Abstract
Objective
Adoption of effective treatments for recurrent binge-eating disorders depends on the balance of costs and benefits. Using data from a recent randomized controlled trial, we conducted an incremental cost-effectiveness analysis (CEA) of a cognitive behavioral therapy guided self-help intervention (CBT-GSH) to treat recurrent binge eating compared to treatment as usual (TAU).
Method
Participants were 123 adult members of an HMO (mean age = 37.2, 91.9% female, 96.7% non-Hispanic White) who met criteria for eating disorders involving binge eating as measured by the Eating Disorder Examination (EDE, Fairburn & Cooper, 1993). Participants were randomized either to treatment as usual (TAU) or TAU plus CBT-GSH. The clinical outcomes were binge-free days and quality-adjusted life years (QALYs); total societal cost was estimated using costs to patients and the health plan, and related costs.
Results
Compared to the group receiving TAU only, those who received TAU + CBT-GSH experienced 25.2 more binge-free days and had lower total societal costs of $427 over 12 months following the intervention (incremental CEA ratio -$20.23 per binge-free day or −$26,847 per QALY). Lower costs in the TAU + CBT-GSH group were due to reduced use of TAU services in that group, resulting in lower net costs for the TAU + CBT group despite the additional cost of CBT-GSH.
Conclusions
Findings support CBT-GSH dissemination for recurrent binge-eating treatment.
Keywords: cost-effectiveness analysis, binge eating, cognitive behavior therapy, guided self-help, evidence-based treatment programs
Major advances have been made in the treatment of eating disorders featuring binge eating as a defining symptom, namely bulimia nervosa (BN) and binge eating disorder (BED), and these advances have resulted in the promulgation of evidence-based treatment guidelines. For example, the 2004 NICE guidelines recommended cognitive-behavioral therapy (CBT) for the treatment of BN and BED. Brownley, Berkman, Sedway, Lohr, and Bulik (2007) have provided more recent support for treating BED with CBT. Efficacious treatments are important for reducing the personal and societal burden of suffering resulting from BN or BED. It has been well established that these eating disorders are associated with significant functional impairment (Agras 2001; Hudson, Hiripi, Pope, & Kessler, 2007; Mond & Hay 2007), decreased health-related quality of life (de la Rie, Noordenbos, & van Furth, 2005; Doll, Petersen, & Stewart-Brown, 2005; Hay, Bacaltchuk, & Stefano, 2004; Las Hayas et al., 2007; Masheb & Grilo, 2004), reduced workplace productivity including excess days missed from work (Mond & Hay, 2007), and elevated health services utilization (Mond & Hay, 2007; Robergeau, Joseph, & Silber, 2006; Simon, Schmidt & Pilling, 2005; Striegel-Moore, Leslie, Petrill, Garvin, & Rosenheck, 2000; Striegel-Moore, Wilson, DeBar, Perrin, Roselli, Kraemer et al., in press). In addition, one recent study suggests that persons with binge eating disorders also incur significant out-of-pocket costs related to their disorder, including costs related to food and over-the-counter medications (e.g., laxatives, diet aids) (Crow, Frisch, Peterson, Croll, Raatz, & Nyman, 2009). Recent reports indicate that persons with recurrent binge eating at a less-frequent level than would be required for a full syndrome diagnosis also report significant psychological distress (Striegel-Moore et al., 2000) and functional impairment (Mond & Hay, 2007), and experts have called for a more inclusive definition of BN or BED to accommodate these cases under the diagnosis of an eating disorder (Wilson & Sysko, in press). Therefore, the present study targeted individuals with recurrent binge eating (defined as reporting a minimum average of one binge eating episode per week for the past three months).
A major challenge in mental health services research concerns the “disconnect” between what is known about effective treatment and what services are being provided to patients in routine care (Proctor et al., 2009), and the treatment of eating disorders is no exception. Notwithstanding the strong evidence in support of using CBT for the treatment of binge eating disorders, few individuals presenting with this clinical picture in community settings receive any treatment specifically targeting their eating disorders (Crow, Peterson, Levine, Thuras, & Mitchell, 2004; Striegel-Moore, Wilson et al., in press); when they do receive targeted treatment, it rarely is CBT-based care (Mussell et al., 2000; Simmons, Milnes, & Anderson, 2008).
Resource constraints (e.g., lack of insurance, time away from work) rank high among reasons for not seeking or receiving mental health care reported by consumers (Cachelin & Striegel-Moore, 2006). Lack of training in CBT and negative expectancies about its efficacy compared to other forms of interventions are common reasons reported by providers for not utilizing CBT when treating individuals with an eating disorder (Simmons et al., 2008). Absence of data on the cost of implementing these interventions, and the cost-effectiveness of these services compared to usual care, is a major reason why health care organizations do not adopt evidence-based treatments. These concerns have contributed to the search for self-help versions of CBT. Guided self-help, derived from manual-based cognitive behavioral therapy (CBT-GSH; Fairburn, 2008) has been shown to be effective in treating patients with BN (Banasiak, Paxton, & Hay, 2007) and BED (Grilo & Masheb, 2005; Wilson, Wilfley, Agras & Bryson, in press). While these studies suggest that CBT-GSH can be efficacious, cost-effectiveness analyses were not reported.
Cost-effectiveness analysis (CEA) is a technique employed for comparing the relative value of treatments and answering the question of whether a new treatment (in this case CBT-GSH) is a better value than current practice. CEA, and other forms of economic evaluation, have been used to evaluate numerous mental health and substance abuse interventions (Hargreaves, Shumway, Hu & Cuffel 1998; Chilsholm, Saxena & Ommeren 2006; Anderson, Chisholm & Fuhr 2009; Bray & Zarkin 2006; French 2001), but no studies have used CEA to evaluate services for binge eating disorders. Ideally, CEA is based on data obtained in settings and from patients that are similar to “the real world,” i.e., reflecting the settings and patients into which the target treatment will likely be applied. Prior efficacy studies of CBT-GSH were carried out in specialty settings and with patients presenting with full syndrome BN or BED (Wilson, Grilo & Vitousek, 2007). Yet, eating disorders are most commonly identified and treated in primary care settings, and a majority of individuals presenting for treatment do not meet full syndrome criteria (Hoek & van Hoeken, 2003; Hudson et al., 2007; Striegel-Moore, Perrin, DeBar, Wilson, Roselli, Kraemer., in press). To address this gap, the present study sought to examine the acceptability, outcome, and cost-effectiveness of CBT-GSH when delivered in a typical health care system, a large health-maintenance organization (HMO). In a separate report we described that CBT-GSH was rated as highly acceptable by patients with recurrent binge eating and that, relative to treatment as usual, significantly more participants in the CBT-GSH group were no longer binge eating at post-treatment and at 6- and 12-month follow-ups. This evidence for the efficacy of CBT-GSH and the effectiveness of CBT-GSH in real-world settings is promising. The purpose of this study is to report our findings of the cost and cost-effectiveness of CBT-GSH.
CEA estimates the relative value of treatments by considering the clinical effectiveness and the cost of each treatment in the form of an incremental cost-effectiveness ratio (ICER). Specifically, the ICER is calculated as the additional costs of a new intervention compared with an alternative treatment divided by the additional clinical effects provided by the new intervention compared to the alternative (e.g., $35/depression-free day). Cost-effectiveness is not synonymous with “less expensive.” Rather, cost-effectiveness means that the new treatment is a good value in terms of an additional outcome and depends in part on how a decision-maker values the health outcome and the budget constraints of the organization.
Guidelines for conducting cost-effectiveness analyses suggest that the preferred clinical outcomes measure the intervention’s effect over time and incorporate its impact on health-related quality of life (Gold, Seigel, Russell, & Weinstein, 1996). For example, quality adjusted life years (QALYs) simultaneously measure gains from reduced mortality (e.g., added life years) and reduced morbidity (e.g., improved quality of life, functioning, or wellbeing) and incorporate these into a single measure. QALYs are calculated by measuring the amount of time spent in different health states (e.g., number of days with symptoms or number of symptom-free days) and multiplying this by utility or preference weights that indicate the relative value of these different health states. For example, in depression studies, depression-free days are assigned a utility weight of 1.0 (full health), while days in a depression episode are assumed to have a lower weight, such as 0.6. QALYs allow for broad comparison of cost-effectiveness outcomes between alternative health care treatments within and across health conditions.
