Abstract
In 2003 Thompson and colleagues reported that daily use of finasteride reduced the prevalence of prostate cancer by 25% compared to placebo. These results were based on the double-blind randomized Prostate Cancer Prevention Trial (PCPT) which followed 18,882 men with no prior or current indications of prostate cancer annually for seven years. Enthusiasm for the risk reduction afforded by the chemopreventative agent and adoption of its use in clinical practice, however, was severely dampened by the additional finding in the trial of an increased absolute number of high-grade (Gleason score ≥ 7) cancers on the finasteride arm. The question arose as to whether this finding truly implied that finasteride increased the risk of more severe prostate cancer or was a study artifact due to a series of possible post-randomization selection biases, including differences among treatment arms in patient characteristics of cancer cases, differences in biopsy verification of cancer status due to increased sensitivity of prostate-specific antigen under finasteride, differential grading by biopsy due to prostate volume reduction by finasteride, and nonignorable drop-out. Via a causal inference approach implementing inverse probability weighted estimating equations, this analysis addresses the question of whether finasteride caused more severe prostate cancer by estimating the mean treatment difference in prostate cancer severity between finasteride and placebo for the principal stratum of participants who would have developed prostate cancer regardless of treatment assignment. We perform sensitivity analyses that sequentially adjust for the numerous potential post-randomization biases conjectured in the PCPT.
Keywords: Causal inference, principal stratification, treatment effects, selection bias
1. INTRODUCTION
The Prostate Cancer Prevention Trial (PCPT) was a multi-center, double blind, randomized trial that studied the effect of finasteride on the period prevalence of prostate cancer in healthy men screened for 7 years (Thompson et al. 2003). The 18,882 men aged 55 years or older with no history or current indicators of prostate cancer (prostate-specific antigen (PSA) ≤ 3.0 nanograms per milliliter (ng/mL) and digital rectal exam (DRE) normal) were randomized to receive either 5 milligrams of finasteride per day or placebo and followed for 7 years. During annual follow-up, participants were referred for a prostate biopsy if their PSA exceeded a threshold or their DRE was abnormal (suspicious for cancer). In addition, all participants not diagnosed with prostate cancer during the study were instructed to undergo an end-of-study prostate biopsy at their seventh and final visit.
Of the 10168 men whose cancer status was known either by a positive mid-study biopsy or a study endpoint biopsy, prostate cancer was detected in 821 (16.6%) of 4951 men on the finasteride arm compared with 1194 (22.9%) of 5217 men on the placebo arm, suggesting that finasteride lowered the risk of prostate cancer (P < 0.001). However, 299 (36.4%) of the 821 finasteride prostate cancer cases were more severe (Gleason score ≥ 7) compared to only 264 (22.1%) of 1194 placebo prostate cancer cases (P < 0.001); see Table 1. Interpretation of the results is therefore challenging since the study suggested that finasteride reduced the overall risk of prostate cancer but accelerated growth of high-grade tumors (Scardino 2003).
Table 1.
Numbers with biopsy, cancer, and high-grade cancer in the Prostate Cancer Prevention Trial.
| Placebo (Z = 0) | Finasteride (Z = 1) | Combined | |
|---|---|---|---|
| Randomized | 8025 | 7966 | 15991 |
| Cancer Status Known by Biopsy (R = 1) | 5217 | 4951 | 10168 |
| Prostate Cancer (S = 1) | 1194 | 821 | 2015 |
| High Grade Prostate Cancer (Y = 1) | 264 | 299 | 563 |
NOTE: These numbers are slightly different from those reported in Thompson et al. (2003) because of early study termination and because we only used participants with complete covariate information. We repeated the analyses reported in Sections 2.2, 3.1, and 3.2 using Thompson and colleagues’ final dataset, and results were nearly identical.
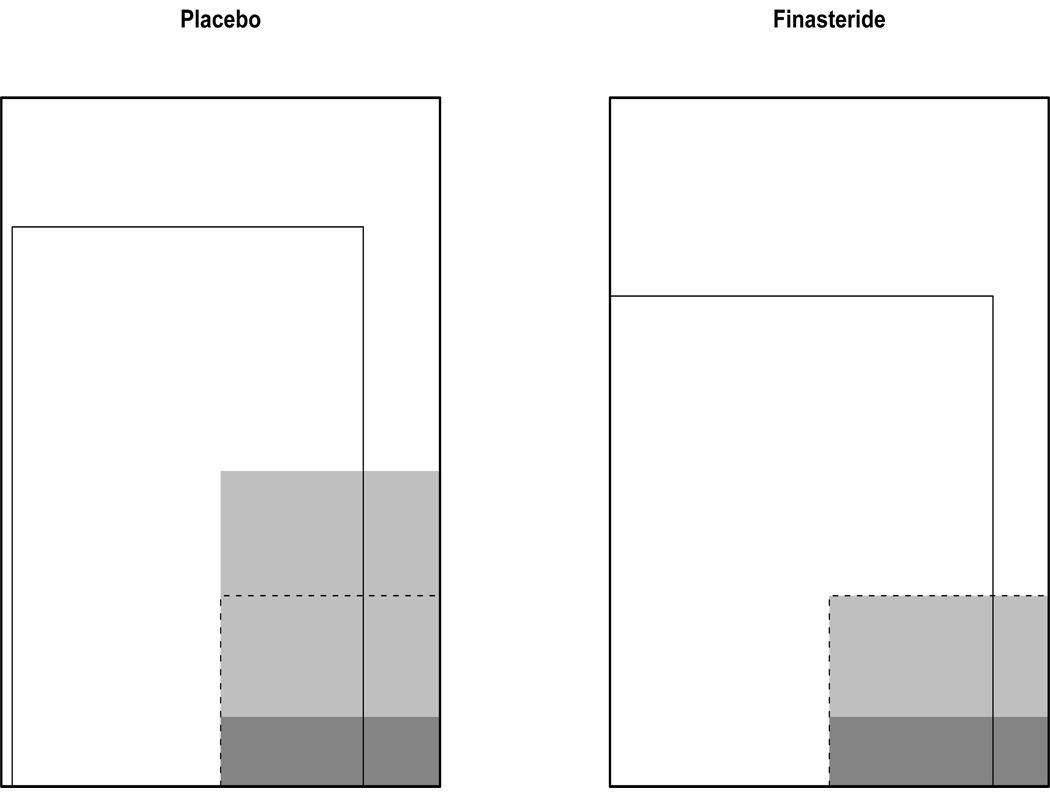
While the apparent proportion of high-grade cancers among those diagnosed with cancer on finasteride is higher than that on placebo, this may not be an appropriate measure of finasteride’s effect on disease severity. Those men diagnosed with cancer are a subset of those men initially randomized in the trial. As this subset was selected after randomization, there could be selection bias (Rosenbaum 1984). If the characteristics of men diagnosed with prostate cancer differ between treatment arms, then the apparent effect of finasteride on prostate cancer grade may be due to correlations between these differing characteristics and cancer grade, rather than the causal effect of finasteride. Additionally, using the number of cancers as the denominator (instead of the number of biopsies or the number randomized) ignores the possibility that finasteride prevented a large fraction of low-grade cases. An example of how post-randomization selection could influence results is shown in Figure 1.
Figure 1.
Hypothetical example of the impact of post-randomization selection in the PCPT analysis. Because of randomization, men in the placebo and finasteride arms are comparable. The shaded regions are those men who developed prostate cancer, the darker shade corresponds to those who developed high-grade cancer. The solid inner boxes in both arms designate those men whose cancer status was determined by biopsy. Notice that the overall proportion of men who develop high-grade cancer is identical between treatment groups. However, the proportion of high-grade cancers among those diagnosed with cancer is much higher in the finasteride arm. The dashed box corresponds to those men who would have gotten cancer regardless of their treatment assignment; a comparison of cancer grade among men in this subpopulation allows one to assess the effect of finasteride on cancer severity.
To limit such potential selection bias, one could compare the prevalence of high-grade cancer among those who received a biopsy (as opposed to among those who were diagnosed with cancer). Of the 4951 men who had a biopsy in the finasteride arm, 6.0% had a high-grade tumor whereas 5.1% of the 5217 men with a biopsy in the placebo arm had a high-grade tumor, a difference that is borderline statistically significant; P = 0.03. Such an analysis may be important for public health purposes. However, it does not directly address the question of determining the effect of finasteride on cancer severity, but presents the combined effects of finasteride on cancer prevalence and on cancer severity among prevalent cases.
