Abstract
Although it was proposed over a century ago that feedback from facial expressions influence emotional experience, tests of this hypothesis have been equivocal. Here we directly tested this facial feedback hypothesis (FFH) by comparing the impact on self-reported emotional experience of BOTOX injections (which paralyze muscles of facial expression) and a control Restylane injection (which is a cosmetic filler that does not affect facial muscles). When examined alone, BOTOX participants showed no pre- to post-treatment changes in emotional responses to our most positive and negative video clips. Between-groups comparisons, however, showed that relative to controls, BOTOX participants exhibited an overall significant decrease in the strength of emotional experience. This result was attributable to a) a pre- vs. post decrease in responses to mildly positive clips in the BOTOX group and b) an unexpected increase in responses to negative clips in the Restylane control group. These data suggest that feedback from facial expressions is not necessary for emotional experience, but may influence emotional experience in some circumstances. These findings point to specific directions for future work clarifying the expression-experience relationship.
Keywords: BOTOX, Emotional Experience, Facial Expression, Facial Feedback, Embodied Emotion
One of the most socially significant of human behaviors is expression of emotions on the face. Accordingly, scientific interest in facial expressions has a long history (Darwin, 1872) and is as alive today as ever (Gross, 1998; Niedenthal, 2007). Much of this interest has concerned the role of emotional expressions as social signals of internal states, and the mechanisms by which we recognize these expressions in others (e.g. Adolphs, 2002; Ekman, Friesen, & Ancoli, 2001; Olsson & Phelps, 2007). Comparatively less attention has been paid, however, to the possibility that facial expressions are not only external manifestations of internal states, but can themselves trigger or modulate emotional experiences. First championed by William James over a century ago (James, 1894), this view is now commonly known as the Facial Feedback Hypothesis (FFH) (Tomkins, 1962).
Though it has been actively pursued since that time, support for the FFH has been somewhat mixed. For example, the FFH predicts two complementary effects: posing a facial expression should increase the intensity of emotional experience, and inhibiting facial expressions should decrease it. However, only the former effect has been found consistently (e.g. Gross, 1998; Keillor, Barrett, Crucian, Kortenkamp, & Heilman, 2002; Strack, Martin, & Stepper, 1988) (for reviews see McIntosh, 1996; Soussignan, 2004). Additionally, strong and weak versions of the FFH have been presented, in which facial feedback either completely determines, or merely influences, emotional experience. Only the weak version has received support (Keillor et al., 2002).
In many of these studies, participants were instructed to hold their faces still, to flex facial muscles for ostensibly non-emotional reasons, or to maintain a specific expression. This leads to two potential sets of problems. First, explicit instructions regarding facial movement or expression could influence participants’ perceptions (and reports) of their emotional experience (Strack et al., 1988). Such instructions can also influence emotional reports through other channels. They may be distracting (Davis, In Press; Davis, Senghas, & Ochsner, In Press), and there is the potential that participants might use cognitive strategies of emotion regulation in order to help limit facial expressivity, even if they do not believe the study hypothesis pertains to emotional expressivity (Davis et al., In Press). Furthermore, any results obtained could be attributed either to a) the mental processes engaged in producing an expression and/or in flexing facial muscles, or b) the feedback from the face once a facial movement occurs or an expression is formed. That is, they may be either productive or reactive.
These problems were partially avoided in a case study of a patient with Guillain-Barre syndrome (Keillor et al., 2002). Despite total facial paralysis, this patient reported normal emotional experience. However, it is difficult to generalize from this finding to typical functioning because the disorder may involve other, more widespread effects (NINDS, 2007). Furthermore, the patient’s pre-paralysis emotional status is not known. Consequently, any changes in emotional experience from before to after the onset of the syndrome would not have been detected.
A more controlled way to investigate the connection between facial expression and emotional experience would be to examine individuals who transiently lose the ability to move facial muscles related to the expression of emotion. This approach was taken in two studies of individuals who received injections of BOTOX® (Allergan, Inc., California, USA) into the corrugator supercilii for cosmetic treatment of glabellar frown lines between the eyes. The active ingredient of BOTOX (botulinum toxin type A, BoNT-A) is a neurotoxin that paralyzes the muscle into which it is injected (Dolly & Aoki, 2006). In the first study, ten patients with ongoing treatment resistant major depression were given BoNT-A injections (Finzi & Wasserman, 2006). Two months after treatment nine of ten participants were no longer clinically depressed, suggesting that BoNT-A injections – and facial feedback – can affect mood. This conclusion is tempered, however, by the facts that participants in this study were aware of the hypothesis, there was no control group, and these findings pertain to people with clinical diagnoses and to the alleviation of their clinical condition, which can include non-emotional as well as emotional changes. The second study used functional imaging to compare brain activity during imitation of angry and sad expressions in groups who either did or did not receive BoNT-A injections (Hennenlotter et al, 2009). They found that during imitation of anger, BoNT-A decreased activity in the amygdala and its coupling with brainstem nuclei involved in autonomic control. Although this ingenious approach establishes a link between the inability to voluntarily contract specific muscles and neural systems implicated in triggering emotional responses, it provides only indirect evidence in favor of the FFH because it did not measure changes in emotional experience.