Mental health experts have raised significant concerns, however, that currently available health-related quality of life measures do not adequately capture all health outcomes relevant to mental health conditions (Brazier, 2008; Dolan, 2008; Knapp & Mangalore 2007). Hence researchers have created additional measures of symptom burden across time, such as depression-free days (Lave, Frank, Schulberg, & Kamlet, 1998; Lynch et al., 2005) or relapse-free days (Palmer et al., 2006). Measures of symptom-free days are advantageous in that they are closely tied to clinical measures for the specific condition, estimate the impact of the condition over time, and can be converted into QALYs if published preference or disability weights are available. QALYs and symptom-free days are complementary and both are often reported.
Workplace productivity has been considered an important indicator of clinical significance or clinical outcome, and expert guidelines on CEA (Gold et al. 1996) recommend including workplace productivity outcomes as a separate secondary outcome in CEA analyses. In the field of eating disorders, only one study has reported on this variable as a clinical correlate of binge eating, and we are unaware of any study examining workplace productivity as an outcome variable. Specifically, in a large community sample of 1,290 men and 1,757 women, Mond and Hay (2007) found that respondents who reported binge eating were significantly more likely to have missed days from work than individuals who did not report binge eating.
Expert guidelines suggest that CEA should approach costs from a “societal perspective” rather than focusing narrowly on costs incurred by the health care system (Gold et al. 1996, pages 60–61). The societal perspective includes costs to the health care systems, other social services sectors, and patient costs such as time and travel costs. Results of the societal perspective can be important when the benefits associated with a new intervention accrue to one group (e.g., patients), while the costs are borne by another group (e.g., health care system). Cost-effectiveness analyses from more limited perspectives, such as the health care system perspective, can also be useful in health system decision-making since most health care decision-makers are only responsible for the benefits and costs that occur within their own system. In the present study, our primary CEA was from the societal perspective, and we also conducted a secondary analysis from the health care perspective.
In health care systems, decisions to adopt a new service are almost always made in the context of budget constraints, and the populations and circumstances of a particular health care system will generally be somewhat different than the context in which research studies have been conducted. In addition, decision-makers in health care systems may be less familiar with complex statistical techniques employed in research studies. For these reasons, experts suggest that uncertainty around empirical estimates be presented using graphical methods that may be more helpful to typical decision-makers such as health plan managers. Following these expert suggestions, we have included recommended graphical presentations to aid in interpretation of the results of our cost-effectiveness analyses.
The present study was designed to determine the cost-effectiveness of CBT-GSH versus standard clinical care (treatment as usual, TAU) in the treatment of recurrent binge eating. To achieve this aim, we needed to measure several outcomes that, due to a dearth of literature, are of interest in their own right, including the costs of the CBT-GSH treatment and the differential impact of the treatments on number of binge-free days and work productivity. The study utilized data from a randomized controlled intervention trial (RCT) that was conducted in a real-world clinical setting, namely a large, group-model HMO (~ 450,000 members) and with excellent capability for capturing objective costs of health services.
Method
Participants
Participants were 123 health plan members (91.9% female, 96.7% white, and 3.3% Hispanic) who enrolled in an RCT to evaluate the effectiveness of CBT-GSH compared to TAU, as described in detail elsewhere (DeBar et al., 2009; Striegel-Moore, Wilson, et al., in press). Recruitment initially drew from a separate epidemiologic study on eating habits and body image (Striegel-Moore, Rosselli, Perrin, DeBar, Wilson, May et al., in press). This study used a brief screener to identify people likely to screen positive for binge eating disorders. The epidemiologic study included a random sample of male and female health plan members. Briefly, health plan members were randomly selected using the electronic medical record (EMR) and sent letters of invitation to complete a short self-assessment about their eating habits, body shape, weight concerns, and other health and demographic factors. Persons who reported binge eating at least once per week during the past three months (“screen-positive”) were invited to participate in further assessment to verify study eligibility.
Detailed information on our recruitment approach and study eligibility are available elsewhere (Debar et al., 2009; Striegel-Moore, Wilson et al. in press). In brief, recruitment initially involved mailings to a random sample of male and female health plan members for an epidemiological study on eating habits and body image (Striegel-Moore, Rosselli, et al., in press). All participants underwent a two-stage case finding procedure involving initial screening followed by a confirmatory diagnostic interview by study staff unaware of the screening status (Striegel-Moore, Perrin, et al., in press). Briefly, 1,092 persons were eligible per the initial screener, 541 of those refused participation [either did not respond (344) or refused further participation in the study (197)], 551 were interviewed with the comprehensive assessment to confirm presence of recurrent binge eating and eating disorder diagnoses based on the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2000). Of the 551 who were interviewed, 379 did not meet this threshold eating disorder criteria, 19 were excluded for other reasons [e.g., were pregnant, had a current diagnosis of anorexia nervosa, or who were severely obese (BMI>45)], and 30 refused participation in the RCT. There were 123 persons who met the criteria for recurrent binge eating per the comprehensive assessment, agreed to participate, and were randomized to receive either the CBT-GSH intervention program or usual care. Patients were assessed at baseline prior to randomization and were then interviewed blind to intervention status by the study team at 12-, 26-, and 52-weeks’ follow-up.
All participants consented to participation in the study, and the study was approved by all participating institutions’ human subjects review boards. The trial was registered with the National Institutes of Health National Library of Medicine Clinical Trials.gov website.
Intervention
The CBT-GSH intervention program, derived from an efficacious CBT program, used the book Overcoming Binge Eating (Fairburn, 1995) to educate participants on the subject and to provide a step-by-step behavioral self-help program to overcome binge eating. CBT-GSH participants were invited to attend eight brief coaching sessions provided by a master’s-level therapist. The first session lasted 60 minutes; each subsequent session lasted 20–25 minutes. The therapist explained the rationale for using the self-help book and helped keep patients focused on using the book as a guide through the program steps. Additional details of the program are reported elsewhere (Striegel-Moore, Wilson et al., in press.)
The master’s-level mental health therapists who provided the CBT-GSH had previous experience in using CBT for depression but no familiarity with eating disorders or CBT-GSH for treating binge eating. Two of the three therapists were trained in clinical psychology and the third in counseling psychology. The therapists had on average six years of clinical experience; they also attended a 3-hour hour training workshop (conducted by GTW) and completed the CBT-GSH program with two pilot patients under close supervision of the senior study staff prior to conducting study intervention sessions. The therapists were supervised on site and participated in biweekly supervision conference calls with all senior clinical investigators (GWT, LD, RSM) who listened to audio recordings of randomly selected sessions.
TAU involved advising all participants upon randomization of the treatment options within the HMO. Available treatment options included seeking help from primary care providers, nutrition care providers, or self-referral to the specialty mental health department of the HMO; care in the HMO mental health department does not require primary care provider referral. Although some HMO mental health providers have been trained in CBT, most provide eclectic therapy combining a variety of techniques and approaches. No formal manualized CBT approach to eating disorders was available in the HMO at the time of the study. All participants could initiate or continue any services normally provided by the HMO and/or outside services, including specialty mental health care and psychiatric medication. Further, all participants could initiate or continue any non–health products or services that they chose to purchase during the study period. Participants in the usual care condition were provided with information about treatment options within the HMO, but no additional services were provided to the usual care control group. No services or products usually available were limited or discouraged in any way.
Measures
Clinical Outcomes
Binge eating disorder diagnoses and symptoms were assessed by evaluators blinded to treatment assignment at baseline and at 12-, 26-, and 52-week follow-ups using the Eating Disorder Examination (EDE; Fairburn & Cooper, 1993). The EDE is a standardized, semi-structured interview that measures the severity of the core clinical features of eating disorders and generates diagnoses of eating disorders. Binge eating episodes were defined as eating episodes that involved consuming in a short period of time (2 hours or less) more than what most people would eat under similar circumstances and experiencing loss of control during the episodes. The reliability and validity of the EDE have been established in several independent studies, and these studies consistently have supported its use (Fairburn, Cooper & O’Connor, 2008).
Following previous cost-effectiveness analyses in mental health (Lave et al. 1998; Simon et al. 2001; Schoenbaum et al. 2001; Lynch et al. 2005), we used this clinical outcome data to create several summary measures of clinical outcome over time for the cost-effectiveness analysis. Specifically, we used the data from the EDE at each assessment time point to create the clinical outcome, binge-free days. The EDE reports were based on symptoms in the past 28 days, and we used linear interpolation to obtain a score for each day in the interval between each assessment. We summed across the follow-up periods to calculate total binge-free days in the 12 months following the intervention. We consider this our primary clinical outcome for the CEA.
