Abstract
Interleukin-17 (IL-17)-secreting T helper 17 cells (Th17) are a recently identified CD4+ T helper subset that has been implicated in various inflammatory and autoimmune diseases. Th17, along with CD4+CD25high Foxp3+ regulatory T cells (Tregs) and other newly emergent T helper subsets, Th9 and Tfh, have expanded the Th1-Th2 paradigm. Although this newly proposed six-subset paradigm significantly improved our understanding on the differentiation of CD4+ T helper cell subsets and the regulation of T helper cells in inflammation and autoimmunity, many questions remain to be answered. In this overview, we will briefly review the following issues: a) Old Th1-Th2 paradigm versus new multi-subset paradigm; b) Structural features of IL-17 family cytokines; c) Th17 cells; d) Effects of IL-17 on various cell types and tissues; e) IL-17 receptor and signaling pathways; f) Th17-mediated inflammations; and g) Protective mechanisms of IL-17 in infections. Lastly, we will look into the interaction of Th17 and Treg in autoimmune diseases and inflammation: Th17 cells interplay with Tregs. Regulation of autoimmunity and inflammation lies in the interplays of the different T helper subsets, therefore, better understanding of these subsets’ interactions with one another would greatly improve our approaches in developing therapy to combat inflammatory and autoimmune diseases.
Keywords: Th17, Tregs, interleukin-17 (IL-17), inflammation, autoimmune diseases
2. Introduction
Interleukin-17 is a pro-inflammatory cytokine derived mainly from T cells, and also from neutrophils, monocytes, macrophages, mast cells and vascular smooth muscle cells(1–5). IL-17 has been implicated in modulating various inflammatory and autoimmune diseases in human and mouse models, including tissue transplant rejection(6), asthma(7), scleritis, uveitis(8), rheumatoid arthritis(9, 10), systemic lupus erythematosus, encephalomyelitis(11), colitis(12, 13), and diabetes(14, 15). Among cytokines and more specifically, pro-inflammatory cytokines, one of the reasons that IL-17 has attracted special attention is that a subgroup of CD4+ T helper cells (Th cells) producing IL-17 has been characterized as Th17 cells. Very importantly, the emerging new multi-subset paradigm with six subsets of T helper cells, including type 1 T helper cells (Th1), type 2 T helper cells (Th2), follicular T helper cells (Tfh)(16, 17), Th9 (IL-9 producing)(18–20), Th17, and CD4+CD25highFoxp3+ (Forkhead box P3 transcription factor) regulatory T cells (Tregs)(21, 22), has replaced the 20 year-old Th1-Th2 paradigm(23). This new paradigm has significantly improved our understanding on the differentiation of functional CD4+ T helper cell subsets and T cell regulation of inflammation and autoimmunity. Of note, inducible Tregs and Th17 arise in a mutually exclusive fashion, depending on whether their differentiation programs are activated in the presence of TGF-β(transforming growth factor-β) or TGF-β plus pro-inflammatory cytokines such as IL-6(24). Despite the remarkable progress made, the interplays between pro-inflammatory IL-17/Th17 and anti-inflammatory Tregs in orchestrating various types of inflammation and autoimmune diseases remain to be elucidated. In this short review, we will focus on the discussion of the current understanding and progress in this front.