A relevant population for addressing the effect of finasteride on cancer severity is the subgroup of patients who would have developed cancer regardless of treatment, but whose treatment may have affected severity (Robins 1995; Rubin 2000; Frangakis and Rubin 2002). The potential outcomes framework (Neyman 1923; Rubin 1978) can be used to define this population. Specifically, as shown in Table 2, participants can be classified into four categories of paired potential outcomes (principal strata (Frangakis and Rubin 2002)) under finasteride and placebo: a participant could have never developed prostate cancer regardless of treatment assignment (stratum NN), a participant could have developed prostate cancer only if they received placebo (stratum CN), developed it only if they received finasteride (stratum NC), or developed prostate cancer regardless of treatment assignment (stratum CC). The total number of high-grade cancers on placebo comes from the number of high-grade cancers in strata CN and CC; the total number of high-grade cancers on finasteride comes from the total number of high-grade cancers in strata NC and CC. While discordance in the contribution of strata CN and NC to the total number of high grades is related to both the ability of finasteride to prevent prostate cancer and affect its severity; within stratum CC, differences in the number of high grade cancers between treatment arms is restricted solely to the effect of finasteride on cancer severity.
Table 2.
Principal Strata in Prostate Cancer Prevention Trial.
| Principal Stratum | Outcome on Placebo | Outcome on Finasteride |
|---|---|---|
| NN | no cancer | no cancer |
| CN | cancer | no cancer |
| NC | no cancer | cancer |
| CC | cancer | cancer |
Hudgens, Hoering, and Self (2003), Gilbert, Bosch, and Hudgens (2003), Hudgens and Halloran (2006), Zhang and Rubin (2003), and Jemiai (2005) have discussed methods, primarily in the context of vaccine trials, for assessing treatment effects on outcomes defined by a post-randomization event. In this manuscript, we first adapt these methods to estimate the effect of finasteride on prostate cancer severity among those who would have had biopsy-detectable cancer regardless of treatment assignment. A key ingredient of our proposed implementation is a sensitivity analysis. In addition to uninformed sensitivity analyses that vary sensitivity parameters over their entire range, we highlight results based on elicited parameters from two subject matter experts. Next we extend these methods to accommodate two additional layers of potential bias in the PCPT: differential biopsy verification and differential cancer grading between finasteride and placebo.
Despite the PCPT protocol’s specification that participants undergo an end-of-study biopsy, not all participants received a biopsy. This was expected as prostate biopsy is an invasive procedure with possible negative side effects (Goodman et al. 2004). As shown in Table 1, 65.0% of men randomized to the placebo arm received a biopsy compared to 62.2% on finasteride (P = 0.0002). This difference is small with statistical significance driven by the large sample size. However, the assumption that biopsies were missing completely at random (MCAR) (Little and Rubin 2002) is unlikely for these data since one of the criteria for interim biopsies was PSA exceeding 4.0 ng/mL or an abnormal DRE (Baker 2000). A differential biopsy verification process between finasteride and placebo is also probable. Finasteride shrinks the volume of the prostate, so it approximately halves the PSA value. Therefore the actual PSA criterion for referral to biopsy on the finasteride arm was referral if an inflation factor (approximately 2.0) × PSA exceeded 4.0 ng/mL (Thompson et al. 2003). Thompson et al. (2006, 2007) showed that the sensitivity of PSA was greatly enhanced on finasteride, and that shrinkage of the prostate gland on finasteride also affected the sensitivity of the DRE test. Since referral to biopsy was strictly mandated by protocol based on observed covariates PSA and DRE, the missing data process is more likely missing at random (MAR) (Little and Rubin 2002; Thompson et al. 2005). Our approach will be to assume MAR by incorporating the observed covariates PSA, DRE, family history of prostate cancer, age, race, and history of a prior negative biopsy (see Table 3) to modify the estimating equations using an inverse weighting approach similar to that proposed by Robins, Rotnitzky and Zhao (1995).
Table 3.
Characteristics of those with and without a study endpoint
| No Biopsy (R = 0) (N=5823) |
Biopsy (R = 1) (N=10168) |
P-valuea | |
|---|---|---|---|
| Finasteride Arm | 3015 (51.8%) | 4951 (48.7%) | 0.0002 |
| Baseline PSA | 1.0 (0.6, 1.7)b | 1.1 (0.7, 1.8) | < 0.0001 |
| Baseline Age | 63 (58, 67) | 62 (58, 66) | 0.0004 |
| Race | < 0.0001 | ||
| White | 5310 (91.2%) | 9471 (93.1%) | |
| African American | 242 (4.2%) | 346 (3.4%) | |
| Other | 271 (4.7%) | 351 (3.5%) | |
| Family History of Prostate Cancer | 785 (13.5%) | 1695 (16.7%) | < 0.0001 |
| Referred to Biopsy because of PSA | 69 (1.2%) | 802 (7.9%) | < 0.0001 |
| Referred to Biopsy because of DRE | 83 (1.4%) | 831 (8.2%) | < 0.0001 |
| Prior Negative Biopsy | 477 (8.2%) | 1348 (13.3%) | < 0.0001 |
Chi-square test for categorical variables, Wilcoxon rank sum test for continuous covariates (baseline PSA and age).
Median (1st quartile, 3rd quartile) reported for continuous covariates.
A second potential bias that is hypothesized to have driven the increased number of high-grade prostate cancer cases on the finasteride arm is due to differential biopsy grading (Lucia et al. 2007). The PCPT used 6-core biopsies which extract six biopsy tissues uniformly spaced across the prostate. Because finasteride shrinks the prostate volume, the 6-core biopsies covered a larger area of the prostate for cases in the finasteride arm and hence were probably more likely to detect high-grade prostate cancer than on the placebo arm. To investigate this hypothesis the PCPT performed a limited, blinded follow-up study of grade on prostatectomy for 531 PCPT participants (225 placebo, 306 finasteride) diagnosed with prostate cancer on biopsy during the study who were later treated by prostatectomy. In the placebo group the sensitivity of biopsy for high-grade detection was 45% (55 biopsy high-grades / 123 prostatectomy high-grades), compared to 66% on finasteride (76 biopsy high-grades / 115 prostatectomy high-grades), suggesting a substantial downward bias in detecting high-grade cancer on placebo relative to finasteride (Table 4). We will consider the impact of this differential biopsy grading by performing an analysis using cancer grades based on prostatectomy. Those men for whom we were able to obtain a prostatectomy tissue sample were not randomly selected from those who were diagnosed with prostate cancer; they tended to be younger, non African American, to have higher PSAs, worse DREs, and have higher grade on biopsy than those without a prostatectomy (Table 5). We use a similar inverse weighting procedure to accommodate this missing data mechanism, incorporating grade on biopsy as an additional covariate.
Table 4.
Comparison of Cancer Grade based on Blinded Biopsy and Blinded Prostatectomy
| Biopsy: | Low Grade | High Grade | Total | ||
|---|---|---|---|---|---|
| Placebo | Prostatectomy | ||||
| Low Grade | 163 | 20 | 183 | ||
| High Grade | 68 | 55 | 123 | ||
| Total | 231 | 75 | 306 | ||
| Finasteride | Prostatectomy | ||||
| Low Grade | 93 | 17 | 110 | ||
| High Grade | 39 | 76 | 115 | ||
| Total | 132 | 93 | 225 |
Table 5.
Characteristics of participants with prostate cancer who had a prostatectomy
| No Prostatectomy (N=1484) |
Prostatectomy (N=531) |
P-valuea | |
|---|---|---|---|
| Finasteride Arm | 596 (40.2%) | 225 (42.4%) | 0.42 |
| Baseline PSA | 1.5 (0.9, 2.1)b | 1.6 (1.0, 2.3) | 0.002 |
| Baseline Age | 65 (60, 69) | 61 (58, 64) | < 0.0001 |
| Race | 0.10 | ||
| White | 1372 (92.5%) | 496 (93.4%) | |
| African American | 79 (5.3%) | 18 (3.3%) | |
| Other | 33 (2.2%) | 17 (3.2%) | |
| Family History of Prostate Cancer | 312 (21.0%) | 122 (22.9%) | 0.38 |
| Referred to Biopsy because of PSA | 336 (22.6%) | 171 (32.2%) | < 0.0001 |
| Referred to Biopsy because of DRE | 265 (17.9%) | 139 (26.2%) | < 0.0001 |
| Prior Negative Biopsy | 205 (13.8%) | 68 (12.8%) | 0.61 |
| High Grade Cancer from Biopsy | 395 (26.6%) | 168 (31.6%) | 0.03 |
Chi-square test for categorical variables, Wilcoxon rank sum test for continuous covariates (baseline PSA and age).