To compare the cost-effectiveness of this intervention with that of other mental health interventions, we also transformed the binge-free days into QALYs by using preference weights from the literature. Preference weights reflect the value of different health or disease states derived from empirical studies or expert opinion. For example, depression-free days are typically assigned a utility weight of 1.0 (full health), while days in a depression episode are estimated to have a lower weight, such as 0.6. To our knowledge, no study to date has directly estimated health-related quality-of-life weights for any type of eating disorder. One international study reported a disability weight for eating disorders in general (Vos & Mathers, 2000), and we used this weight. The weight reported was derived by three expert panels of physicians who developed disability weights on 52 disease categories from the ICD-9 using the person trade-off method of valuation (Southard et al. 2000). The expert panel suggested a disability weight of .28; this estimated weight is comparable to weights for moderate depression reported in the literature. This disability weight suggests that eating disorders decrease a person’s quality of life, such that on days when the person is symptomatic their utility weight would be 0.28 lower than when they are in full health (full health = utility weight of 1.0). Based on this expert-derived disability weight, we assigned a utility weight of 1.0 (full health) to binge-free days, while days that were not binge free are assumed to have a lower weight of 0.72. This approach allows us to compare our results broadly to other mental health interventions; however, because there is so little empirical data estimating utility weights for eating disorders, we consider this a secondary clinical outcome.
Workplace Productivity
In the present study, we obtained self-report data on days lost from work or days of reduced productivity while at work as an additional clinical outcome measure. As recommended by the Public Health Service guidelines on reporting cost-effectiveness analyses (Gold et al., 1996), we reported this productivity measure separately from the main cost-effectiveness outcomes and do not value the lost productive time or include this in the cost-effectiveness analysis.
At each assessment we asked participants two questions. First we asked them to report on the number of full days lost from work due to eating disorder symptoms or concerns since the last assessment. The second question asked participants to report on days of reduced productivity while at work due to eating disorder symptoms since the previous assessment. We then summed across these measures to estimate total days lost due to eating or weight-related symptoms or concerns.
Cost outcomes: Intervention costs
We estimated the total cost of CBT-GSH services from clinical trial records and study staff estimates. Study and HMO accounting records provided payroll costs, cost of facilities and overhead, and information on purchases of goods and services. Study staff estimated the time to complete each intervention task. For example, the intervention therapists kept logs of time spent with participants. Study staff also reported use of capital equipment, space, and supplies needed to produce the intervention.
We included all costs of conducting the intervention, including the individual coaching sessions, bi-weekly expert supervision conference calls with therapists, therapists’ training, and all session materials (workbooks, handouts, etc.). We excluded research-specific costs. We define research specific costs as costs of activities related to participation in a research study that are provided to all participants (both TAU and TAU plus CBT-GSH), and that would not be replicated by a health system if the new intervention was adopted into practice (Lynch et al. 2004). Research specific activities include costs for staff to consent and randomize subjects, costs related to research assessment that would not be replicated in clinical settings, and costs of supervising research related activities.
Cost outcomes: Treatment-as-usual (non–protocol) services
We created comprehensive profiles of usual care HMO services from the EMR and other electronic HMO administrative data. These data, used in previous studies, accurately represent services paid for by the HMO (Hornbrook & Goodman 1996; Lynch et al., 2005). We supplemented HMO data with a self-report survey called the Participant Health Care Utilization Survey (PHCUS), which was developed by members of the study team (FLL, LLD) for a previous study (Clarke et al., 2005) and was adapted for use with patients with eating disorders. The PHCUS asked participants to report any services that they received for their eating disorder or weight-related symptoms or concerns. The survey included health care services as well as social and other types of services received by participants during the study period. At baseline, participants were asked to report on any services they had received or products purchased in the previous three months; at all follow-up points, they were asked to report service and product use since the previous assessment. We cross-checked information reported on the PHCUS with the HMO records (both data sources include information on dates, names of providers, and types of services) and then removed duplicates. We grouped health services into four mutually exclusive categories: weight and eating disorder-related services, medications for mental health problems, other medications, and “all other services.”
The PHCUS survey also included non–medical costs that persons with eating disorders might be likely to incur. For each service reported we asked participants to report out-of-pocket costs, travel, and wait times. We also asked participants to report all expenditures during the study period on products and services to assist in management of eating disorder or weight concerns. The PHCUS survey was interviewer-administered at the major assessment time points of the RCT (baseline and 12-, 26-, and 52-week follow-ups).
For the HMO services, we estimated costs by applying unit costs developed and tested in previous studies (Hornbrook, Goodman & Bennett 1991; Hornbrook & Goodman, 1995; Hornbrook & Goodman, 1996; Lynch et al., 2005) to the HMO utilization measures. These final cost variables represent HMO expenditures. For non–HMO services and products, we used local market unit costs to create final cost variables (unit cost details are available upon request).
We estimated participant costs based on patient utilization data and participant self-reports of estimates of out-of-pocket expenditures and travel and wait time. From these data, we created profiles of participant time spent for the intervention, usual care services, travel to services, and waiting. Economic experts have suggested several approaches to valuing patient time (Gold et al., 1996; Koopmanschap & van Ineveld, 1992; Sculpher, 2001). Wages have been widely used as a proxy value for time spent in interventions or lost from work due to illness (Gold et al., 1996), and this study used wages to value participants’ time. Because the RCT did not collect information on participants’ wages, we used a combination of annual income, marital status, and education to derive estimates of hourly wages for participants as other studies of the cost-effectiveness of mental health programs have done (Chisholm et al., 2001).
Statistical Methods
The purpose of the economic evaluation is to estimate the additional cost of the new intervention (CBT-GSH) over and above the cost of TAU for the additional benefit of the new intervention (CBT-GSH) over and above the benefit of TAU; this is also called the incremental cost-effectiveness. Following methods developed by the Public Health Service’s Task Force on Cost-effectiveness in Health and Medicine (see pages 77–79 of Gold et al for a detailed discussion), we estimate incremental cost-effectiveness by first estimating the average cost and clinical effect in each group (TAU, CBT-GSH), next creating an incremental cost-effectiveness ratio using these averages, and then using non-parametric bootstrapping to create a distribution of incremental cost and effect pairs from the observed data that retain the joint distribution of incremental costs and incremental effects which often are correlated. This distribution is to assess uncertainty around our estimate (O’Brien & Briggs 2002).
The economic evaluation was conducted from a societal perspective and from the health systems perspective. In each instance, an incremental cost-effectiveness ratio was computed as the mean cost difference between CBT-GSH group and TAU group divided by the mean difference in binge-free days (Gold et al., 1996; pages 77–79). In the first analysis, all costs borne by the health care system, other service sectors, and patients were included to represent the societal perspective. In the second analysis, costs were limited to include only those paid for by the health system providing the intervention.
Analyses were carried out on an intention-to-treat basis. We had complete clinical outcome and health services data on 75% of the study participants. For the remainder of the participants, clinical outcome and service-use data were missing at some time points on some measures. Missing data were imputed using multiple imputation with chained equations (Little & Rubin, 2002; Royston, 2004; Royston, 2005) using STATA statistical software. Our procedures assumed that data were missing at random. We included all non–missing values at all time points and baseline demographics in the models that generated imputed estimates of outcomes. We created five imputation datasets and combined estimates such that the standard errors reflect the variability introduced by the imputation process (Little & Rubin, 2002).
The primary CEA was of total societal costs for one year post-randomization. As is typical, costs were not normally distributed. We used the net benefit regression method (Hoch, Briggs, & Willan, 2002; Stinnett & Mullahy, 1998) with ordinary least squares regression analyses to examine cost-effectiveness. This method restates the incremental cost-effectiveness ratio in a linear form thus allowing the use of standard regression approaches to evaluate cost-effectiveness. We used non–parametric bootstrapping with a single model with 1000 replications using the bias corrected and accelerated method (O’Brien & Briggs, 2002; Thompson & Barber, 2000).
Adjusted differences between the CBT-GSH and TAU groups were estimated using ordinary least squares regression models with bootstrap interval estimates; all analyses were adjusted for baseline characteristics including age, gender, and BMI. Hypotheses tests for the clinical and cost outcomes were based on the significance of the group variable in the bootstrapped multiple regression equations (Knapp, McCrone, Fombonne, Beecham, & Wostear, 2002; Mooney & Duval, 1993). For our primary clinical outcomes, binge-free days and days missed from work, we also calculated effect size using Cohen’s d (Cohen, 1988).