The present study built on and attempted to address some of the methodological limitations of prior work by investigating the connection between facial expression and emotional experience by comparing healthy participants’ self-reports of emotional experience before and after they received one of two types of cosmetic facial injections used to treat facial wrinkles. One group received injections of BOTOX®. The second group received injections of Restylane® (Medicis Pharmaceutical Corp., Arizona, USA) whose active ingredient is hyaluronic Acid (HA). The mechanism of action of HA differs critically from that of BoNT-A in that HA is a filler and has no effect on facial muscles (Brandt & Cazzaniga, 2007).
We predicted that if facial feedback can influence emotional experience, then individuals who received BoNT-A injections would show a drop in self-reported emotional experience relative to any change shown by individuals who received HA injections. To test this prediction, we assessed emotional response to positive and negative video clips before and after treatment.
Methods
All procedures were carried out with approval of the Columbia University Institutional Review Board.
Participants
Seventy-two women between the ages of 27 and 60 who planned to receive cosmetic facial injections took part in the experiment. Four did not return for the second session, and their data were therefore excluded from all analyses, leaving 33 in the BoNT-A group (Mean age = 46, SD = 6.7 years) and 35 in the HA group (Mean age = 45, SD = 7.3 years). None had had the procedures before. None were taking medications for the diagnosis of depression. See Table 1 for demographic information regarding level of education, race, and income.
Table 1. Demographic Information.
| Restylane (HA) | BOTOX (BoNT-A) | |
|---|---|---|
| Education | ||
|
| ||
| HS or less | 4 | 4 |
| Some college | 5 | 11 |
| College | 13 | 12 |
| Some grad | 3 | 1 |
| Graduate | 10 | 5 |
|
| ||
| Race | ||
|
| ||
| White | 25 | 29 |
| Black | 6 | 1 |
| Hispanic* | 3 | 1 |
| Mixed | 1 | 2 |
|
| ||
| Annual Household Income Range | ||
|
| ||
| <$30K | 9 | 4 |
| $30-60K | 11 | 10 |
| $60-100K | 9 | 14 |
| $100-200K | 64 | 5 |
Participants who indicated Hispanic and did not provide additional information. (Other categories may include people of Hispanic origin).
Recruitment
Participants were recruited via newspaper advertisements and through notification by their physician. Participants were compensated for participation in the psychological experiment by receiving their cosmetic treatment for no fee. Thus, there was little or no value to participation unless one wanted to receive the cosmetic procedure. Compensation in this form was made possible by donation of BOTOX® Cosmetic (BoNT-A) by Allergan, Inc., California, USA, and Restylane® (HA) by Medicis Pharmaceutical Corp., Arizona, USA.
Participants elected whether to have a procedure, and which procedure they wished to have. Thus, the study was a quasi-experimental design. This design was necessary to avoid coercing participants to obtain a procedure they would not otherwise have chosen. To prevent self-selection bias from influencing group differences, participants elected their procedure after completing Session 1 and upon meeting with the physician for treatment.
The physician who administered all treatments (F.B.) has offices in both New York City and Coral Gables, Florida. Participants were recruited in both locations. Of the 68 who completed both sessions, 49 were in New York (23 BoNT-A and 26 HA), and 19 in Coral Gables (ten BoNT-A and nine HA). As described below, analyses tested for and found no significant effects of testing location.
Stimuli
Both positive and negative video clips were used so that a decrease in the strength of self-reported emotional experience could be distinguished from an overall shift towards greater positivity or negativity. Two additional weakly emotional video clips were included to mask our interest in the strongly positive and negative clips that were of primary interest, and to provide additional observations. Informal pre-testing suggested that finding truly neutral clips for this purpose would be difficult. We therefore selected mildly positive emotional clips to serve as these filler stimuli. The first clip, which was always the same for each participant, was presented at the beginning of the session as a training trial and was not analyzed.
Stimuli thus consisted of eight video clips (mean length = 2 min, 20 sec, SD = 12 sec), divided into two sets, set A and set B, one to be shown during each session. Order of sets was counterbalanced such that half of the subjects from each group viewed set A in the first session and B in the second, and vice-versa. Each set contained one negative, one positive, and two mildly positive clips. The negative clips were from the NBC™ television show Fear Factor (for example, one depicted a man eating a live worm sausage). The mildly positive clips were from documentary footage and television (for example, a segment on the painter Jackson Pollock). The positive clips were selections from the ABC™ television show America’s Funniest Videos. One set of the stimuli (including one negative, one positive, and two mildly positive video clips) has been used in prior research, and has been shown to elicit expected levels of emotional facial expressions in a normal population (Davis et al., In Press).