Median (1st quartile, 3rd quartile) reported for continuous covariates.
In section 2, we formulate our problem using a causal modeling framework. We propose three assumptions that identify the average causal effect of finasteride on severity of cancer among participants who would have developed biopsy-detectable cancer regardless of treatment assignment. In section 3, we perform sensitivity analyses investigating the implications of plausible violations to these assumptions. Sensitivity analyses are performed by systematically relaxing assumptions, estimating sharp bounds and using sensitivity parameters to examine a gradation of assumption violations. Results from the PCPT are shown over sensitivity parameter ranges elicited from two subject matter experts. In section 4, we perform an analysis accounting for possible differential biopsy grading. And in section 5, we discuss our findings and the general analytic approach. Relevant estimating equations are found in the Appendix.
2. CAUSAL MODELING FRAMEWORK
2.1 Potential Outcomes and Observed Data
As previously mentioned, to define our target of interest we use a causal modeling framework which employs potential outcomes (Neyman 1923; Rubin 1978). Let Z = 0 or 1 denote assignment to placebo or finasteride, respectively. Let Si(z) be the indicator of biopsy-detectable prostate cancer if, possibly contrary to fact, subject i is assigned Zi = z. (It should be noted that for brevity, throughout this paper we will often write “got cancer” or “developed cancer” instead of the more precise “had a biopsy-detectable prostate cancer.” The implications of any differential sensitivity of the 6-core biopsies used to detect prostate cancer in this trial will be discussed in section 5.) Similarly, let Ri(z) be the indicator that Si(z) is known if Zi = z; consistent with the primary analysis of the PCPT, Ri(z) = 1 if a participant has an end of study biopsy or a positive mid-study biopsy given Zi = z. Let Yi(z) be the indicator of high-grade prostate cancer (Gleason grade ≥ 7) if Zi = z. (Gleason grades are subjective, ordinal scores which we have chosen to dichotomize at the traditional value to be consistent with the previous PCPT analyses and subsequent controversy.) In this setting, if a subject does not have prostate cancer, then the severity of their cancer does not exist. Specifically, we set Yi(z) = * if Si(z) = 0. Additionally, let Xi(z) be a vector of covariates if Zi = z. Note that Xi(0) = Xi(1) for baseline covariates.
In order to link potential outcomes to observed data we assume that
| (1) |
where Ri is the observed indicator that prostate cancer status is known, Si is the realized indicator of prostate cancer (missing if Ri = 0), Yi is the realized indicator of high-grade prostate cancer (missing if Ri = 0 and not defined if Si = 0), and Xi are the observed covariates. This notation implicitly assumes that the potential outcomes of each trial participant are not influenced by the treatments of other participants, known as the Stable Unit Treatment Value Assumption (SUTVA) (Rubin 1978) or “no interaction between units” (Cox 1958). Let the observed data Oi = (Zi, Ri, Si, Yi, Xi) for i = 1,⋯,N, be independent and identically distributed (i.i.d.) copies of O = (Z, R, S, Y, X), where if Ri = 0, Si and Yi are not included in the observed data vector.
A causal estimate of the treatment effect on severity of prostate cancer among patients who would have developed biopsy-detectable prostate cancer regardless of treatment assignment can be expressed as a risk ratio, odds ratio, or absolute risk difference; we use the latter, referred to here as the average causal effect:
Within each treatment arm the group of subjects who developed cancer is a mixture of subjects who would have always had cancer and those who would not have had cancer had they received the other treatment; see Table 2. It is important to note therefore that ACE is not necessarily equal to the difference of observable conditional expectations, E(Y |S = 1, R = 1, Z = z), for z = 0, 1.
We will make the independence assumption
| (2) |
which is ensured by randomization. Under (1) and (2), E(Y (z)|R(z) = 1, S(z) = 1) = E(Y |R = 1, S = 1, Z = z). Because we do not know who would have developed cancer regardless of treatment assignment, ACE is not identifiable under (1) and (2) alone, and requires additional assumptions for estimation.
2.2 Assumptions to Identify the Average Causal Effect
In a first analysis to identify ACE we make the following additional assumptions:
| (3) |
| (4) |
| (5) |
where for random variables A,B, and C, A ╨ B|C indicates conditional independence of A and B given C. Assumption (3) states that obtaining a biopsy is independent of cancer status and severity, and is equivalent to the assumption that cancer status is MCAR. Under this assumption, E (Y (z)|S(z) = 1, R (z) = 1) = E(Y (z)|S(z) = 1), that within treatment arm the average risk of high-grade cancer among cancers for men whose cancer status was known equals the average risk of high grade cancer among cancers for men whether or not their cancer status was known.
Assumption (4) is often referred to as monotonicity and implies that everyone who developed biopsy-detectable prostate cancer in the finasteride arm also would have developed biopsy-detectable cancer if randomized to placebo. Under this assumption, the probability that a participant who developed cancer under placebo would have developed cancer had they instead been randomized to receive finasteride, P(S (1) = 1|S (0) = 1), is equal to P(S(1) = 1)/P(S(0) = 1), which under (1) and (2) can be estimated as the relative risk of cancer. While assumption (4) is not consistently testable, there was no evidence suggesting its violation since finasteride appeared to have a beneficial effect of partially preventing prostate cancer across all covariate-defined subgroups (Thompson et al. 2003).
Assumption (5) states that among subjects who developed prostate cancer in the placebo arm, their cancer status had they been randomized to finasteride is independent of the observed severity of their cancer. In other words, the placebo arm distribution of the severity of prostate cancer is the same in the always diseased principal stratum (S(0) = S(1) = 1; stratum CC in Table 2) and the protected principal stratum (S(0) = 1, S(1) = 0; stratum CN).
Assumptions (1)–(5) and the observed data identify ACE
where the first line is by (4), the second by (5), the third by (3), and the final by (1) and (2). The ACE for the PCPT data is therefore estimated as 299/821 − 264/1194 = 0.14 (95% Wald confidence interval of 0.10, 0.18), indicating that finasteride caused a statistically significant 14% absolute risk increase in high-grade prostate cancer compared to the placebo arm.
Assumptions (3)–(5) are not consistently testable from empirical data and are refutable in the PCPT. These asumptions are not the only assumptions that identify ACE as equal to the difference of observable conditional expectations. We consider (3)–(5) because they provide a reasonable and interpretable platform for facilitating a sensitivity analysis in the context of the PCPT.
3. SENSITIVITY ANALYSES
3.1 Relaxing Assumption (5)
Assumption (5) states that the grade of prostate cancer among cases on placebo is unrelated to whether or not they would have developed cancer had they been on finasteride. This assumption may be implausible since finasteride may well be less effective against more aggressive prostate cancer.
Gilbert, Bosch, and Hudgens (2003) (GBH) proposed a flexible approach for relaxing (5) by assuming the model
| (6) |
where logit a =log(a/(1−a)), α0 is an unknown parameter, and β0 is fixed and known. For men who developed cancer on placebo, the logistic model (6) uses a sensitivity parameter β0 to link observed cancer grade to the probability of developing cancer if on finasteride. The β0 is interpreted as the difference in log-odds (so exp(β0) is the odds ratio) of prostate cancer on the finasteride arm for high- versus low-grade prostate cancer on the placebo arm. The β0 is not identifiable from the observed data; it is a fixed sensitivity parameter and varied as part of a sensitivity analysis. Setting β0 = 0 corresponds to the conditional independence assumption (5). Setting β0 = ±∞ corresponds to the bounds for ACE given by Hudgens, Hoering, and Self (2003) (HHS), and implies that those diagnosed with cancer in placebo with the most (or least) severe cancer are those who would have been diagnosed with cancer if randomized to finasteride.