3. Old Th1-Th2 Paradigm versus New Multi-Subset Paradigm
3.1. The Th1-Th2 Paradigm’s Crisis
The Th1-Th2 cell paradigm, which has been used to explain how hosts initiate different T cell responses to eradicate the invasion of various pathogens, was first proposed 20 years ago by Mosmann and Coffman(23). Th1 cells are associated with cell surface expression of chemokine receptors CCR5 (Chemokine C-C motif receptor 5) and CXCR3 (CXC-chemokine receptor 3), and Th2 cells with CCR3 and CCR4(25–27). Th1 associated cell surface molecules include CD26 and lymphocyte activation gene-3 whereas Th2 associated surface molecules include CD62L, T1/ST2 and CD30(28). The signature cytokines for Th1 and Th2 are interferon-γ(IFN-γ) and IL-4, respectively. Th1 cells also secrete IL-2, tumor necrosis factor-α(TNF-α), and lymphotoxin (LT), which are important in mediating delayed type hypersensitivity responses and macrophage activation. Th2 cells also secrete IL-5, IL-9, IL-10 and IL-13. Th1 cells are important in cellular immunity against virus and intracellular pathogens. Th2 cells are critical in humoral immunity, aid in B cell proliferation and are important in allergic responses and protection against infection of helminthic parasites(23, 29). This paradigm has been the cornerstone to our understanding of T cell responses until recent years. However, it is apparent that there are many complicated pathological conditions that cannot be simply clarified by the Th1-Th2 paradigm. For a long time, autoimmune diseases are thought to be mainly driven by the auto-reactivity of Th1 cells as shown in T cell passive transfer studies. For example, Th1 cells were thought to have a pathogenic function in the development of experimental autoimmune encephalomyelitis (EAE), which suggested that IFN-γ producing Th1 cells have specificity for self-antigen and are pathogenically required for the induction of organ-specific autoimmunity in EAE. However, the depletion of IFN-γ gave contradictory results. IFN-γ-deficient and IFN-γ receptor-deficient mice and mice lacking other factors involved in Th1 lineage differentiation develop more severe disease rather than being protected from the disease(30, 31). Another example of how Th1-Th2 paradigm fails to address autoimmunity is seen in the relationship between IL-12 and IL-23. IL-12 is a cytokine important in Th1 differentiation. IL-12 and IL-23, each made of two subunits and these two cytokines share a common subunit, p40 and each have a unique subunit, p35 (IL-12) and p19 (IL-23)(32), respectively. It is shown that loss of IL-23 render animals highly resistant to the development of autoimmunity and inflammation whereas the lack of IL-12 did not. The results of loss of IL-12 suggest that Th1 cells are not required for the induction of autoimmune-mediated inflammation(33). Taken together, thus, Th1-Th2 paradigm could not completely explain these new results, which lead investigators to search for new subsets and propose a new multi-subset paradigm.
3.2. New CD4+ Th subsets – Tregs, Th17, Th9, Tfh, IL-10 IFN-γ-secreting CD4+ T cells and CD4+CD28null T cells
Since 1990, several new CD4+ Th subsets have been characterized: 1) Tregs: Tregs have an important role in suppressing both innate and adaptive immune responses(34–36). The naturally occurring Tregs have a major role in modulating the activity of self-reactive cells. The identification of Foxp3 as the critical determinant for Tregs development and function has generated expanded interest in studying the balance between autoimmunity and regulatory mechanisms in human autoimmune diseases. 2) Th17: Th17 is a distinctive subset of CD4+RORγt+IL23R+CCR6+CD161+ IL-17-producing T cells (37–39), which is different from Th1, Th2, Tfh(16), Th9(18–20), and Treg cell subsets. Th17 cells and their effector cytokines are involved in the pathogenesis of various autoimmune diseases and in mediating host defensive mechanisms against various infections such as extracellular bacterial infections. 3) Th9: In the presence of TGF-β and IL-4, IL-9 producing cells are distinct from Th1, Th17 and Foxp3+ Tregs. Th9 does not express any well-characterized transcription factors, such as T-box expressed in Th1 cells (T-bet), trans-acting Th2-cell-specific transcription factor GATA3, Th17-specific retinoid-related orphan receptor γt (RORγt) and Tregs-specific Foxp3. Th9 cells are neither anergic nor suppressive, and Th9 cells vigorously proliferate and enhance T cell proliferation. These results suggest that Th9 cells represent a new subset of T helper cells. The specific transcription factor uniquely expressed by Th9 cells remains to be identified(18–20). 4) Tfh: After the requirement of T cell association for B cell production of antibody was observed in 1960s, it took more than 30 years before the follicular CD4+ T cell subset was characterized. Tfh cells have a unique ability to home to the B cell follicles in germinal centers owing to their expression of CXC-chemokine receptor 5 (CXCR5) and subsequently to induce antibody production(16, 17). Of note, Th1, Th2, Th9, Th17 and Tregs are localized in T cell zone and circulation, whereas Tfh cells are the only CD4+ T helper cells localized in the B cell follicles(17). New evidences suggest some aspects of the relationship between Th subsets: a) Tfh can be generated from Tregs, which challenges the current view that Tfh and Tregs are different subsets(40); b) Tfh can produce IL-17 without expressing Th17-specific transcription factor RORγt(41, 42); and c) inducible Tregs and Th17 cells arise in a mutually exclusive manner(24). 5) IL-10 IFN-γ-secreting CD4+ T cells: A new CD4+ T cell subset, IL-10 IFN-γ-secreting CD4+ T cells were first reported in the early 1990s. They are suppressive T cells, which are able to inhibit cytotoxic T lymphocytes. These Foxp3−Tbet+ cells have a similar function but are distinct from conventional Tregs. IL-10 IFN-γ-secreting CD4+ T cells are activated in chronic infection and are responsible for prolonged infection. However, the relationships between IL-10 IFN-γ-secreting CD4+ T cells and other CD4+ T cell subsets remain unclear(43). 6) CD4+CD28null T cells: It has been reported that CD4+CD28null T cells are an unusual subset of helper cells, which expand and have deleterious effects in atherosclerosis and coronary artery disease. However, it is unclear whether CD4+CD28null T cells have overlaps with Th1 and Th17 subsets(44).