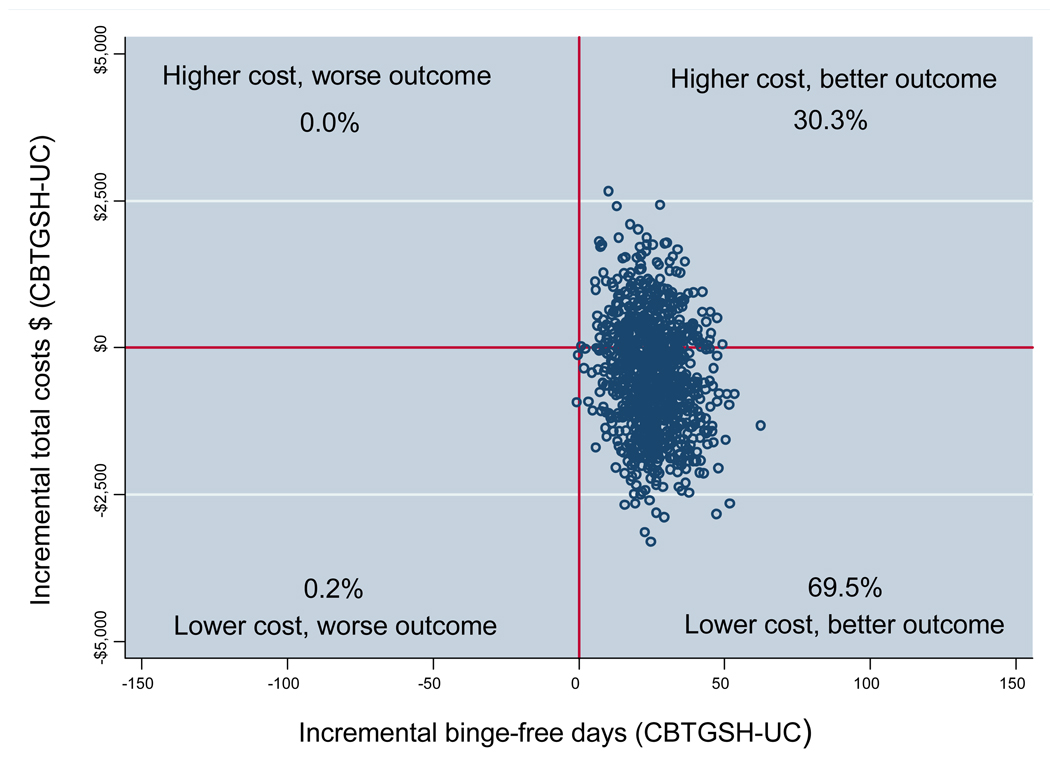
We represent uncertainty in our estimates in two ways. First, we used the bootstrap observations to estimate a 95% confidence interval around the average incremental cost-effectiveness ratios and then we plotted the bootstrapped incremental cost and effect pairs to create an incremental cost-effectiveness plane (Fenwick & Byford, 2005). The incremental cost-effectiveness plane is divided into four quadrants, centered at zero. Incremental cost-effect pairs that fall into the southeast quadrant indicate that the new intervention is less costly and more effective than the alternative treatment. Incremental cost-effect pairs that fall into the northwest quadrant indicate that the new intervention is more costly and less effective than the alternative treatment. The northeast quadrant indicates that the new intervention is more costly and more effective, and the southwest quadrant indicates that the new intervention is less costly and less effective than the alternative treatment. Often the scatter plot of incremental cost and effect pairs falls within more than one of the four quadrants, indicating that there is uncertainty about whether or not the new intervention is cost-effective compared to the alternative.
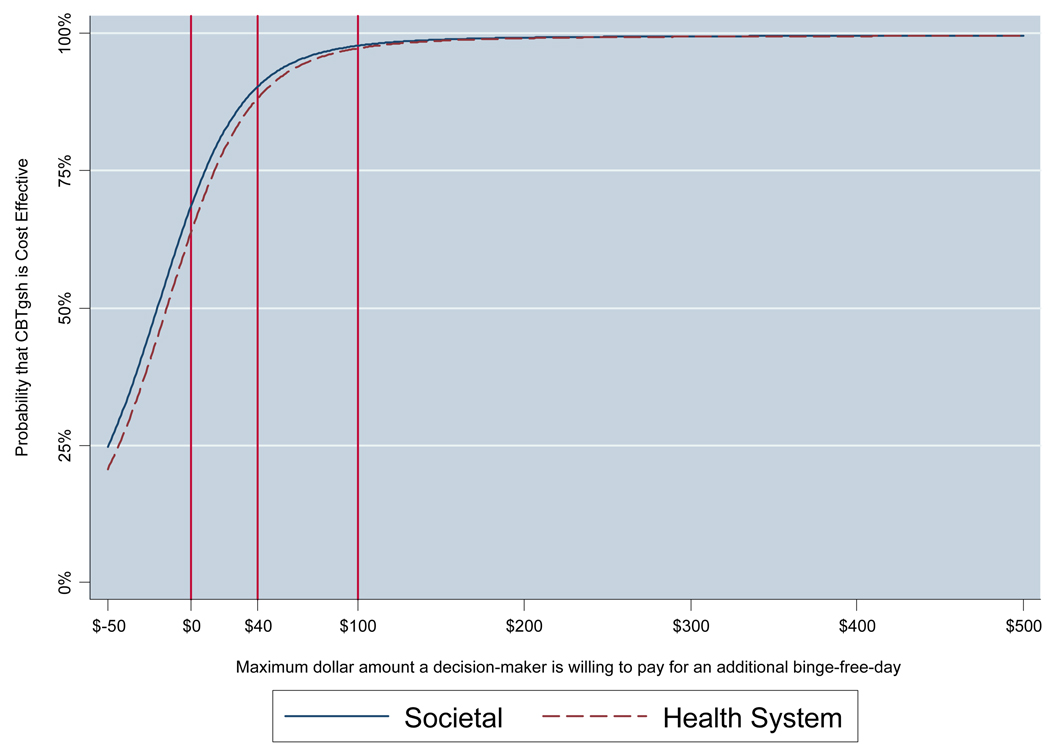
A common result is that a new intervention is more costly and more effective than an alternative. In this situation the decision-maker needs to know the probability that a new intervention is cost-effective compared to the alternative treatment, given the budget available to pay for an additional unit of clinical improvement. A second graphical method, the cost-effectiveness acceptability curve (CEAC; Fenwick & Byford, 2005), is used to address this situation. The CEAC presents the probability that a new intervention is cost-effective compared to its alternative over a range of maximum dollar levels that a decision-maker is willing and able to pay for an additional increment of the clinical outcome (e.g., binge-free day), given budgetary and other constraints.
Results
Sample Description
Table 1 reports selected baseline characteristics of randomized participants; detailed group comparisons have been reported elsewhere (DeBar et al., 2009; Striegel-Moore, Wilson et al., in press). There were no statistically significant differences between the CBT-GSH intervention and TAU groups at baseline.
Table 1.
Baseline Demographic and Clinical Characteristics of Study Sample
| Mean/% (SD/N) | Mean/% (SD/N) | |
|---|---|---|
| Variable | TAU (N=64) | CBT-GSH (N=59) |
| Age | 36.6 (7.9) | 37.8 (7.6) |
| Female | 92.2% (59) | 91.5% (54) |
| White (%) | 95.3% (61) | 98.3% (58) |
| Hispanic (%) | 3.1% (2) | 3.4% (2) |
| ≥ Some college | 79.7% (51) | 84.7% (50) |
| EDE diagnosis | ||
| Bulimia nervosa | 9.4% (6) | 10.2% (6) |
| BED | 42.2% (27) | 47.5% (28) |
| Recurrent binge eating | 48.4% (31) | 42.4% (25) |
| BMI | 30.9 (6.7) | 31.7 (5.7) |
| Workplace productivity | ||
| Total days lost | 4.3 (7.8) | 4.1 (8.3) |
Note: TAU = Treatment As Usual.
CBT-GSH =Guided Self-help Cognitive Behavioral Therapy.