Under (1)–(4) and (6), GBH proposed estimating α0 using the fact that P(S(1) = 1|S(0) = 1) = Σy=0,1 P(S(1) = 1|S(0) = 1, Y (0) = y)P(Y (0) = y|S(0) = 1), and by recognizing that P(S(1) = 1|S(0) = 1) can be estimated as the observed relative risk of prostate cancer (discussed in section 2.2) and that P(Y (0) = y|S(0) = 1) can be estimated as the observed proportion of placebo cancer cases that are high- /low-grade. Once α0 has been estimated, the probability of high-grade cancer for placebos who would have developed cancer under either treatment is estimated by plugging in estimates to the expectation of the biased sample model:
Given that under (1)–(4), E(Y (1)|S(0) = S(1) = 1) = E(Y |R = 1, S = 1,Z = 1) (discussed in section 2.2), estimation of ACE follows, and the variance can be estimated via the bootstrap or as described in the Appendix.
We elicited plausible ranges for β0 from two subject matter experts from independent institutions, one a clinician who is particularly enthusiastic concerning finasteride treatment and the other an epidemiologist who has been more pessimistic. We prompted these experts with the question, “Given two men assigned placebo who got cancer during the course of the trial: who do you believe would be more likely to have gotten cancer if, contrary to fact, they were assigned finasteride? ____the person with the higher Gleason score, ____the person with the lower Gleason score, or ____the two are equally likely.” We then elicited odds ratios and ranges. Both experts felt that the man with high Gleason on placebo would more likely have developed prostate cancer on finasteride, and provided ranges for the odds ratio exp(β0) of (1.05, 1.35) and (2.00, 4.00), respectively. Note that neither interval contains 1, implying that neither expert believed assumption (5) scientifically plausible.
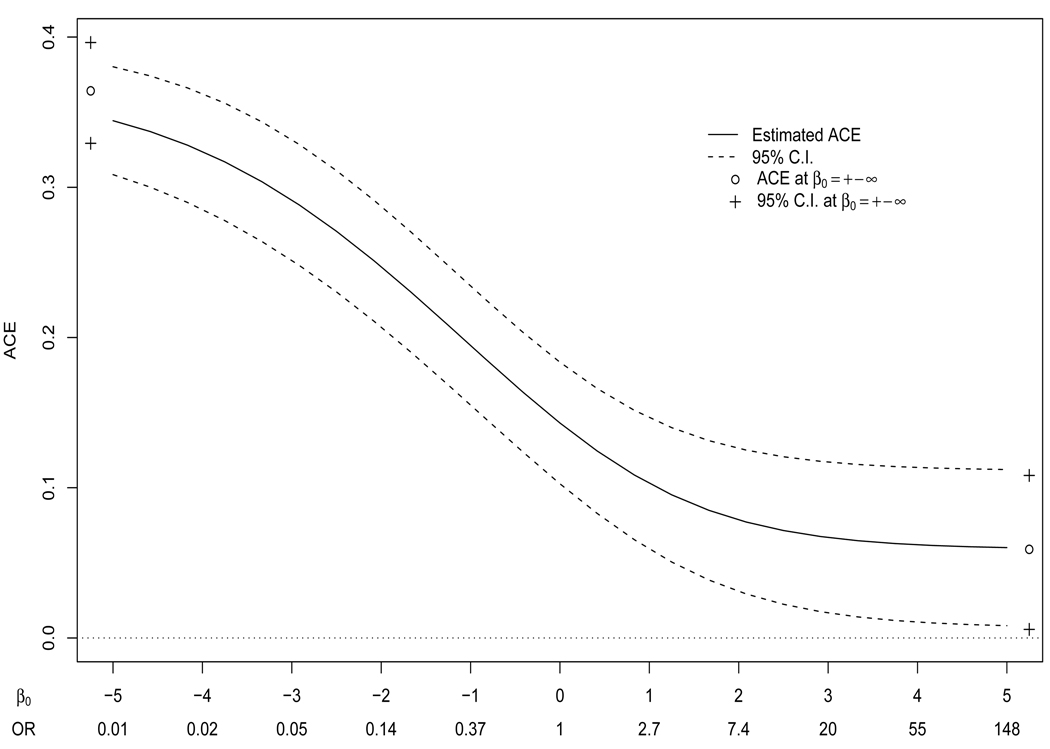
Figure 2 shows estimation of ACE under (1)–(4), and (6) repeated for β0 in the broad interval [−5, 5], i.e. odds ratio exp(β0) ϵ [.007, 148], with 95% Wald confidence intervals constructed using the asymptotic expression for the variance of the estimated ACE. (Confidence intervals were similar for percentile bootstrap intervals based on 1000 replications.) Figure 2 also includes estimates and percentile bootstrap confidence intervals for β0 = ±∞. For this analysis the elicited ranges did not come into play since for all β0, including ±∞, the null hypothesis that ACE = 0 was rejected at the 0.05 level. Therefore under (1)–(4) and (6), no matter what the hypothesized relationship between high-grade prostate cancer on placebo and the risk of prostate cancer on finasteride, among people who would have had prostate cancer detected on biopsy on either arm of the study, those on finasteride had a statistically significant higher risk of developing high-grade prostate cancer.
Figure 2.
Sensitivity analysis of the average causal effect (ACE) of finasteride on severity of prostate cancer under (1), (2), and (3)–(6). The sensitivity parameter is given in terms of β0 (log(OR)) and as odds ratios (OR).
3.2 Relaxing Assumptions (4) and (5)
The assumption of monotonicity (4), which states that everyone with cancer in finasteride would have gotten cancer if randomized to placebo, is strong and may not be plausible (Dawid, 2000), even though finasteride appeared to reduce the risk of prostate cancer across all trial subgroups. In a Ph.D. dissertation with Andrea Rotnitzky, Jemiai (2005) proposed sensitivity analysis methods that relaxed (4) in addition to (5). Following their arguments, it can be shown that in addition to (1), (2), and (3), ACE is identified under (6) and the following two assumptions:
| (7) |
| (8) |
where α0 and α1 are unknown parameters to be estimated and ϕ, β0, and β1 are fixed sensitivity parameters to be varied as part of the sensitivity analysis. By relaxing monotonicity, we no longer assume that all men with detectable cancer on finasteride would have developed detectable cancer on placebo. Assumption (7) specifies the probability of getting detectable cancer in placebo given detectable cancer in finasteride and assumption (8), which is analogous to (6), links this probability to cancer grade. The sensitivity parameter β1 has a similar definition to β0 described in Section 3.1. In the PCPT, the sensitivity parameter ϕ may lie anywhere between 0 and 1, with ϕ = 1 corresponding to monotonicity, ϕ = P(S(0) = 1) corresponding to independence between S(0) and S(1), and ϕ = 0 implying that no one with cancer on finasteride would have developed cancer if on placebo. For fixed ϕ, β0 and β1, estimating equations analogous to those of Jemiai’s dissertation used to estimate ACE are shown in the Appendix.
For performing a sensitivity analysis we first note that bounds on ACE can be constructed by not restricting any of the sensitivity parameters. When this is done the bounds on ACE are −1 and 1, the minimum and maximum possible values of ACE without even looking at the data, so that these bounds are uninformative. Note that the method proposed by Zhang and Rubin (2003) would obtain identical bounds. Therefore to extract any useful information from this analysis it is necessary to establish plausible ranges of the sensitivity parameters as done in Section 3.1.
In addition to eliciting a plausible range for β0 (discussed in the previous section), we also elicited plausible ranges for β1 and ϕ from our two subject matter experts. Our pessimist chose the ranges ϕ ϵ [0.8, 0.95], exp(β0) ϵ [2, 4], and exp(β1) ϵ [0.25, 0.50]. These ranges reflect a belief that monotonicity is slightly violated (between 5 and 20% of those with detectable cancer in the finasteride arm would not have gotten detectable cancer if randomized to placebo), that those with more severe forms of cancer in the placebo arm are more likely those who would have gotten detectable cancer if randomized to the finasteride arm, and that those with less severe forms of cancer in the finasteride arm are those who are more likely to have gotten detectable cancer if randomized to the placebo arm. Our optimist chose the ranges ϕ ϵ [1.0, 1.0], exp(β0) ϵ [1.05, 1.35], and exp(β1) ϵ [1.05, 1.35]. Notice from his range for ϕ that this expert believed the monotonicity assumption. Therefore, his range for β1 is irrelevant, because in (8), P(S(0) = 1|S(1) = 1, Y (1) = y) = 1 irrespective of the value of y, and therefore the analysis of Section 3.1 (Figure 2) is the appropriate sensitivity analysis of ACE corresponding to this expert’s opinions.