Information regarding the cosmetic treatments
BoNT-A blocks the release of the neurotransmitter acetylcholine in the terminal bouton of the motor neuron at the junction with the muscle into which it is injected. Critically, after BoNT-A injections, nerve signals may remain intact as they travel from the brain and ultimately down the axon to the muscle. At the muscle synapse, however, no neurotransmitter is released, the muscle lies inert, and thus no feedback about muscle movement is sent back to the brain from the periphery. BoNT-A can take up to two weeks to take full effect, and its effects typically persist for four to six months, until the neuron regenerates function (Dolly & Aoki, 2006). HA, in contrast, is hydrophilic, and reduces the appearance of wrinkles by filling the area under the skin with water captured by the acid. Its effects typically last six months (Brandt & Cazzaniga, 2007).
Participants who elected BoNT-A received injections into the glabellar region (the corrugator supercilii muscles involved in furrowing the brow) and into the region lateral to the eyes (the orbicularis oculi muscles involved in producing “laugh lines” or “crow’s feet”). Six participants received injections into the corrugator supercilii only, and one into the orbicularis oculi only. Participants who elected HA received injections into the nasolabial folds (the lines beginning at the nose and extending down around the sides of the mouth). See Figure 1.
Figure 1.
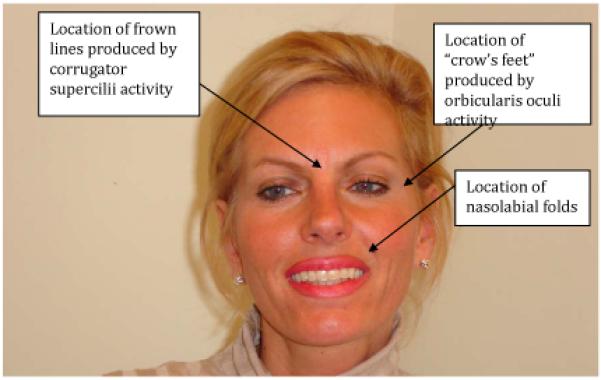
Locations of wrinkles targeted by procedures.
Procedures
The sequence of the procedures is illustrated in Figure 2.
Figure 2.
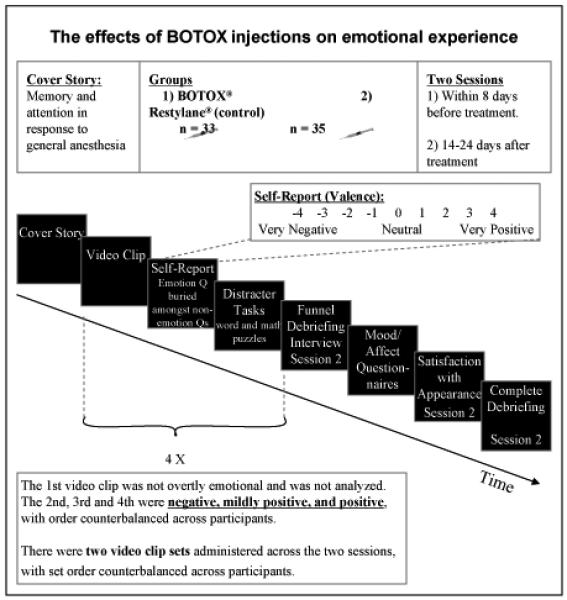
An illustration of the sequence of steps within the protocol.
Cover Story
To limit the degree to which participants believed that the study pertained specifically to BoNT-A or HA, participants were told that the experiment was testing patients receiving a variety of medical procedures in order to study the effects of general anesthesia on memory and attention, and in particular, whether such effects could be found up to two or three weeks following treatment. As their procedure did not include general anesthesia, all participants were told that they were in the non-anesthesia comparison group. By mentioning our interest in the longer-term effects of their procedure, this story provided a motivation for asking participants to return for a second session without needing to make reference to the two-week window necessary for BoNT-A to take full effect. We nonetheless brought participants back as soon after the procedures had taken full effect as possible, to control for longer term changes in participants’ lives. In this way, if indeed there were an effect on emotional experience, we would have the maximum potential to detect it.