Clinical Outcomes
The CBT-GSH group reported significantly more binge-free days (mean = 330.7, SD = 41.0) than the TAU group (mean = 305.5, SD = 60.3) (z=3.02, p=0.002, d=0.47) over the 12 months of the study. The CBT-GSH group reported fewer days lost from work due to eating-disorder related impairment over the 12 months of the study (mean = 5.3, SD = 18.2) than the TAU group (mean = 11.0, SD = 25.4), but this difference was not statistically significant (z=−1.55, p=0.121, d=0.25). Details of clinical outcomes may be found in our clinical outcomes report (Striegel-Moore, Wilson, et al., in press).
Treatment Costs and Total Costs
The average cost per participant of delivering the CBT-GSH intervention was $167 SD=100); by definition, TAU patients did not receive CBT-GSH, thus incurring no such costs. The direct labor cost of providing the intervention was $115 per participant (average mental health therapist time spent with participants = 5.2 hours), direct non-labor costs of providing the intervention (e.g., intervention materials, space, equipment) were $37 per participant, and other costs of providing the intervention (supervision of staff, and staff training) were $16 per participant.
Both TAU and CBT-GSH participants used a variety of TAU health care services in the 12 months following the intervention. The proportion of TAU participants who saw a TAU mental health provider for eating disorder symptoms or concerns during the 12 months following the intervention was 6.3% and for CBT-GSH was 5.1%. Table 2 presents unadjusted patterns of health care and other costs by group and type of service for the 12 months post-randomization. In the 52 weeks following randomization, 40.4% of the usual care participants reported specialty-health-service use for weight or eating disorders compared to about 16.7% of the CBT-GSH group. The proportion of TAU participants and CBT-GSH participants who used any prescription medications and the distribution of the number of dispensings of prescription medications over the 12 month study period were similar between the groups. However, the average cost of prescription medications was higher in the TAU group.
Table 2.
Unadjusted Mean Cost (in 2006 Dollars) of Services and Patient Costs Following Randomization into CBT-GSH (N = 59) and TAU (N = 64).
| Percent with anyuse |
Mean cost (SD) |
|||
|---|---|---|---|---|
| Type of Service | TAU | CBTgsh | TAU | CBTgsh |
| Health Care Services | ||||
| Weight & eating disorder costs | 40.4% | 16.7% | 287.32 (597.14) | 81.23 (274.95) |
| Other medical costs | 91.2% | 96.3% | 2174.19 (2206.48) | 2432.85 (4642.66) |
| Psychiatric medications | 56.1% | 57.4% | 396.70 (631.81) | 247.10 (632.11) |
| Other medications | 86.0% | 85.2% | 1092.37 (3479.57) | 498.01 (768.21) |
| Total prescription costs | 87.7% | 90.7% | 1489.07 (3588.41) | 745.11 (1275.86) |
| (1) Total TAU health care costs | 95.0% | 98.3% | 3806.38 (5259.28) | 3359.61 (6235.94) |
| (2) Total cost CBTgsh intervention | 167.00 (100.00) | |||
| Patient Costs | ||||
| Time and expenditures for health care services | 62.3% | 31.3% | 126.93 (262.10) | 46.64 (116.50) |
| Non–health services | 52.8% | 29.2% | 118.55 (227.48) | 69.08 (199.72) |
| Over-the-counter medications | 24.5% | 8.3% | 20.04 (47.62) | 1.90 (8.54) |
| Other weight loss products | 52.8% | 33.3% | 26.38 (46.18) | 20.22 (43.13) |
| (3) Total patient costs | 92.8% | 68.5% | 291.64 (356.96) | 143.02 (276.52) |
| (4) Total Costs | 97.8% | 100.0% | 4098.02 (5306.32) | 3670.63 (6254.99) |
| 50th percentile | 2854.58 | 1952.11 | ||
Use of other medical care services (not related to eating disorder or weight) was similar between the groups. The proportion of people using any psychiatric and other medications was similar in both groups, but the CBT-GSH group had lower average costs for both types of medications. Total non-protocol health care costs were $447 lower in the CBT-GSH group on average (see the row labeled “1” in Table 2). Patient time and out-of-pocket costs for all health care services, including non–protocol and intervention health services were lower in the CBT-GSH group compared to usual care. CBT-GSH participants were also less likely to incur non–health-related costs related to weight and eating disorders. TAU participants were more likely to use non-health services (e.g., commercial weight loss programs, self-help programs such as Overeater’s Anonymous, alternative medical care such as hypnosis), over-the-counter medications (e.g., diet pills, weight loss supplements, laxatives), and weight loss products (e.g., DVDs, books, magazines). Total patient costs (see the row labeled “3” in Table 2) were $149 lower in the CBT-GSH group. Mean total costs, the sum of total non–protocol health care (“1”) and intervention costs (“2”), and all patient costs (“3”) were $427 lower in the CBT-GSH group, but this difference was not statistically significant (p=.30, d=0.11).
Cost-effectiveness Analysis
Our primary CEA took the societal perspective, which accounts for all costs and savings incurred within and outside of the health care system and to the patients themselves. Using binge-free days as the clinical outcome, the adjusted incremental cost-effectiveness ratio (ICER) for 12 months following the intervention was negative: ICER= −$20.23 (95% CI=−154.06-113.61). The negative value of the ICER indicates that the intervention is cost saving. Thus the intervention resulted in an incremental cost savings of $20.23 per binge-free day from the societal perspective. Using binge-free days as the clinical outcome, the intervention was also cost-saving from the health plan perspective: ICER= −$14.67 (95% CI=−92.68–107.80). Using QALYs as the clinical outcome, we estimated an adjusted incremental cost per QALY of −$26,847 (95% CI=−312,835 - 38,085) from the societal perspective.
Figure 1 presents the incremental cost-effectiveness plane, a scatter plot in which each point in the figure represents an incremental total societal cost and binge-free-day pair for a single bootstrap replication. Of the observations, 69% indicated that CBT-GSH had better clinical effects and lower total societal costs in comparison to TAU. An additional 30% of the pairs indicated that the intervention had better clinical effects and higher total societal costs. Thus, nearly all (99%) indicate better clinical effects with more than two-thirds of the cases actually showing a cost savings following the intervention.
Figure 1.
Incremental cost-effectiveness plane (N = 123).
Figure 2 presents two cost-effectiveness acceptability curves (CEAC) showing the probability that CBT-GSH is cost-effective compared to TAU over a range of maximum dollar values a decision-maker would be willing and able to pay for an additional binge-free day. The two CEAC represent the societal (solid line) and the health care (broken line) cost perspectives respectively. The x-axis shows maximum dollar amounts a decision-maker is able and willing to pay to achieve an additional binge-free day. The y-axis shows the probability that, given the data, the true cost-effectiveness ratio falls at or below these maximum dollar value amounts (van Hout, Al, Gordon, & Rutten, 1994). To provide concrete examples of how a decision-maker might interpret the CEAC, Figure 2 has two vertical bars showing examples of different levels of payment a decision-maker might be willing and able to pay. For example, if a decision-maker is willing and able to pay a maximum of $40 per additional binge-free day, the probability that the intervention is cost-effective compared to usual care is 90%. In another example, if a decision-maker is willing and able to pay a maximum of $100 per additional binge-free day, then the probability that the intervention is cost-effective compared to usual care is 98%.
Figure 2.
Cost-effectiveness acceptability curve (CEAC): Binge-free days at 12 months following randomization.
Sensitivity analysis with one high-cost outlier removed produced a similar pattern of results to our primary analysis, with cost savings of $39.97 per binge-free day (ICER = −$39.97 [95% CI=−136.62-11.40]) from the societal perspective. Sensitivity analyses using only cases with complete data found similar results (ICER = −$11.65 [95% CI: −126.12 to 140.02]). For days lost from work, results analyzing cases with complete data only found a similar pattern of results as the primary analysis, the CBT-GSH group reported fewer days lost from work due to eating-disorder related impairment over the 12 months of the study (mean = 4.9, SD = 17.8) than the TAU group (mean = 11.9, SD = 27.1) and in this case the difference was statistically significant (z=−2.27 p=0.02, d=0.30).
Discussion
This paper reports results of the first economic evaluation of an evidence-based treatment for recurrent binge eating versus standard clinical care using primary data collected from a randomized clinical trial. We found that CBT-GSH for persons with recurrent binge eating resulted in an average incremental cost savings of $20.23 per binge-free day. Symptom-free days have been used in other areas of psychopathology (e.g., depression) to facilitate comparisons of treatment effects across disorders and to aid in the calculation of estimates of the disorder’s impact on quality of life. Because there are no CEA studies of interventions to treat recurrent binge eating, we compare our result to CEA studies of depression treatments when “depression-free days” was the outcome to help put our results in context of mental health policy in general. Most studies indicate that the average incremental cost per depression-free day is in the range of $15–100 (2006 dollars), yet none of these studies indicates that the depression interventions are cost-saving (Lynch et al., 2005; Schoenbaum et al., 2001; Simon et al., 2001; Simon et al., 2002). Although our study is not directly comparable to this work, our results suggest that CBT-GSH is likely to be at least as cost-effective as many accepted depression treatments.