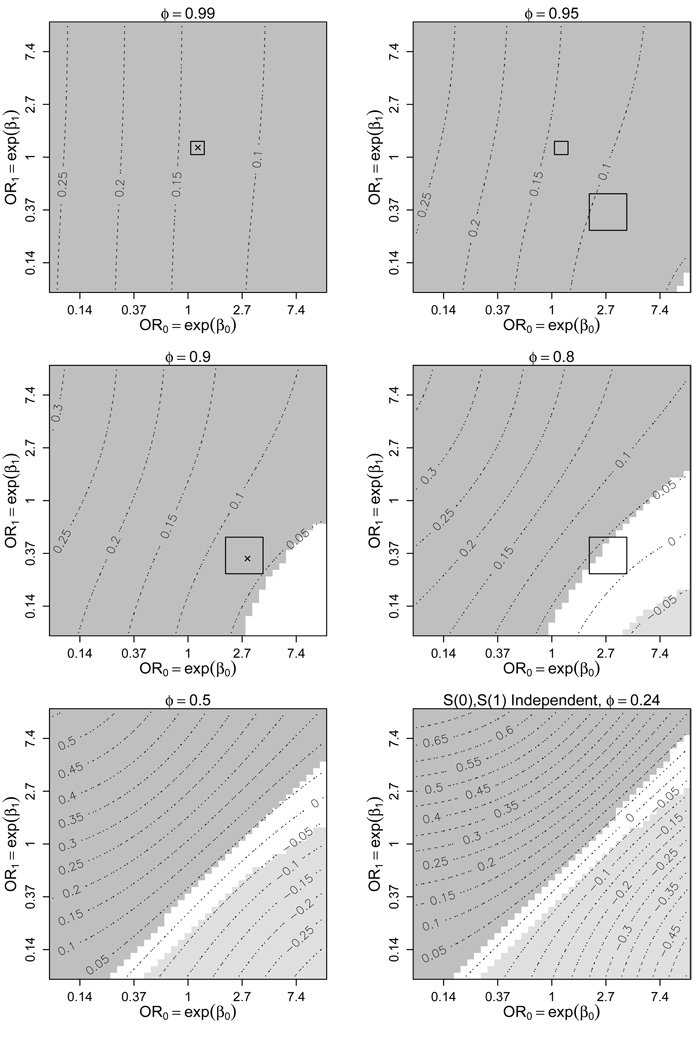
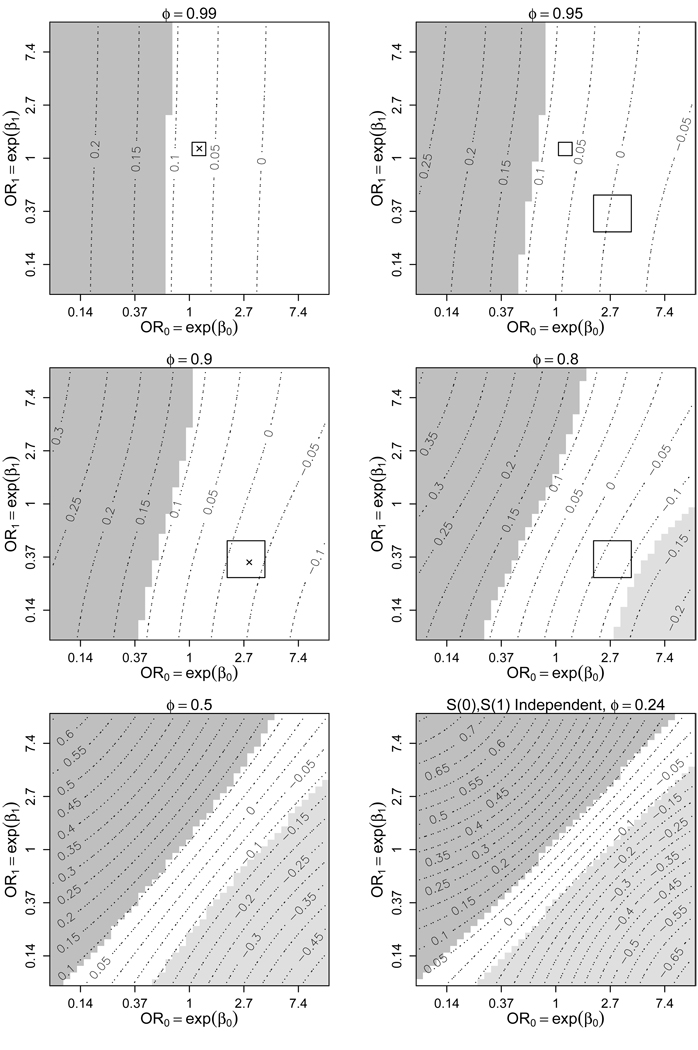
Figure 3 shows a sensitivity analysis of ACE, varying ϕ, β0, and β1. It seems unlikely that developing cancer on the finasteride arm is independent of developing cancer on the placebo arm (ϕ = 0.24). Even more unlikely is that S(0) and S(1) are negatively correlated, so plots assuming ϕ < 0.24 are not shown. For display purposes the range for β0 (and β1) was condensed from [−5, 5] in Figure 2 to [−2.5, 2.5] in Figure 3, corresponding to odds ratios between 0.08 and 12.2. Contours represent the estimated ACE at a given ϕ, β0, and β1. Shaded regions correspond to those sensitivity parameter values where the Wald-based 95% confidence interval for ACE does not contain 0. Therefore, estimates in the dark-shaded regions imply that among those who would have gotten cancer regardless of treatment assignment finasteride caused high-grade cancer, the light-shaded regions imply that finasteride lowered cancer grade, and estimates in the unshaded region fail to reject H0 : ACE = 0 at the 0.05-level. Notice in Figure 3 that at ϕ = 0.99 (a minor violation of monotonicity), H0 : ACE = 0 is rejected for all β0 and β1 between [−2.5, 2.5], consistent with the analysis performed under the assumption of monotonicity (Figure 2). As ϕ moves farther from 1, the range of estimates for ACE increases (bottom two graphs of Figure 3). Our experts’ ranges for ϕ, β0, and β1 are included as rectagles in Figure 3, with × corresponding to their selections of the most likely values of the sensitivity parameters. (For illustrative purposes we have included our optimist’s range for β0 and β1, at ϕ = 0.95 and ϕ = 0.99, although Figure 2, not Figure 3, is the appropriate sensitivity analysis corresponding to this expert’s opinions.) The hypothesis H0 : ACE = 0 is rejected for most values of ϕ, β0, and β1 within the experts’ ranges; evidence that finasteride is causing higher grades of cancer. The exception is when ϕ is at the far end of our pessimist’s plausible range, e.g. if ϕ = 0.8, exp(β0) = 4, and exp(β1) = 0.25 there is insufficient evidence to reject H0.
Figure 3.
Contour plots of for varying levels of the sensitivity parameters β0, β1, and ϕ under (1), (2), (3), and (6)–(8). ϕ is the probability of prostate cancer if randomized to placebo given prostate cancer in the finasteride arm. OR0 (OR1) is the odds ratio for prostate cancer in finasteride (placebo) based on cancer grade, given cancer in placebo (finasteride). The shaded regions represent those choices of β0, β1, and ϕ where H0 : ACE = 0 is rejected at the 0.05-level. The darker (lighter) shades are where ACE > 0 (ACE < 0), implying a higher (lower) risk of high-grade prostate cancer on finasteride. The rectangles represent ranges of our sensitivity parameters selected by two subject matter experts. The × represent their most likely sensitivity parameter values.
3.3 Relaxing Assumptions (3), (4), and (5)
Assumption (3), that receipt of a biopsy is independent of cancer status and severity is refutable in the PCPT, particularly because men were referred to biopsy based on their PSA and DRE. Verification bias in the PCPT could also have differed by treatment arm since sensitivity of PSA for prostate cancer detection on biopsy was higher on the finasteride arm. To relax (3), we assume
| (9) |
that conditional on covariates (baseline and post-baseline), receipt of biopsy is independent of cancer status and severity. In the PCPT, assumption (9) is much more reasonable than (3), as the study rigorously measured covariates related to both reciept of a biopsy and to prostate cancer outcome: PSA and DRE at each yearly visit, family history of prostate cancer, age, race, and prior negative biopsies.