Presentation of stimuli
Participants took part in two experimental sessions, one before and one after treatment, specifically, within the eight days before, and 14 to 24 days after treatment.1
Session 1 began with the training video clip. This clip was used to acquaint participants with the experimental procedure and the questions that followed each clip. Data pertaining to this first clip were excluded from the analyses. The second, third, and fourth clips were negative, mildly positive, and positive, presented in counterbalanced order across participants and groups. Following each video were a number of filler questions, the majority of which pertained to aspects of memory and attention that were of no interest. Amidst the filler questions was the item pertaining to our variable of interest: the number that most closely represented how participants felt as a result of watching the video clip, given a scale from “Very Negative” at -4, to “Neutral” at 0, to “Very Positive” at +4. After all self-report items were completed, participants completed math and word puzzles to clear their minds and reduce potential carryover effects from one stimulus to the next, and to further distract attention from our interest in emotional response. At the end of Session 1, participants completed the Beck Depression Inventory (BDI) (Beck, Steer, & Garbin, 1988) and the Positive and Negative Affect Schedules (PANAS) (Watson, Clark, & Tellegen, 1988) in order to control for potential baseline differences between the groups on variables related to daily affect and mood.
Session 2 was identical to Session 1, except as follows: Participants who had seen one set of video clips in Session 1 saw the complementary set in Session 2, with set order counterbalanced across participants and groups. A “funnel” debriefing interview (described below) followed the final video clip. Participants then reported on their satisfaction with their appearance as a result of their procedures, using a nine-point Likert scale from 1: “Completely unsatisfied” to 9: “Completely satisfied”, in order to investigate whether this variable influenced emotional self-reports. At the completion of the study participants were fully debriefed, including an explanation of the cover story and the true purposes of the research. They were encouraged to ask questions to ensure full understanding.
Data was unavailable for one participant from each of the PANAS and satisfaction with appearance measures. Missing data points were replaced with group means.
Debriefing interview
A funnel debriefing interview, modeled after those used by Bargh and colleagues (Bargh, Chen, & Burrows, 1996), incrementally guided participants towards the hypothesis with each of seven questions, culminating by asking participants to imagine what the study might be about if it were other than the effects of general anesthesia on memory and attention.
To assess awareness of the hypothesis, two coders who were blind to experimental condition coded the responses from the interview as to whether participants guessed that the study pertained to facial expression and emotional experience.
To create a liberal criterion, erring on the side of a correct guess, participants did not need to explicitly state that the hypothesis corresponded to the connection between facial expression and emotional experience, but merely that the hypothesis pertained to both emotional experience and facial expression in some way. Thus, participant responses were coded separately for emotional experience and for facial expression. As there were seven questions in the interview, coders gave scores of one through seven for each of the two factors, indicating the question at which a participant guessed that the hypothesis pertained to that factor. When participants never guessed that the hypothesis pertained to emotional experience or facial expression they were scored an eight for that factor.
Results
Self-report of emotional experience
As the hypothesized effects concerned the magnitude of emotional experience, self-report ratings for the negative video clips were multiplied by minus one. In this way, relatively stronger emotional experience was indicated by more positive scores for all video clip types, regardless of valence.
Order of video clip set presentation across the two sessions was included as a factor in our analyses to control for any order effects.
To test our hypothesis that BoNT-A participants should experience a decrease in emotional response across sessions relative to HA participants, we conducted a 2 (treatment group) × 2 (session) × 3 (video clip type) × 2 (video clip set order) mixed design ANOVA on participants’ strength of emotion scores. The analysis revealed a significant interaction of session by group, F(1,64) = 4.54, p = .04, partial η2 = .07.2 This interaction reflected a relative decrease from Session 1 to 2 for the BoNT-A group when compared to the HA group (Figure 3 and Table 2). Planned comparisons of Session 1 and Session 2 scores for each video clip revealed that the BoNT-A group did not, however, show the predicted decrease in reported emotional experience to either the positive or the negative clips (Fs < 1). Instead, the significant session x group interaction was attributable to BoNT-A participants showing a significant decrease in response to the mildly positive clips, F(1,64) = 5.46, p = .02, d = .38, and the HA group showing a significant increase in reported emotional experience in response to the negative video clips, F(1,64) = 3.91, p = .05, d = .26.
Figure 3.
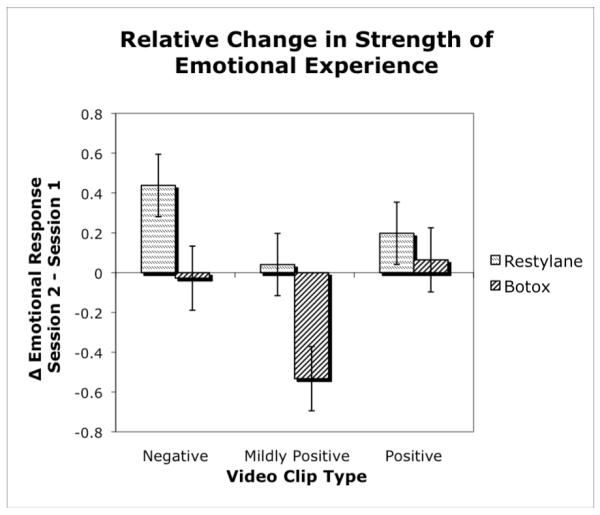
Participants who received BOTOX injections experienced a decrease in strength of emotional response relative to those who received Restylane, particularly in response to a mildly positive (intended neutral) video clip. Values represent change in strength of emotional experience from pre- to post-treatment. Lower scores indicate a relative decrease in the strength of emotional experience. Error bars represent SE. (Using the pooled error variance from the session by group effect, SE was estimated for each group as ).