Based on recommendations from the U.S. Public Health Service Panel on Cost-Effectiveness in Health and Medicine (Gold et al., 1996), a cost-effectiveness ratio for a new intervention that is less than $40,000 per QALY gained is a reasonable investment for the health care system. Our base-case analysis and all our sensitivity analyses meet or exceed this criterion. This suggests that our results present strong evidence that the CBT-GSH intervention is cost effective under current criteria for adopting a new intervention. Although experts have recommended using QALYs as the primary clinical outcome in CEA, we chose to include QALYs as a secondary clinical outcome in this analysis. We chose to take this approach because to date there are no studies that have directly estimated utility weights for any type of eating disorder. We used a weight from the one study that has reported a disability weight for eating disorders to date. This international study reported a disability weight for eating disorders in general (Vos & Mathers, 2000) and the weight was estimated by expert panels of physicians, primarily general practitioners. Future research that directly estimates utility weights for persons with eating disorders in general, and recurrent binge eating in particular, could improve estimation of the relative value of interventions for eating disorders and could help improve the accuracy of policy decisions regarding allocation of resources for eating disorders.
Although our pattern of results suggests lower health care and patient costs in the CBT-GSH group compared to usual care, these results were not statistically significant, and represent a small effect. Of interest is the fact that we found that study participants reported using a considerable amount of non–health plan-related products and services to manage weight and eating disorder concerns. The pattern of results suggests that the CBT-GSH intervention may have reduced the use of these non–health plan-related products and services. To our knowledge, this is the first study that has measured non–health costs related to eating disorders in the context of a randomized clinical trial. Given the prevalence of use identified in this study, future studies of eating disorders may want to consider measuring patient costs for services and products outside the health care system.
Participants in the CBT-GSH group reported significantly more binge-free days than did TAU participants. Our CEA did not consider all costs incurred by patients as a direct result of binge eating and therefore may have underestimated the cost-effectiveness of CBT-GSH. Specifically, we did not include food costs of eating binges. Only one study to date has examined the food costs of eating binges, using one-week food records obtained from a sample of ten women entering treatment for BN (Crow et al., 2009). The estimated average annual costs per person for foods consumed during bulimic episodes was $787.93, which translated into 5.3% of pre-tax income when assuming median earnings for women of comparable demographic characteristics. This suggests that including food costs would likely have resulted in a more substantive difference in overall patient costs between those in the CBT-GSH group compared to TAU.
Diminished workplace productivity has not yet been examined widely in eating disorders. Noteworthy is the fact that even though our sample included a substantial subset of individuals who did not meet full syndrome criteria, the average number of days with diminished workplace productivity due to binge eating was similar to the annual number of days lost from work due to moderate depression (Broadhead, Blazer, George, & Tse 1990; Simon, Revicki, Heiligenstein, Grothaus, VonKorff, Katon, & Hylan 2000), attesting to the clinical significance of recurrent binge eating. This study is the first to report on the impact of treatment on workplace productivity in patients with an eating disorder. Although our primary analysis did not find significant intervention effects on CBT-GSH participants’ workplace productivity, our sensitivity analysis using only the sample of patients with complete data suggests that CBT-GSH may improve workplace productivity outcomes; more information on the effects of eating disorders on workplace outcomes could aid in future policies about treatments for these conditions.
This study has several limitations. The sample in this study was relatively small, had limited racial and ethnic diversity, and was relatively highly educated. Although our sample does limit the generalizability of our results, the size and characteristics of our sample are not atypical in clinical trials of eating disorders (e.g., Byford et al., 2007; Schmidt et al. 2007). Replication of this result in multiple sites and with populations that have greater ethnic and racial diversity would provide valuable information for decision-makers. Future work that included samples with a greater range of educational backgrounds could also help decision-makers to identify whether groups with different levels of literacy and education could benefit equally from this intervention. We included workplace outcomes; however we did not validate these using employer reports of missed days from work, which might have strengthened this measure. Participation in the research assessments might have increased awareness of the negative consequences of recurrent binge eating-related behavior leading to increased help-seeking for binge eating-related services. Similarly, the research assessment could have provided some positive clinical benefit for study participants in either group. Our study was not designed to address these issues directly; however, both TAU and TAU+CBT group participants received the same research assessments such that both groups should have been equally likely to be influenced by the assessments. We included several types of non-health care costs that study participants might have had during the study period, however we did not include the cost of food. Participation in the intervention might have influenced participant food costs, for example, by reducing costs due to reduced binges. Inclusion of food costs could have provided a more comprehensive assessment of the impact of the intervention. Our study compared TAU to TAU+CBT rather than CBT-GSH only compared to TAU. We chose this comparison because it represents how the intervention would be implemented in practice in health care organizations; the new treatment would be offered as an additional treatment, and health plan members would not be denied other regularly available services. However, the limitation of this approach is that some TAU services could also have had a beneficial clinical effect, and our approach does not allow us to rule out this possibility. If TAU services were effective, they could have led to improved clinical outcomes and reduced health care or other costs. Although this is a possibility given our study design, total TAU health care services were not statistically different between the groups. Although we attempted to implement the intervention in a manner that would represent the “real world,” it is likely that our supervision and training standards exceeded usual practice standards. These standards may have led to better outcomes and greater costs than would be experienced in a typical health plan.
Our findings suggest that health plans, and other integrated systems of health care, can intervene to treat recurrent binge eating for a cost similar to or more attractive than other generally accepted medical interventions. However, replication of our results in other settings and populations is needed prior to widespread dissemination of CBT-GSH. In addition, there may be other barriers to adoption of this evidence-based treatment. For example, medical providers often do not recognize eating disorders in their patients, and they may be particularly unaware of the potential negative impact of recurrent binge eating, which may be less familiar than some other types of eating disorders.
Few evidence-based services for recurrent binge eating are currently available to patients in everyday clinical settings, and previous studies indicate that managed care organizations and other insurers that are concerned about increasing costs may be reluctant to cover new services that might attract persons at risk for mental health problems. However, our results indicate that it is possible to provide evidence-based care for recurrent binge eating in a cost-effective manner. In order for this intervention to be adopted in real-world settings, several societal shifts may be necessary—including heightened awareness of the morbidity and costs associated with recurrent binge eating, more therapists trained in the use of manual-based CBT, and the creation of new public policy initiatives to provide incentives for implementing evidence-based programs for recurrent binge eating.
Acknowledgments
This work was supported by grant number MH066966 (to principal investigator R.S.M.) from the the National Institute of Mental Health (NIMH) and the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK) (awarded to Kaiser Foundation Research Institute). The contents of this study are solely the responsibility of the authors and do not necessarily represent the official viewpoints of the NIH, NIMH, NIDDK, or the Kaiser Foundation Research Institute. We thank Dana Foley and Terresa Fair for their excellent editorial and administrative support respectively. Trial registry name: Guided Self-Help Treatment for Binge Eating Disorders (BEST)
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/ccp
Registration number: NCT00158340
URL for registry: http://clinicaltrials.gov/show/NCT00158340
Contributor Information
Frances L. Lynch, Center for Health Research, Kaiser Permanente Northwest, Portland, Oregon, and Department of Public Health and Preventive Medicine, Oregon Health & Sciences University.
Ruth H. Striegel-Moore, Department of Psychology, Wesleyan University.
John F. Dickerson, Center for Health Research, Kaiser Permanente Northwest, Portland, Oregon.
Nancy Perrin, Center for Health Research, Kaiser Permanente Northwest, Portland, Oregon, and School of Nursing, Oregon Health & Sciences University..
Lynn DeBar, Center for Health Research, Kaiser Permanente Northwest, Portland, Oregon, and Department of Public Health and Preventive Medicine, Oregon Health & Sciences University..
G. Terence Wilson, Graduate School of Applied and Professional Psychology, Rutgers, The State University of New Jersey..
Helena C. Kraemer, Department of Psychiatry and Behavioral Sciences, Stanford University.