4. Structure Features of IL-17 Family Cytokines
IL-17 was originally cloned from an activated T cell hybridoma using subtractive hybridization method in rodents. Initially, it was named cytotoxic T-lymphocytes-associated antigen 8 (CTLA-8). This protein was shown to have 57% homology with the 13th ORF of a T-lymphotropic virus, herpes virus Saimiri virus (initially HVS13, later vIL-17)(45), the significance of which was not completely understood. The human IL-17 cytokine cDNA was cloned from a CD4+ T cell library and it was found to have 63% amino acid homology to mouse IL-17. IL-17 (also IL-17A) is the founding member of the IL-17 cytokine family, which consists of six cytokines designated IL-17A, IL-17B, IL-17C, IL-17D, IL-17E and IL-17F. IL-17 consists of 155 amino acids, and it is secreted as a disulfide-linked homodimeric glycoprotein(46). Of the six related cytokines identified in the family, IL-17 and IL-17F have the greatest homology, approximately 55%; IL-17F is also secreted as a disulfide-linked dimer(47, 48). IL-17B and IL-17C have approximately 28% amino acid homology with IL-17(49). IL-17E has about 20% homology with IL-17, IL-17B, and IL-17C(50). IL-17F, like IL-17, is produced by T cells, whereas other members are produced from non-T cell tissues(47, 49, 50). Using techniques such as immunoprecipitation followed by immunoblot, enzyme-linked immunosorbent assay (ELISA), and mass spectrometry, IL-17 and IL-17F are found to exist either as homodimers or heterodimers with each other in vitro and in activated human CD4+ T cells. All three forms of IL-17 and IL-17F are also found to have biological activities. IL-17F is found to express at the highest concentration followed by IL17A/F heterodimer whereas IL-17 homodimer detection is minimal in activated T cells from human donor(51). In mouse, IL-17A/F heterodimer has also been detected, which has an important role in neutrophil recruitment in the airways and activation of fibroblast and macrophages to produce other pro-inflammatory cytokines(52, 53). The 3-dimensional crystal structure of IL-17F reveals that IL-17 family members form a canonical pseudo-cystine knot(47). Several important questions remain unclear: (a) how the expressions of these IL-17 family members are regulated; and (b) how these IL-17 family members and other cytokines functionally interact in regulation of inflammation and autoimmunity.
5. Th17 Cells
The first member of IL-17 cytokine family has been characterized for over 15 years. But it was not until recent years that the cells, that uniquely produce these cytokines, were characterized. In addition to CD4+ T cells, IL-17 and IL-17F were found to be produced by a number of other cell types such as γδ T Cells, natural killer T cells (NKT), neutrophils, eosinophils, and monocytes(54, 55) and vascular smooth muscle cells(4). However, these two cytokines are mainly secreted by active CD4+ T cells(48, 56, 57). Studies in IL-17 and IL-17F lead to the classification of a unique CD4+ T cell subset named T-helper 17(58, 59). Besides, IL-17 and IL-17F, Th17 cells also secrete other cytokines such as IL-21, IL-22, IL-26 and CCL20(60–62).
As discussed above, Th17 is a distinctive subset of CD4+ T cells, which is different from Th1, Th2, Tfh(16), Th9(18–20), and Treg cell subsets. Th17 cells and their effector cytokines are involved in the pathogenesis of various autoimmune diseases and in mediating host defensive mechanisms against various infections, such as extracellular bacterial infections. Differentiation of naïve CD4+ T cells into effector T helper cells requires initiation by their T-cell receptor and co-stimulatory molecules. Th17 is unique from the other lineages in that it requires co-stimulatory molecules distinct from those required by other T lineage(33, 61, 63–68), and that Th17 is implicated in immune responses different from those of Th1, Th2 and Tregs. Th1 cells require IL-12 for development and they produce primarily IFN-γ, IL-2, and LT to orchestrate T cell-mediated immunity(69). Th2 cells develop in the presence of IL-4, and the differentiated Th2 cells in turn produce IL-4, IL-5, and IL-13 to facilitate humoral immune response(70). It has been found that cytokines and transcription factors unique to other subsets have the ability to inhibit the development of Th17 cells. For example, IFN-γ produced by Th1, and IL-4, product of Th2 cells, negatively regulate Th17 development and IL-17 expression. Th17 cell differentiation is inhibited by transcriptional factors specific for Th1 and Th2 cells, STAT1 (Signal Transducer and Activator of Transcription), STAT4 and T-bet, STAT6 and GATA3, respectively(57, 58). Furthermore, Th17 promoting cytokines also suppress the development of other T cell lineages(64). This finding suggests that the balance of the T cell lineage is regulated by the products of each subset, and that during an infection, the proper T helper cell activation is crucial for the host to sufficiently combat and control the infection. However, in some studies it has been observed that a subset of Th17 cells can co-produce IFN-γ and IL-17, suggesting that IFN-γ may not always inhibit IL-17 production but rather plays a role in the pathogenic functions of Th17(37, 71, 72). The function of IFN-γ in Th17-mediated pathogenesis remains to be investigated.