Table 2. Means of reported strength of emotional experience for each group at each session. SE given in parentheses.
| Negative | Mildly Positive | Positive | ||||
|---|---|---|---|---|---|---|
| Session 1 | Session 2 | Session 1 | Session 2 | Session 1 | Session 2 | |
| Restylane (HA) | ||||||
|
| ||||||
| Strength of Emotional Experience |
2.51 (0.30) |
2.95 (0.26) |
0.78 (0.25) |
0.82 (0.22) |
1.68 (0.30) |
1.88 (0.29) |
|
| ||||||
| BOTOX (BoNT-A) | ||||||
|
| ||||||
| Strength of Emotional Experience |
2.82 (0.31) |
2.80 (0.27) |
1.29 (0.26) |
0.75 (0.23) |
2.08 (0.31) |
2.14 (0.30) |
Because research ethics precluded assigning individuals to treatment conditions, it was important to verify that the observed effects were not due to baseline differences between the two groups in affect or demographics. To address this possibility two kinds of analyses were conducted. The first was a planned 2 (treatment group) × 3 (video clip type) × 2 (video clip set order) ANOVA on scores at Session 1 that did not reveal a significant between groups difference in self-reported emotion prior to treatment (p = .09). The second repeated the 2 (treatment group) × 2 (session) × 3 (video clip type) × 2 (video clip set order) mixed design ANOVA, while adding education, income level, and Session 1 and 2 BDI and PANAS scores as covariates (Tables 1 and 3). The session-by-group interaction remained significant in each case.
Table 3. Means of Beck Depression Inventory (BDI) and Positive and Negative Affect Schedule (PANAS) for each group at each session. SE given in parentheses.
| Restylane (HA) | BOTOX (BoNT-A) | |||
|---|---|---|---|---|
| Session 1 | Session 2 | Session 1 | Session 2 | |
| BDI | 6.92 (1.04) |
4.94 (0.76) |
7.15 (0.99) |
8.15 (1.10) |
| PANAS Positive Affect |
34.11 (1.31) |
33.44 (1.09) |
36.19 (1.49) |
33.33 (1.51) |
| PANAS Negative Affect |
18.04 (1.12) |
16.97 (1.07) |
17.85 (1.38) |
19.82 (1.55) |
Debriefing interview
Inter-rater reliability correlation coefficients between judges regarding the question at which participants stated that the hypothesis pertained to emotional experience and facial expression were r = .71 and r = 1, respectively. As no Pearson r can be calculated when scores remain constant (in this case a score of eight for each participant for both coders), an r value of 1 was assigned to indicate perfect correlation, reflecting that no participants guessed at any point during the interview that the hypothesis pertained to facial expression. An inter-rater reliability coefficient for whether participants guessed that the study pertained to both emotional experience and facial expression was thus also r = 1; that is, both judges fully agreed that no participants fell into this category.
Discussion
To test the facial feedback hypothesis (FFH), this study asked whether the strength of self-reported emotional experience would be decreased by BoNT-A induced paralysis of specific facial muscles, which render an individual unable to generate facial expressions and therefore to experience facial feedback. Comparing pre-, vs. post-injection self-reports for a group of BoNT-A participants and their matched control group, our findings provide mixed support for the FFH.
On one hand, when examined alone, the BoNT-A group did not show a post-injection drop in self-reported emotional experience in response to the negative and positive stimulus clips. On the other hand, between group comparisons showed that relative to HA participants, BoNT-A participants exhibited an overall post-injection decrease in the magnitude of emotional experience. Follow-up tests revealed that this effect was driven by a) a decrease in responses to the mildly positive control clips for the BoNT-A group, combined with b) an unexpected increase in responses to the negative film clips for the HA group.
Implications for emotion theory and research
Taken together, these data have at least three kinds of implications for emotion theory and research. First and foremost, they do not support strong versions of the FFH that posit a necessary role for facial feedback in emotional experience, but are consistent with weaker versions of the FFH suggesting that there are circumstances in which facial feedback contributes to, but is not the sole determiner of, emotional experience (Keillor et al., 2002; McIntosh, 1996).