References
- Agras WS. The consequences and costs of the eating disorders. The Psychiatric Clinics of North America. 2001;24:371–379. doi: 10.1016/s0193-953x(05)70232-x. [DOI] [PubMed] [Google Scholar]
- Anderson P, Chisholm D, Fuhr DC. Effectiveness and cost-effectiveness of policies and programmes to reduce the harm caused by alcohol. Lancet. 2009 Jun 27;373(9682):2234–2246. doi: 10.1016/S0140-6736(09)60744-3. [DOI] [PubMed] [Google Scholar]
- Banasiak SJ, Paxton SJ, Hay PJ. Perceptions of cognitive behavioural guided self-help treatment for bulimia nervosa in primary care. Eating Disorders. 2007;15:23–40. doi: 10.1080/10640260601044444. [DOI] [PubMed] [Google Scholar]
- Bray JW, Zarkin GA. Economic evaluation of alcoholism treatment. Alcohol Research & Health. 2006;29(1):27–33. [PMC free article] [PubMed] [Google Scholar]
- Brazier J. Measuring and valuing mental health for use in economic evaluation. Journal of Health Services Research & Policy. 2008;13 Suppl. 3:70–75. doi: 10.1258/jhsrp.2008.008015. [DOI] [PubMed] [Google Scholar]
- Broadhead WE, Blazer DG, George LK, Tse CK. Depression, Disability Days, and Days Lost From Work in a Prospective Epidemiologic Survey. Journal of the American Medical Association, 264. 1990;19:2524–2528. [PubMed] [Google Scholar]
- Brownley KA, Berkman ND, Sedway JA, Lohr KN, Bulik CM. Binge eating disorder treatment: a systematic review of randomized controlled trials. The International Journal of Eating Disorders. 2007;40:337–348. doi: 10.1002/eat.20370. [DOI] [PubMed] [Google Scholar]
- Byford S, Barrett B, Roberts C, Clark A, Edwards V, Smethurst N, Gowers SG. Economic evaluation of a randomised controlled trial for anorexia nervosa in adolescents. The British Journal of Psychiatry. 2007;191:436–440. doi: 10.1192/bjp.bp.107.036806. [DOI] [PubMed] [Google Scholar]
- Cachelin FM, Striegel-Moore RH. Help seeking and barriers to treatment in a community sample of Mexican American and European American women with eating disorders. The International Journal of Eating Disorders. 2006;39:154–161. doi: 10.1002/eat.20213. [DOI] [PubMed] [Google Scholar]
- Chisholm D, Godfrey E, Ridsdale L, Chalder T, King M, Seed P, et al. Chronic fatigue in general practice: economic evaluation of counseling versus cognitive behaviour therapy. The British Journal of General Practice. 2001;51:15–18. [PMC free article] [PubMed] [Google Scholar]
- Chilsholm D, Saxena S, Ommeren M. Geneva: World Health Organization; Dollars, DALYs and Decisions: Economic Aspects of the Mental Health System. 2006
- Clarke G, Debar L, Lynch F, Powell J, Gale J, O’Connor E, et al. A randomized effectiveness trial of brief cognitive-behavioral therapy for depressed adolescents receiving antidepressant medication. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:888–898. [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Crow SJ, Peterson CB, Levine AS, Thuras P, Mitchell JE. A survey of binge eating and obesity treatment practices among primary care providers. The International Journal of Eating Disorders. 2004;35:348–353. doi: 10.1002/eat.10266. [DOI] [PubMed] [Google Scholar]
- Crow SJ, Frisch MJ, Peterson CB, Croll J, Raatz SK, Nyman JA. Monetary costs associated with bulimia. The International Journal of Eating Disorders. 2009;42:81–83. doi: 10.1002/eat.20581. [DOI] [PubMed] [Google Scholar]
- de la Rie SM, Noordenbos G, van Furth EF. Quality of life and eating disorders. Quality of Life Research. 2005;14:1511–1522. doi: 10.1007/s11136-005-0585-0. [DOI] [PubMed] [Google Scholar]
- DeBar LL, Yarborough BJ, Striegel-Moore RH, Rosselli F, Perrin N, Wilson GT, et al. Recruitment for a guided self-help binge eating trial: potential lessons for implementing programs in everyday practice settings. Contemporary Clinical Trials. 2009;30:326–333. doi: 10.1016/j.cct.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan P. Developing methods that really do value the ‘Q’ in the QALY. Health Economics, Policy, and Law. 2008;3:69–77. doi: 10.1017/S1744133107004355. [DOI] [PubMed] [Google Scholar]
- Doll HA, Petersen SE, Stewart-Brown SL. Eating disorders and emotional and physical well-being: Associations between student self-reports of eating disorders and quality of life as measured by the SF-36. Quality of Life Research. 2005;14:705–717. doi: 10.1007/s11136-004-0792-0. [DOI] [PubMed] [Google Scholar]
- Domino ME, Burns BJ, Silva SG, Kratochvil CJ, Vitiello B, Reinecke MA, et al. Cost-effectiveness of treatments for adolescent depression: results from TADS. The American Journal of Psychiatry. 2008;165:588–596. doi: 10.1176/appi.ajp.2008.07101610. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z. The eating disorder examination. 12th ed. New York: Guilford Press; 1993. [Google Scholar]
- Fairburn CG. Overcoming Binge Eating. New York: Guilford Publications; 1995. [Google Scholar]
- Fairburn CG, Beglin SJ. Eating Disorder Examination Questionnaire. New York: Guilford Press; 2008. [Google Scholar]
- Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. The British Journal of Psychiatry. 2005;187:106–108. doi: 10.1192/bjp.187.2.106. [DOI] [PubMed] [Google Scholar]
- French MT. Economic evaluation of alcohol treatment services. Recent Developments in Alcoholism. 2001;15:209–228. doi: 10.1007/978-0-306-47193-3_12. [DOI] [PubMed] [Google Scholar]
- Gold MR, Seigel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. Oxford: Oxford University Press; 1996. [Google Scholar]
- Grilo CM, Masheb RM. A randomized controlled comparison of guided self-help cognitive behavioral therapy and behavioral weight loss for binge eating disorder. Behaviour Research Therapy. 2005;43:1509–1525. doi: 10.1016/j.brat.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Hay PJ, Bacaltchuk J, Stefano S. Psychotherapy for bulimia nervosa and binging. Cochrane Database of Systematic Reviews. 2004:CD000562. doi: 10.1002/14651858.CD000562.pub2. [DOI] [PubMed] [Google Scholar]
- Hargreaves WA, Shumway M, Hu TW, Cuffel B. Cost-Outcome Methods for Mental Health. San Diego, CA: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Economics. 2002;11:415–430. doi: 10.1002/hec.678. [DOI] [PubMed] [Google Scholar]
- Hornbrook MC, Goodman MJ, Bennett MD. Assessing health plan case mix in employed populations: ambulatory morbidity and prescribed drug models. Advances in Health Economics and Health Services Research. 1991;12:197–232. [PubMed] [Google Scholar]
- Hornbrook MC, Goodman MJ. Assessing relative health plan risk with the RAND-36 health survey. Inquiry. 1995;32:56–74. [PubMed] [Google Scholar]
- Hornbrook MC, Goodman MJ. Chronic disease, functional health status, and demographics: a multi-dimensional approach to risk adjustment. Health Services Research. 1996;31:283–307. [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp M, McCrone P, Fombonne E, Beecham J, Wostear G. The Maudsley long-term follow-up of child and adolescent depression: 3. Impact of comorbid conduct disorder on service use and costs in adulthood. The British Journal of Psychiatry. 2002;180:19–23. doi: 10.1192/bjp.180.1.19. [DOI] [PubMed] [Google Scholar]
- Knapp M, Mangalore R. The trouble with QALYs…. Social Psychiatry and Psychiatric Epidemiology. 2007;16:289–293. doi: 10.1017/s1121189x00002451. [DOI] [PubMed] [Google Scholar]
- Koopmanschap MA, van Ineveld BM. Towards a new approach for estimating indirect costs of disease. Social Science & Medicine. 1992;34:1005–1010. doi: 10.1016/0277-9536(92)90131-9. [DOI] [PubMed] [Google Scholar]
- Las Hayas C, Quintana JM, Padierna JA, Bilbao A, Munoz P, Francis CE. Health-related Quality of Life for Eating Disorders questionnaire version-2 was responsive 1-year after initial assessment. Journal of Clinical Epidemiology. 2007;60:825–833. doi: 10.1016/j.jclinepi.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Lave JR, Frank RG, Schulberg HC, Kamlet MS. Cost-effectiveness of treatments for major depression in primary care practice. Archives of General Psychiatry. 1998;55:645–651. doi: 10.1001/archpsyc.55.7.645. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2nd ed. New Jersey: John Wiley and Sons; 2002. [Google Scholar]