Our approach is to perform a sensitivity analysis similar to Section 3.2, only assuming (9) instead of (3). Under (1), (2), (6)–(9), ACE can be estimated by augmenting the estimating equations by weights corresponding to the inverse probability of having a biopsy, in a manner similar to that described by Robins, Rotnitzky, and Zhao (1995). Specifically, we use all participants to estimate the probability of having a biopsy based on covariates and treatment assignment, P(R = 1|X = x,Z = z); the estimating equations for an individual who had a biopsy are then weighted by the inverse of their estimated probability of getting a biopsy.
In the PCPT many participants had biopsies during the course of the trial. Consistent with the original analysis, we have defined S = 1 as a positive biopsy at any time during the trial, S = 0 as a negative biopsy 7 years after randomization (as well as all prior biopsies), and R = 0 if no biopsy was taken 7 years after randomization and all prior biopsies (if any) during the course of the study were negative. Therefore, cancer status within 7 years of randomization was unknown (R = 0) for two reasons: 1) dropout before 7 years of follow-up without a cancer diagnosis, or 2) staying in the study for 7 years with no positive interim biopsy and deciding not to have an end-of-study biopsy. One can therefore think of P(R = 1|X = x, Z = z) = P(A = B = 1|X = x, Z = z) = P(B = 1|A = 1, X = x, Z = z)P(A = 1|X = x, Z = z), where A is the indicator of staying in the study until a cancer status (S) ascertaining biopsy and B is the indicator of having a biopsy. We modeled and estimated both probabilities separately, multiplying the two together to estimate P(R = 1|X = x, Z = z). The probability of not dropping out of the study before diagnosis of S was modeled using logistic regression with Z and covariates baseline PSA, age, race, and family history of prostate cancer. Given that A = 1, the probability of choosing to have a biopsy was modeled using logistic regression with Z, baseline PSA, age, race, family history of prostate cancer, abnormal DRE at last visit and its interaction with treatment, biopsy recommendation based on high PSA at last visit and its interaction with treatment, and prior negative biopsy during the course of the trial. Estimation details are given in the Appendix.
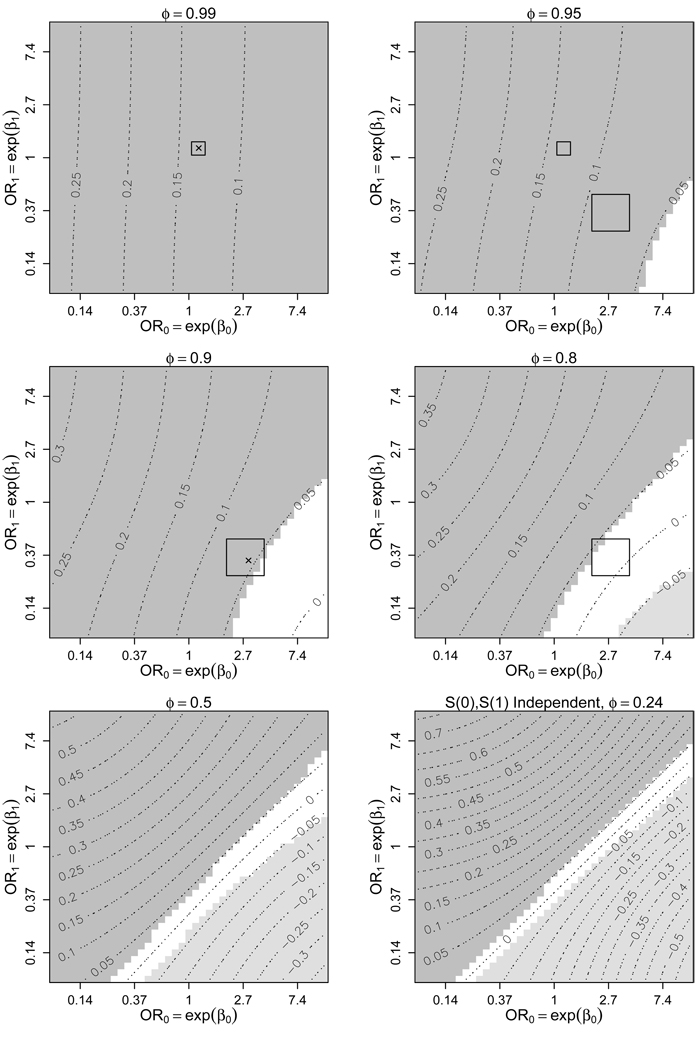
Results under (9) shown in Figure 4 are very similar to the results under (3) shown in Figure 3. The estimated ACE is slightly lower under (9) than under (3) for the same sensitivity parameter values, but this difference is not substantial. That results under (9) are similar to results under (3) is consistent with analyses of the receiver-operating characteristic (ROC) curves for both arms of the study, where Thompson and colleagues found no difference in the area under the ROC for analyses accounting for and not accounting for potential verification bias by incorporating biopsy-predicting covariates (Thompson et al. 2005, 2006).
Figure 4.
Contour plots of for varying levels of β0, β1, and ϕ under (1), (2), and (6)–(9). See Figure 3’s caption.
4. DIFFERENTIAL BIOPSY GRADING
We re-performed the sensitivity analyses of Section 3.3 (assuming (1), (2), (6)–(9)), accounting for differential biopsy high-grade detection by using the prostatectomy results from the 531 participants in Table 4. Cancer grade was reported as missing for those diagnosed with cancer but without a prostatectomy. To account for potential bias due to our excluding those without a prostatectomy, we again employed inverse probability weights. Among those diagnosed with prostate cancer, we first estimated the probability of having a prostatectomy based on treatment, original cancer severity, and covariates using a linear logistic model. Next, the components of our estimating equations which incorporated cancer severity were multiplied by an indicator of getting a prostatectomy and the subject-specific inverse probability of prostatectomy estimated using the logistic model. Variance estimates were altered to account for this weighting. Details are in the Appendix.
Figure 5 is the resulting sensitivity analysis of ACE, using prostatectomy-defined cancer grades. Notice now that over the entire range of sensitivity parameters chosen by our experts, there is insufficient evidence to reject H0 : ACE = 0. The inability to reject the null is due to two factors: First, for a given β0, β1, and ϕ, the estimated ACE decreased when using prostatectomy measures of cancer severity (Figure 5) compared to the original measurement (Figure 4). Second, the variance of the estimated ACE increased when using prostatectomy measures. This loss of power is not surprising, as prostatectomies were only obtained in about a quarter of those who were diagnosed with prostate cancer.
Figure 5.
Contour plots of using cancer grade based on prostatectomy for varying levels of β0, β1, and ϕ under (1), (2), and (6)–(9). See Figure 3’s caption.
It should also be noted that results using inverse probability weights can be highly variable when there are extreme weights. The weights in this prostatectomy analysis were similar between treatment arms, but ranged from 1.7 to 49.2. There were two individuals with extreme weights (> 46), one in finasteride with Y = 1 and one in placebo with Y = 0. If these two individuals were removed the maximum weight was much smaller, 25.9, and for given sensitivity parameters, the estimated ACE and its standard error decreased (e.g., at ϕ = 0.9, exp(β0) = 3 and exp(β1) = 1/3, with standard error=0.053 compared to with standard error 0.060 in the analysis of Figure 5). In general, conclusions were similar (data not shown).
5. DISCUSSION
So does finasteride affect the severity of prostate cancer? It depends on the assumptions one is willing to make. If one does not make any assumptions, ACE can take any value between −1 and 1, implying that one can draw any conclusion one would like from this data. Ignoring the prostatectomy results, over most of the sensitivity parameter ranges chosen by our subject matter experts, estimates and 95% confidence intervals for ACE were greater than 0, implying that finasteride does increase the severity of cancer (Figure 2–Figure 4). However, using the more recent prostatectomy measures of disease severity to account for potential bias due to differential biopsy grading, over all sensitivity parameter ranges chosen by our experts there was in-sufficient evidence to reject H0 : ACE = 0 (Figure 5). These latter estimates were less precise, as they were based on a smaller proportion of trial participants. More efficient methods of accounting for misclassification of Y warrant further study.