Second, the unexpected but intriguing finding that BoNT-A injections affected responses to mildly but not strongly emotional clips suggests that one critical factor determining when facial feedback may matter is the strength of the emotional impulse. Current theories suggest that emotional experience arises from the operation of multiple different systems involved in emotional appraisal as well as perception, judgment and emotion expressive behavior (Barrett, Mesquita, Ochsner, & Gross, 2007). In the context of the present data, it is plausible to suggest that strong impulses may be less susceptible to influence by any single factor – such as facial feedback – because they are over-determined by responses in multiple systems. On this view, weaker emotional impulses are influenced more by the contributions of any single type of relevant input – such as facial feedback. Although intriguing, this conclusion must at present remain a tentative hypothesis because the present study was not designed to test it explicitly. Indeed, this study included only weakly positive, strongly positive, and strongly negative clips, and so cannot be used to determine whether the BoNT-A injections influence weak impulses in general or just weak positive ones. Future work including both strong and weak positive and negative film clips will be necessary to address this issue.
Third, multiple aspects of this study’s design suggest our results are attributable to the absence of facial feedback alone – and not other factors that could have influenced the results of prior studies. The use of BoNT-A as an indirect means of manipulating facial feedback – rather than explicitly instructing participants to hold their faces in a particular expression – reduces the possibility that expectations or experimenter demand influenced emotion reports. In keeping with this, detailed debriefings showed that all participants believed the cover story that the experiment had to do with memory and attention instead of emotion and facial expression. In addition, the finding that the most significant changes across sessions occurred in response to the mildly emotional stimuli also suggests that the effects of BoNT-A injections are not due to expectations that the treatment should affect one’s emotional reactions – if they had been, the greatest changes in emotional response would have been expected for the most overtly emotional stimuli. Furthermore, unlike prior patient work that examined emotion experience only after paralysis had set in (Keillor et al, 2002), the use of a within-participant, pre- vs. post-treatment design controlled for individual differences in pre-paralysis experience. Additionally, the inclusion of the HA comparison group controlled for any incidental factors that might influence emotional experience, including expectations and motivations (as both sought cosmetic treatments), condition (facial wrinkles), method of application (injectables), and invasiveness (cf.Finzi and Wasserman, 2006). Finally, unlike the prior case of facial paralysis, which was due to a disorder with potentially widespread effects (Keillor et al, 2002), the effects of BoNT-A are known to be highly selective. BoNT-A injections leave intact the neural and psychological processes that generate a motor command, but keep this command from being translated into action by rendering injected facial muscles inert, thereby eliminating the subsequent feedback to the brain that expressive muscle activity occurred.
Limitations and future directions
Future work could build on and address limitations of the present study in a number of ways.
First, it could follow up on the unexpected finding that BoNT-A injections influenced reactions to low intensity positive but not reactions to high intensity positive and negative stimuli. Using BoNT-A injections (or a comparable manipulation) and both positive and negative stimuli of varying emotional intensity, such studies could directly test the hypothesis that facial feedback more strongly influences low intensity emotional responses.
Second, future work could further clarify the extent to which the present findings are related to the specific injection sites used for each procedure. Whereas BoNT-A was applied to the wrinkles of the upper face, HA was applied to regions closer to the mouth, such as the nasolabial folds. For ethical and practical reasons, participants received treatments in locations where each drug is typically used. BoNT-A is not injected under the nasolabial folds, for example, because paralysis there could interfere with eating and speaking. Thus there is a potential confounding of injection site with group. This difference in injection site raises two issues. First, there is the question of whether differences in injection site could lead to differences in relative satisfaction with each treatment, which in turn could influence emotional state. This was not the case; when participants’ self-reports of satisfaction with their appearance after the procedure are entered into the analyses as a covariate, there are no significant changes in the results. Second, perhaps the relative differences between the groups were due to the differing effects on musculature or sensory feedback at the specific injection sites. The muscles injected with BoNT-A were the corrugator supercilii (frown lines between the eyes) and the orbicularis oculi (laugh lines, or “crows feet”). By contrast, HA was injected subcutaneously into the nasolabial folds (running from below the nose around the sides of the mouth), which rest above portions of both the levator labii (raising upper lip in disgust) and zygomaticus major (smiling with the mouth). These differences in injection site may have impacted responses to the negative film stimuli. These film clips predominantly elicited disgust, which has been associated with activity in the corrugator supercilii and levator labii (Ekman et al., 2001). By paralyzing only the former muscle group, our manipulation may have been a relatively weak one for the BoNT-A participants. Thus, it remains possible that BOTOX injections would impact negative emotional experiences that differ in how much they involve movement of the corrugator, such as different degrees and types of disgust, anger, sadness, or worry. Future research might gain more power by examining multiple negative emotions, with varying degrees of association with corrugator supercilii activity.