- Lynch F, Valanis B, Whitlock E, Smith S. Cost-effectiveness of a tailored intervention to increase screening in HMO women overdue for pap test and mammography services. Prev Med. 2004;38(4):403–411. doi: 10.1016/j.ypmed.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Lynch FL, Hornbrook M, Clarke GN, Perrin N, Polen MR, O’Connor E, et al. Cost-effectiveness of an intervention to prevent depression in at-risk teens. Archives of General Psychiatry. 2005;62:1241–1248. doi: 10.1001/archpsyc.62.11.1241. [DOI] [PubMed] [Google Scholar]
- Masheb RM, Grilo CM. Quality of life in patients with binge eating disorder. Eating and Weight Disorders. 2004;9:194–199. doi: 10.1007/BF03325066. [DOI] [PubMed] [Google Scholar]
- Mond JM, Hay PJ. Functional impairment associated with bulimic behaviors in a community sample of men and women. The International Journal of Eating Disorders. 2007;40:391–398. doi: 10.1002/eat.20380. [DOI] [PubMed] [Google Scholar]
- Mooney CZ, Duval RD. Bootstrapping: A Nonparametic Approach to Statistical Inference. Vol. 95. Thousand Oaks: Sage Publications; 1993. [Google Scholar]
- Mussell MP, Crosby RD, Crow SJ, Knopke AJ, Peterson CB, Wonderlich SA, et al. Utilization of empirically supported psychotherapy treatments for individuals with eating disorders: A survey of psychologists. The International Journal of Eating Disorders. 2000;27:230–237. doi: 10.1002/(sici)1098-108x(200003)27:2<230::aid-eat11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- O’Brien BJ, Briggs AH. Analysis of uncertainty in health care cost-effectiveness studies: an introduction to statistical issues and methods. Statistical Methods in Medical Research. 2002;11:455–468. doi: 10.1191/0962280202sm304ra. [DOI] [PubMed] [Google Scholar]
- Palmer S, Davidson K, Tyrer P, Gumley A, Tata P, Norrie J, et al. The cost-effectiveness of cognitive behavior therapy for borderline personality disorder: results from the BOSCOT trial. Journal of Personality Disorders. 2006;20:466–481. doi: 10.1521/pedi.2006.20.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor EK, Landsverk J, Aarons G, Chambers D, Glisson C, Mittman B. Implementation research in mental health services: an emerging science with conceptual, methodological, and training challenges. Administration and Policy in Mental Health. 2009;36:24–34. doi: 10.1007/s10488-008-0197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robergeau K, Joseph J, Silber TJ. Hospitalization of children and adolescents for eating disorders in the State of New York. The Journal of Adolescent Health. 2006;39:806–810. doi: 10.1016/j.jadohealth.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Royston P. Multiple imputation of missing values. Stata Journal. 2004;4:227–241. [Google Scholar]
- Royston P. Multiple imputation of missing values: update. Stata Journal. 2005;5:118–201. [Google Scholar]
- Schmidt U, Lee S, Beecham J, Perkins S, Treasure J, Yi I, et al. A randomized controlled trial of family therapy and cognitive behavior therapy guided self-care for adolescents with bulimia nervosa and related disorders. The American Journal of Psychiatry. 2007;164:591–598. doi: 10.1176/ajp.2007.164.4.591. [DOI] [PubMed] [Google Scholar]
- Schoenbaum M, Unutzer J, Sherbourne C, Duan N, Rubenstein LV, Miranda J, et al. Cost-effectiveness of practice-initiated quality improvement for depression: results of a randomized controlled trial. The Journal of the American Medical Association. 2001;286:1325–1330. doi: 10.1001/jama.286.11.1325. [DOI] [PubMed] [Google Scholar]
- Sculpher M. The role and estimation of productivity costs in economic evaluation. Oxford, England: Oxford University; 2001. [Google Scholar]
- Simmons AM, Milnes SM, Anderson DA. Factors influencing the utilization of empirically supported treatments for eating disorders. Eating Disorders. 2008;16:342–354. doi: 10.1080/10640260802116017. [DOI] [PubMed] [Google Scholar]
- Simon GE, Revicki D, Heiligenstein J, Grothaus L, VonKorff M, Katon WJ, Hylan TR. Recovery from Depression, Work Productivity, and Health Care Costs Among Primary Care Patients. General Hospital Psychiatry. 2000;22:153–162. doi: 10.1016/s0163-8343(00)00072-4. [DOI] [PubMed] [Google Scholar]
- Simon GE, Katon WJ, VonKorff M, Unutzer J, Lin EH, Walker EA, et al. Cost-effectiveness of a collaborative care program for primary care patients with persistent depression. The American Journal of Psychiatry. 2001;158:1638–1644. doi: 10.1176/appi.ajp.158.10.1638. [DOI] [PubMed] [Google Scholar]
- Simon GE, Von KM, Ludman EJ, Katon WJ, Rutter C, Unutzer J, et al. Cost-effectiveness of a program to prevent depression relapse in primary care. Medical Care. 2002;40:941–950. doi: 10.1097/00005650-200210000-00011. [DOI] [PubMed] [Google Scholar]
- Simon J, Schmidt U, Pilling S. The health service use and cost of eating disorders. Psychological Medicine. 2005;35:1543–1551. doi: 10.1017/S0033291705004708. [DOI] [PubMed] [Google Scholar]
- Southard ME, Essink-Bot ML, Bonsel GJ. Disability weights for diseases: A modified protocol and results for a Western European region. European Journal of Public Health. 2000;10:24–30. [Google Scholar]
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Medical Decision Making. 1998;18:S68–S80. doi: 10.1177/0272989X98018002S09. [DOI] [PubMed] [Google Scholar]
- Striegel-Moore RH, Leslie D, Petrill SA, Garvin V, Rosenheck RA. One-year use and cost of inpatient and outpatient services among female and male patients with an eating disorder: evidence from a national database of health insurance claims. The International Journal of Eating Disorders. 2000;27:381–389. doi: 10.1002/(sici)1098-108x(200005)27:4<381::aid-eat2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Striegel-Moore RH, Perrin N, DeBar L, Wilson GT, Rosselli F, Kraemer HC. Screening for binge eating disorders using the Patient Health Questionnaire in a community sample. International Journal of Eating Disorders. doi: 10.1002/eat.20694. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striegel-Moore RH, Rosselli F, Perrin N, Debar L, Wilson GT, May A, et al. Gender difference in the prevalence of eating disorder symptoms. International Journal of Eating Disorders. doi: 10.1002/eat.20625. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striegel-Moore RH, Wilson GT, Debar L, Perrin N, Kramer H, Rosselli F, et al. Effectiveness of guided self-help for binge eating disorders, results of the BEST study. Journal of Consulting and Clinical Psychology. (in press) [Google Scholar]
- Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed? British Medical Journal. 2000;320:1197–1200. doi: 10.1136/bmj.320.7243.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and C/E-ratios alongside a clinical trial. Health Economics. 1994;3:309–319. doi: 10.1002/hec.4730030505. [DOI] [PubMed] [Google Scholar]
- Vos T, Mathers CD. The burden of mental disorders: a comparison of methods between the Australian burden of disease studies and the Global Burden of Disease study. Bulletin of the World Health Organization. 2000;78:427–438. [PMC free article] [PubMed] [Google Scholar]
- Wilson GT, Grilo CM, Vitousek KM. Psychological treatment of eating disorders. The American Psychologist. 2007;62:199–216. doi: 10.1037/0003-066X.62.3.199. [DOI] [PubMed] [Google Scholar]
- Wilson GT. Psychological treatments of binge eating disorder. Archives of General Psychiatry. doi: 10.1001/archgenpsychiatry.2009.170. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GT, Wilfley DE, Agras W, Bryson SW. Psychological treatments of binge eating disorder. Archives of General Psychiatry. doi: 10.1001/archgenpsychiatry.2009.170. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GT, Sysko R. Frequency of Binge Eating Episodes in Bulimia Nervosa and Binge Eating Disorder: Diagnostic considerations. International Journal of Eating Disorders. doi: 10.1002/eat.20726. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]