Our sensitivity analyses do not yield a single answer, which some might find unattractive. In contrast, we could have chosen a range of plausible sensitivity parameters, put a distribution on this range, and then integrated ACE over this distribution. Or more formally, we could have performed a fully Bayesion analysis, putting a prior on our sensitivity parameters and estimating the posterior distribution of ACE (e.g., Scharfstein et al. 2003). Such approaches are reasonable and may yield simpler answers. However, as the estimated ACE is highly dependent on the sensitivity parameters and hence the choice of prior, we prefer to show results under a wide range of sensitivity parameters, letting the reader draw conclusions based on his/her personal beliefs.
Of course, in order to make sense of a sensitivity analysis of this type, interpretation of the sensitivity parameters is key. Although choosing a range for counterfactual sensitivity parameters can be challenging, we believe that our subject matter experts understood them. We specifically chose an expert who we thought would be an optimist and another expert who we thought would be a pessimist. Differences between the chosen sensitivity parameter ranges probably reflect differences of opinion rather than a poor understanding of the parameters. A discussion of the challenges of eliciting and interpreting similar sensitivity parameters, as well as a survey similar to the one we used to elicit our ranges is found elsewhere (Shepherd, Gilbert, and Mehrotra 2007). We recognize that other subject matter experts may have opinions very different than those elicited from our two experts. A more thorough picture of expert opinion about the sensitivity parameter would require elicitation from more experts. Although one can imagine experiments that may favor choosing certain ranges for the sensitivity parameters, it should be recognized that no experiment can truly estimate these parameters as they are indeed counterfactual.
In Section 3.3, we relaxed assumption (3) by assuming (9), that having a biopsy was independent of cancer status or severity conditional on covariates. Instead, we could have performed sensitivity analyses following the general approach of Rotnitzky et al. (1998) and assumed the following model for missing biopsy:
| (10) |
for z = 0, 1, s = 0, 1, and y = 0, 1, where η = (η0, η1) are unknown scalars and τ0, τ1, τ2, and τ3 are sensitivity parameters. However, this approach introduces 4 new sensitivity parameters, bringing us to a total of 7 sensitivity parameters – too many to be of practical use – and we believe the PCPT gathered the right covariates to make (9) reasonable.
There are a few additional issues we did not address in our sensitivity analyses. The first is that biopsy does not perfectly detect prostate cancer and that detection may vary by treatment. Throughout this paper we have defined S as biopsy-detectable prostate cancer. Therefore, interpretation of ACE is limited to the effect of finasteride on severity of cancer among those with biopsy-detectable prostate cancer under either treatment. While it is reasonable to assume that a subject with cancer detected on biopsy truly has cancer, a negative biopsy does not necessarily mean that there is no cancer present. It is also likely that the negative predictive value (NPV) of biopsy for prostate cancer on finasteride is larger than the NPV on placebo because of finasteride’s tendency to shrink the prostate. Hence, more cancers were likely missed on the placebo arm than on the finasteride arm. The implications of this potential differential cancer detection on the estimated ACE depend on the grade of those possibly undetected cancers. As we do not have this information nor estimates of the NPV on either treatment arm, this potential misclassification would have to be addressed by additional sensitivity analyses. Second, we have ignored compliance, so our conclusions can only be interpreted as the causal effect of randomization to finasteride, not actually taking finasteride. Baker (2000) proposed methods which address noncompliance in the context of the PCPT.
Finally, it should be noted that while prostate cancer grade is important, of greater clinical importance is whether finasteride decreased prostate cancer mortality. Unfortunately, the PCPT cannot answer this question because death by prostate cancer is rare; an answer would require longer follow-up and/or more participants. Perhaps the most clinically important question the PCPT can answer is “Does finasteride reduce the risk of severe prostate cancer?” We have addressed a different question: “What is the effect of finasteride on cancer severity among those who would be diagnosed with cancer regardless of treatment?” Our question addresses the controversy of the PCPT’s conflicting results and finasteride’s causal mechanisms. However, our question is not as important from a clinical or public health perspective because it is not known which men will be diagnosed with cancer irrespective of treatment.
Although we have focussed on the PCPT, these methods are more generally applicable. One example is HIV vaccine trials where there is interest in estimating the effect of vaccination on post-infection outcomes among those who would have been infected regardless of treatment assignment (HHS, GBH). Proposed methods in this context have assumed monotonicity and have ignored missing data (missing infection status and/or missing post-infection outcome if infected). The methods presented here relax monotonicity, account for missing data, and can be applied without alteration when the outcome is continuous. Another possible application of these methods is for examining the causal effect of treatment on an outcome that only exists in survivors (e.g., Hayden, Pauler, and Schoenfeld 2005; Egleston et al. 2007).
In conclusion, our sensitivity analyses offer new insights about potential explanations of the increased number of high-grade cancers on the finasteride arm of the PCPT. Although not completely exhaustive, we believe they account for most of the potential biases that could artificially induce the conflicting results of an increased absolute number of high-grade prostate cancer cases on the finasteride arm in the face of a 25% reduction in biopsy-detectable prostate cancer. This finding appears not to be due to differential biopsy verification but could be due to the improved sensitivity of biopsy for detecting high grade disease in finasteride compared to placebo, which when accounted for removes the statistical significance of the average causal effect of increased high-grade prostate cancer by finasteride.
ACKNOWLEDGEMENTS
We would like to thank Catherine Tangen, Phyllis Goodman, Ian Thompson, Alan Kristal, and William Dupont for their help with this manuscript. This article was supported in part by Public Health Service grant CA37429 from the National Cancer Institute.
APPENDIX: ESTIMATING EQUATIONS
A.1 Estimating equations for Section 3.2
Let μz ≡ E(Y (z)|S(0) = S(1) = 1) and pz ≡ P(S(z) = 1) for z = 0, 1. Therefore ACE = μ1 − μ0. Define θ ≡ (p0, p1, α0, α1, μ0, μ1), where α0, α1 are given by (6), (8). Our sensitivity parameters are β0, β1, and ϕ. Let N denote the total number of subjects with an end-of-study biopsy (i.e., the analysis is only performed on those with R = 1).
To estimate θ under (1), (2), (3), (6), (7), and (8), Jemiai (2005) proposed an estimating equation of the form, , Where
Jemiai showed that the resulting estimate, θ̂ is asymptotically normal with where C = Γ−1ΩΓ−1′, and Ω = E[U(θ)U(θ)′]. C is estimated in the usual manner by replacing expectations with and plugging in θ̂ for θ. The variance of is estimated as (Ĉ55 + Ĉ66 − 2 Ĉ56)/N, where Ĉij corresponds to the ith row and jth column of the estimate of C. Estimates and confidence intervals for other smooth functions of θ, such as the causal risk ratio μ1 / μ0, can be similarly constructed through an application of the continuous mapping theorem.
Computation is straightforward
p0 and p1 are first estimated and then plugged into the 3rd and 4th lines of Ui(θ) to estimate α0 and α1 separately, using a one-dimensional optimizer (e.g., R’s function optimize). Using the estimates of p0, p1, α0, and α1, the remaining parameters μ0 and μ1 are estimated. This results in the unique solution of the estimating equation.
Note that the approach of GBH employed in Section 3.1 is equivalent to solving Σ Ui(θ) = 0 with Ui(θ) as defined above only setting ϕ = (1+exp(−α1 − β1Yi))−1 = 1, and removing the fourth line of Ui(θ) because α1 is no longer estimated.
A.2 Estimating equations for Section 3.3
Let N = 15991 (i.e., we are including all participants, both R = 0 and R = 1). Let Vi(θ, η̂) = Ui(θ)λi(η̂)−1 Ri, where λi(η) = P(Ri = 1|Zi = z, Xi = x) = P(Bi = 1|Ai = 1, Xi = x, Zi = z)P(Ai = 1|Xi = x, Zi = z) = λai(ηa)λbi(ηb) with η = (ηa, ηb), η̂is the solution to the score equation, and mi(η) is the first derivative with respect to η of the logarithm of λai(ηa)Ai(1 − λai(ηa))1−Ai(λbi(ηb)Bi(1 − λbi(ηb))1 − Bi)Ai. We employ the models where Xi =(baseline PSA, age, age2, white race indicator, African American indicator, indicator of family history of prostate cancer) and where Xi =(baseline PSA, age, age2, white race indicator, African American indicator, indicator of family history of prostate cancer, indicator of biopsy referral due to PSA, Z × (indicator of biopsy referral due to PSA), last DRE, Z × (last DRE), indicator of prior negative biopsy). From Newey and McFadden (1994), where and C is estimated in the usual way, replacing expectations with and plugging in θ̂ and η̂ for θ and η, respectively.