Third, such work on differences between injection sites may also help us unpack the unexpected finding that the HA group showed stronger post-injection responses to the negative film clips. Because HA has no known effect on muscle movement, replications are needed, and at present we can only speculate as to why this finding was obtained. That being said, the role of the levator labii muscle group (pulling up the upper lip) in disgust may be relevant. By attracting water molecules, HA increases swelling in the region into which it is injected. This swelling may have amplified sensation, thereby increasing the feedback-based contribution to emotional experience of the levator labii. While this interpretation is consistent with the FFH, future research should examine changes in sensation due to HA-induced swelling, and whether it is sufficient to modify emotional experience.
Fourth, within the BoNT-A group, the injection sites for BoNT-A merits future consideration, as well. BoNT-A injections were placed in two different muscle groups related to emotional expression – the corrugator supercilii, which is important for furrowing the brow, as in disgust or anxiety, and the orbicularis oculi, which is involved in smiling (Ekman et al., 2001). Consequently, we cannot determine the relative importance of paralyzing each muscle group individually. The fact that both the corrugator supercilii and orbicularis oculi were injected suggests the effects might generalize to mild emotions regardless of valence. Future research will be necessary to better dissociate the effects of specific muscle paralysis on specific emotions.
Fifth, future work could replicate the present findings to determine whether a trend towards a baseline difference between the groups contributed to some of the observed changes across sessions. For example, given that the BoNT-A group had a numerically stronger response to the mildly positive stimulus at Session 1, one might wonder whether their score at Session 2 reflects regression to the mean. While we await future work, we note now that this explanation seems unlikely for a number of reasons, however: a) the difference between the groups at baseline was not significant, b) by including a repeated measure (before to after treatment) as well as a between groups comparison, we statistically take into account baseline differences, and c) there is a theoretical reason to expect BoNT-A to have diminished affective responses, whereas there is no reason to expect that regression to the mean would occur for one but not all conditions.
Sixth, future work could further clarify the neural pathway linking facial feedback to emotional experience. The present pre- vs. post-treatment design could be combined with functional imaging to determine, for example, whether changes in emotional experience depend upon changes in activation and functional connectivity of the amygdala, as suggested by prior work (Hennenlotter et al., 2009).
Seventh, it could be interesting to determine whether the effects of BoNT-A on emotional experience interact with one’s clinical status. For example, prior work combining the present methods with the study of depression could determine whether prior findings that depressive symptoms may be at least partially alleviated by BoNT-A injections depends on changes in the capacity to experience specific kinds of positive or negative emotions (Finzi & Wasserman, 2006).
Finally, as we consider the generalizability of these findings, we should keep in mind that only women were included in this study. We have no theoretical reason to expect that the present findings would not generalize across genders. However, far greater numbers of women elect to receive the treatments of interest than men, and consequently we were unable to recruit men in numbers that would allow us to balance gender, along with our other demographic variables, across groups. As more men elect to have cosmetic treatments, we may be able to determine if they show similar effects to those we found here.
Conclusion
Research on embodied cognition and emotion increasingly suggests that, at least in some circumstances, body state information can affect judgment, knowledge, and experience (Barsalou, 2008; Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004; Damasio, 1999; Niedenthal, 2007). The present study joins this research by pointing towards specific circumstances in which facial feedback may influence emotional experience. William James famously proposed that emotional experiences are perceptions of bodily processes. Our data suggest that the nature of the connection between mind and body may be more complex than even he suspected.
Acknowledgments
We thank members of the clinical research team at Dermatology Research Institute for their invaluable help with recruitment, and Alex Cazzaniga, Director, for consultation regarding dermatological research and organizational assistance. This research was supported by Allergan, Inc. (Irvine, CA 92623), who supplied the BOTOX® Cosmetic product, by Medicis Pharmaceutical Corp. (Scottsdale, AZ 85258), who supplied the Restylane® product, and by grant MH071637 to K.N.O. from NIH. Financial disclosure: F.B. serves as an investigator and consultant for both Allergan, Inc., and Medicis Pharmaceutical Corp.
Footnotes
Two participants took part in Session 1 more than eight days prior to treatment because they rescheduled their appointment for treatment.
Planned analyses were conducted to investigate the effects of city (Coral Gables vs. NYC). With city included in the analysis, a 2 (treatment group) × 2 (session) × 3 (video clip type) × 2 (video clip set order) × 2 (city) ANOVA revealed a significant interaction of city by session, F(1,60) = 5.31, p = .03, partial η2 = .08, reflecting a pattern wherein there was a slight increase in emotional experience from Session 1 to 2 for New York City participants and a slight decrease for Coral Gables participants. There was also a significant main effect of city, in which the Coral Gables participants rated their emotions as stronger than did the New York City participants, F(1,60) = 6.06, p = .02, d = .67. Crucially, city did not interact with treatment group, and could therefore safely be excluded from the interpretation of the primary analysis presented.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/emo.
