- 28.Gard DL, Cha BJ, Schroeder MM. Confocal immunofluorescence microscopy of microtubules, microtubule-associated proteins, and microtubule-organizing centers during amphibian oogenesis and early development. Curr Top Dev Biol. 1995;31:383–431. doi: 10.1016/s0070-2153(08)60234-3. [DOI] [PubMed] [Google Scholar]
- 29.Burbank KS, Groen AC, Perlman ZE, Fisher DS, Mitchison TJ. A new method reveals microtubule minus ends throughout the meiotic spindle. J Cell Biol. 2006;175:369–75. doi: 10.1083/jcb.200511112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjerknes M. Physical theory of the orientation of astral mitotic spindles. Science. 1986;234:1413–6. doi: 10.1126/science.3787253. [DOI] [PubMed] [Google Scholar]
- 31.Reinsch S, Gonczy P. Mechanisms of nuclear positioning. J Cell Sci. 1998;111 (Pt 16):2283–95. doi: 10.1242/jcs.111.16.2283. [DOI] [PubMed] [Google Scholar]
- 32.Pecreaux J, Roper JC, Kruse K, Julicher F, Hyman AA, Grill SW, Howard J. Spindle oscillations during asymmetric cell division require a threshold number of active cortical force generators. Curr Biol. 2006;16:2111–22. doi: 10.1016/j.cub.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 33.Tournebize R, Popov A, Kinoshita K, Ashford AJ, Rybina S, Pozniakovsky A, Mayer TU, Walczak CE, Karsenti E, Hyman AA. Control of microtubule dynamics by the antagonistic activities of XMAP215 and XKCM1 in Xenopus egg extracts. Nat Cell Biol. 2000;2:13–9. doi: 10.1038/71330. [DOI] [PubMed] [Google Scholar]
- 34.Hamaguchi MS, Hiramoto Y. ANALYSIS OF THE ROLE OF ASTRAL RAYS IN PRONUCLEAR MIGRATION IN SAND DOLLAR EGGS BY THE COLCEMID-UV METHOD. Development Growth & Differentiation. 1986;28:143–56. doi: 10.1111/j.1440-169X.1986.00143.x. [DOI] [PubMed] [Google Scholar]
- 35.Hays TS, Wise D, Salmon ED. Traction force on a kinetochore at metaphase acts as a linear function of kinetochore fiber length. J Cell Biol. 1982;93:374–89. doi: 10.1083/jcb.93.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonczy P, Pichler S, Kirkham M, Hyman AA. Cytoplasmic dynein is required for distinct aspects of MTOC positioning, including centrosome separation, in the one cell stage Caenorhabditis elegans embryo. J Cell Biol. 1999;147:135–50. doi: 10.1083/jcb.147.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brachet A. Experimental polyspermy as a means of analysis of fecundation. Arch Entwicklungsmech Org. 1910;30:261–303. [Google Scholar]
- 38.Herlant M. Recherches sur les oeufs di-et-trispermiques de grenouille. Archs Biol. 1911;26:103–328. [Google Scholar]
- 39.Glotzer M. The 3Ms of central spindle assembly: microtubules, motors and MAPs. Nat Rev Mol Cell Biol. 2009;10:9–20. doi: 10.1038/nrm2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andreassen PR, Palmer DK, Wener MH, Margolis RL. Telophase disc: a new mammalian mitotic organelle that bisects telophase cells with a possible function in cytokinesis. J Cell Sci. 1991;99 (Pt 3):523–34. doi: 10.1242/jcs.99.3.523. [DOI] [PubMed] [Google Scholar]
- 41.Selman GG. DETERMINATION OF THE 1ST 2 CLEAVAGE FURROWS IN DEVELOPING EGGS OF TRITURUS-ALPESTRIS COMPARED WITH OTHER FORMS. Development Growth & Differentiation. 1982;24:1–6. doi: 10.1111/j.1440-169X.1982.00001.x. [DOI] [PubMed] [Google Scholar]
- 42.Zotin AI. The Mechanism of Cleavage in Amphibian and Sturgeon Eggs. J Embryol Exp Morphol. 1964;12:247–62. [PubMed] [Google Scholar]
- 43.Hu CK, Coughlin M, Field CM, Mitchison TJ. Cell polarization during monopolar cytokinesis. J Cell Biol. 2008;181:195–202. doi: 10.1083/jcb.200711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howard J, Hyman AA. Microtubule polymerases and depolymerases. Curr Opin Cell Biol. 2007;19:31–5. doi: 10.1016/j.ceb.2006.12.009. [DOI] [PubMed] [Google Scholar]