A.3 Estimating equations for Section 4
Define Qi as the indicator of subject i obtaining a prostatectomy. Re-define Yi as the cancer high-grade indicator (0 or 1) based on the prostatectomy. Let be the previous cancer high-grade indicator based on biopsy. Model the probability of prostatectomy given prostate cancer with where Xi =(baseline PSA, age, white race indicator, African American indicator, indicator of family history of prostate cancer, indicator of biopsy referral due to PSA, last DRE, indicator of prior negative biopsy). Let η̂q denote the estimate of ηq. Define
| (11) |
where (V1i(θ, η̂),⋯, V6i(θ, η̂)) = Vi(θ, η̂) as defined in A.2. Estimation is obtained by solving where N = 15991. Notice that this estimating equation is the same as that given in A.2 except the last four components of the equation used to estimate (α0, α1, μ0, μ1) only use prostatectomy results, weighting by the inverse probability of having a prostatectomy.
The variance is estimated in the same manner as described in A.2, except we now replace η of A.2 with η = (ηa, ηb, ηq), and mi(η) is the first derivative of the logarithm of and V (·) is replaced with W (·).
Contributor Information
Bryan E. Shepherd, Department of Biostatistics, Vanderbilt University, Nashville, TN, 37232, USA
Mary W. Redman, Fred Hutchinson Cancer Research Center, Seattle, WA, 98109, USA
Donna P. Ankerst, Department of Biostatistics, University of Munich, Munich, Germany
REFERENCES
- Baker SG. Analyzing a randomized cancer prevention trial with a missing binary outcome, an auxiliary variable, and all-or-none compliance. Journal of the American Statistical Association. 2000;95:43–50. [Google Scholar]
- Cox DR. Planning of Experiments. New York: Wiley; 1958. [Google Scholar]
- Egleston BL, Scharfstein DO, Freeman EE, West SK. Causal inference for non-mortality outcomes in the presence of death. Biostatistics. 2007;8:526–545. doi: 10.1093/biostatistics/kxl027. [DOI] [PubMed] [Google Scholar]
- Frangakis CE, Rubin DB. Principal stratification in causal inference. Biometrics. 2002;58:21–29. doi: 10.1111/j.0006-341x.2002.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PB, Bosch RJ, Hudgens MG. Sensitivity analysis for the assessment of causal vaccine effects on viral load in HIV vaccine trials. Biometrics. 2003;59:531–541. doi: 10.1111/1541-0420.00063. [DOI] [PubMed] [Google Scholar]
- Goodman PJ, Tangen CM, Crowley JJ, Carlin SM, Ryan A, Coltman CA, Jr, Ford LG, Thompson IM. Implementation of the Prostate Cancer Prevention Trial (PCPT) Controlled Clinical Trials. 2004;25:203–222. doi: 10.1016/j.cct.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Hayden D, Pauler DK, Schoenfeld D. An estimator for treatment comparisons among survivors in randomized trials. Biometrics. 2005;61:305–310. doi: 10.1111/j.0006-341X.2005.030227.x. [DOI] [PubMed] [Google Scholar]
- Hudgens MG, Halloran ME. Causal vaccine effects on binary postinfection outcomes. Journal of the American Statistical Association. 2006;101:51–64. doi: 10.1198/016214505000000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgens MG, Hoering A, Self SG. On the analysis of viral load endpoints in HIV vaccine trials. Statistics in Medicine. 2003;22:2281–2298. doi: 10.1002/sim.1394. [DOI] [PubMed] [Google Scholar]
- Jemiai Y. unpublished Ph.D. dissertation under the supervision of A. Rotnitzky. Harvard School of Public Health, Department of Biostatistics; 2005. Semiparametric methods for inferring treatment effects on outcomes defined only if a post-randomization event occurs. [Google Scholar]
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. New York: Wiley; 2002. [Google Scholar]
- Lucia MS, Epstein JI, Goodman PJ, Darke AK, Reuter VE, Civantos F, La Rosa F, Kattan MW, Tangen CM, Lippman SM, Parnes HL, Coltman CA, Thompson IM. Finasteride and high-grade prostate cancer in the Prostate Cancer Prevention Trial. Journal of the National Cancer Institute. 2007;99:1375–1383. doi: 10.1093/jnci/djm117. [DOI] [PubMed] [Google Scholar]
- Newey WK, McFadden D. Large sample estimation and hypothesis testing. In: Engle RF, McFadden DL, editors. Handbook of Econometrics. Volume IV. Elsevier Science B.V.; 1994. [Google Scholar]
- Neyman J. On the application of probability theory to agricultural experiments: Essay on principles. Translated in Statistical Science. 1923;5:465–480. [Google Scholar]
- Robins JM. An analytic method for randomized trials with informative censoring: Part I. Lifetime Data Analysis. 1995;1:241–254. doi: 10.1007/BF00985759. [DOI] [PubMed] [Google Scholar]
- Robins JM, Rotnitzky A, Zhao LP. Analysis of semiparametric regression models for repeated outcomes in the presence of mising data. Journal of the American Statistical Association. 1995;90:106–121. [Google Scholar]
- Rosenbaum PR. The consequences of adjustment for a concomitant variable that has been affected by the treatment. Journal of the Royal Statistical Society, Series A. 1984;147:656–666. [Google Scholar]
- Rotnitzky A, Robins JM, Scharfstein DO. Semiparametric regression for repeated outcomes with nonignorable nonresponse. Journal of the American Statistical Association. 1998;93:1321–1339. [Google Scholar]
- Rubin DB. Bayesian inference for causal effects: the role of randomization. The Annals of Statistics. 1978;6:34–58. [Google Scholar]
- Rubin DB. Causal inference without counterfactuals. In: Dawid AP, editor. Journal of the American Statistical Association. Vol. 95. 2000. pp. 435–437. Comment on. [Google Scholar]
- Scardino PT. The prevention of prostate cancer – the dilemma continues. The New England Journal of Medicine. 2003;349:295–297. doi: 10.1056/NEJMe038109. [DOI] [PubMed] [Google Scholar]
- Scharfstein DO, Daniels MJ, Robins JM. Incorporating prior beliefs about selection bias into the analysis of randomized trials with missing outcomes. Biostatistics. 2003;4:495–512. doi: 10.1093/biostatistics/4.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd BE, Gilbert PB, Mehrotra DV. Eliciting a counterfactual sensitivity parameter. The American Statistician. 2007;61:56–63. [Google Scholar]
- Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA. The influence of finasteride on the development of prostate cancer. The New England Journal of Medicine. 2003;349:213–222. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- Thompson IM, Ankerst DP, Chi C, Lucia MS, Goodman P, Crowley JJ, Parnes HL, Coltman CA. The operating characteristics of prostate-specific antigen in a population with initial PSA of 3.0 ng/ml or lower. Journal of the American Medical Association. 2005;294:66–70. doi: 10.1001/jama.294.1.66. [DOI] [PubMed] [Google Scholar]
- Thompson IM, Chi C, Ankerst DP, Goodman P, Tangen C, Lippman S, Lucia MS, Parnes HL, Coltman CA. Effect of finasteride on the sensitivity of PSA for detecting prostate cancer. Journal of the National Cancer Institute. 2006;98:1128–1133. doi: 10.1093/jnci/djj307. [DOI] [PubMed] [Google Scholar]
- Thompson IM, Tangen CM, Goodman PJ, Lucia MS, Parnes HL, Lippman SM, Coltman CA. Finasteride improves the sensitivity of digital rectal examination for prostate cancer detection. Journal of Urology. 2007;177:1749–1752. doi: 10.1016/j.juro.2007.01.071. [DOI] [PubMed] [Google Scholar]
- Zhang JL, Rubin DB. Estimation of causal effects via principal stratification when some outcomes are truncated by ‘death, ’. Journal of Educational and Behavioral Statistics. 2003;28:353–368. [Google Scholar]