Contributor Information
Joshua Ian Davis, Barnard College of Columbia University.
Ann Senghas, Barnard College of Columbia University.
Fredric Brandt, Dermatology Research Institute, Coral Gables, Florida and Private Practice in Coral Gables, Florida, and Manhattan, NY.
Kevin N. Ochsner, Columbia University
References
- Adolphs R. Recognizing emotion from facial expressions: psychological and neurological mechanisms. Behavioral & Cognitive Neuroscience Reviews. 2002;1(1):21–62. doi: 10.1177/1534582302001001003. [DOI] [PubMed] [Google Scholar]
- Bargh JA, Chen M, Burrows L. Automaticity of social behavior: Direct effects of trait construct and stereotype activation on action. Journal of Personality and Social Psychology. 1996;71(2):230–244. doi: 10.1037//0022-3514.71.2.230. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, Gross JJ. The experience of emotion. Annual Review of Psychology. 2007;58:373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsalou LW. Grounded cognition. Annual Review of Psychology. 2008;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Brandt FS, Cazzaniga A. Hyaluronic acid fillers: Restylane and Perlane. Facial Plastic Surgery Clinics of North America. 2007;15:63–76. doi: 10.1016/j.fsc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio A. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. Harcourt Brace & Company; New York: 1999. [Google Scholar]
- Darwin CR. The Expression of the Emotions in Man and Animals. John Murray; London: 1872. [Google Scholar]
- Davis JI. Representations of facial expressions can be viewed as integral to emotional experience. In: Freitas-Magalhães A, editor. Emotional Expression: The Brain and the Face. University Fernando Pessoa Press; Porto: In Press. [Google Scholar]
- Davis JI, Senghas A, Ochsner KN. How does facial feedback modulate emotional experience? Journal of Research in Personality. doi: 10.1016/j.jrp.2009.06.005. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolly JO, Aoki KR. The structure and mode of action of different botulinum toxins. European Journal of Neurology. 2006;13(Suppl. 4):1–9. doi: 10.1111/j.1468-1331.2006.01648.x. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen W, Ancoli S. Facial signs of emotional experience. In: Parrott GW, editor. Emotions in social psychology: Essential readings. Psychology Press; New York: 2001. pp. 255–264. [Google Scholar]
- Finzi E, Wasserman E. Treatment of Depression with Botulinum Toxin A: A case Series. Dermatologic Surgery. 2006;32(5):645–650. doi: 10.1111/j.1524-4725.2006.32136.x. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74(1):224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Hennenlotter A, Dresel C, Castrop F, Baumann AOC, Wohlschlager AM, Haslinger B. The link between facial feedback and neural activity within central circuitries of emotion - New insights from botulinum toxin-induced denervation of frown muscles. Cerebral Cortex. 2009;19(3):537–542. doi: 10.1093/cercor/bhn104. [DOI] [PubMed] [Google Scholar]
- James W. The physiological basis of emotion. Psychological Review. 1894;1:516–529. [Google Scholar]
- Keillor JM, Barrett AM, Crucian GP, Kortenkamp SA, Heilman KM. Emotional experience and perception in the absence of facial feedback. Journal of the International Neuropsychological Society. 2002;8:130–135. [PubMed] [Google Scholar]
- McIntosh DN. Facial feedback hypothesis: Evidence, Implications, and directions. Motivation and Emotion. 1996;20(2):121–147. [Google Scholar]
- Niedenthal PM. Embodying Emotion. Science. 2007;316:1002. doi: 10.1126/science.1136930. [DOI] [PubMed] [Google Scholar]
- NINDS Guillain-Barre Syndrome Fact Sheet. 2007 from http://www.ninds.nih.gov/disorders/gbs/detail_gbs.htm.
- Olsson A, Phelps EA. Understanding social evaluations: What we can (and cannot) learn from neuroimaging. In: Wittenbrink B, Schwarz N, editors. Implicit Measures of Attitudes. Guilford Press; New York: 2007. pp. 159–175. [Google Scholar]
- Soussignan R. Regulatory function of facial actions in emotion processes. In: Shohov SP, editor. Advances in Psychology Research. Vol. 31. Nova Science Publishers, Inc.; Hauppauge, NY, US: 2004. pp. 173–198. [Google Scholar]
- Strack F, Martin L, Stepper S. Inhibiting and facilitating conditions of the human smile: A non-obtrusive test of the facial feedback hypothesis. Journal of Personality and Social Psychology. 1988;54(5):768–777. doi: 10.1037//0022-3514.54.5.768. [DOI] [PubMed] [Google Scholar]
- Tomkins S. Affect, Imagery, and Consciousness: The Positive Affects. Vol. 1. Springer; New York: 1962. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]