In recent years, the cytokines and transcription factors involved in the generation, differentiation, and expansion of Th17 have been examined. However, there are still controversies to the exact conditions for Th17 development, especially in humans. TGF-β, IL-6, IL-21, IL-23, and IL-1β are the factors that have been found to be involved in Th17 differentiation and development in humans and rodents with some variance among the species. RORγt, RORα, STAT3, interferon-regulatory factor-4 (IRF4)(73) and the activator protein (AP)-1 B cell-activating transcription factor (Batf) are the five transcription factors identified in promoting the differentiation of Th17 from naïve T cells(1, 74). RORγt is a transcriptional factor specific for Th17 cell development, and the up-regulation of RORγt is dependent on STAT3(75–77).
TGF-β, produced by a variety of cells, is a critical cytokine in the development, homeostasis and tolerance of T cells. TGF-β is found to be an essential differentiation factor in the development of Tregs. Tregs inhibit inflammation and autoimmunity by counteracting the effects of other T helper cells. TGF-β, produced by Foxp3+ (as we recently reviewed(78)) Tregs, inhibits Th1 cell differentiation(79). Numerous reports have shown that TGF-β is crucial in Th17 development in mice and humans(65, 80, 81). However, others report that TGF-β may not be necessary for Th17 lineage commitment in humans, but a common consensus regarding which factors drives Th17 cells development in humans remains elusive(82–84). In mice, TGF-β and IL-6 are important in driving the development of Th17 in naïve T cells while IL-21 and IL-23 are critical in maintaining and expanding Th17(61, 64). In vitro study has shown that TGF-β stimulation of T cells drives the development of Tregs(85), but the addition of pro-inflammatory cytokines such as IL-6 to T cells in the presence of TGF-β favors Th17 differentiation (Figure 1). Also, the effect of IL-6 and TGF-β to induce Th17 cells is amplified when IL-1β and TNF-α are used as co-stimulators(63, 67). TGF-β is required for the induction of IL-17 and IL-23 receptor (IL-23R) in naïve T cells, which make Th17 responsive to IL-23. IL-23 is another cytokine important in Th17 cell differentiation(86). IL-23 stabilizes the expansion of Th17 cells, and the IL-23R is induced in T cells after stimulation with IL-6 plus TGF-β(65). However, high concentration of TGF-β has been shown to inhibit the expression of IL-23R on T cells, suggesting that TGF-β has a biphasic function in T cell development(1). At the transcriptional level, TGF-β up-regulates RORγt expression on T cells, but it inhibits the expression of IL-17 in naïve T cells and drives Treg differentiation. Only the addition of pro-inflammatory cytokines such as IL-6 is this inhibition mitigated(87). IL-6 induces the expression of IL-21, which in turn induces IL-23R expression as observed. The induction of IL-23R is nearly abolished in the absence of IL-21R. Since IL-21 is produced by Th17 cells, IL-21 can induce Th17 cells(66) and IL-17 production via positive feedback. IL-21 is found to suppress TGF-β-induced expression of Foxp3 during Treg differentiation(60). IL-6, IL-21, and IL-23 all have the ability to independently induce RORγt expression, which is dependent on STAT3. In T cells lacking STAT3, IL-17 induction is completely lost in the presence of IL-6 or IL-21 with TGF-β(68). However, they are not sufficient to induce Th17 differentiation alone. Th17 expression is only seen when TGF-β is also present, suggesting that RORγt alone is not enough to induce Th17 differentiation either. Additional factors induced by TGF-β are required to drive Th17 proliferation. Taken together, thus IL-6, IL-21, and IL-23 act in conjunction with TGF-β to induce RORγt-dependent differentiation of Th17 cells. STAT3 is also found to be activated downstream of IL-6, IL-21, and IL-23. STAT3 and RORγt act together to induce maximal IL-17 differentiation. In addition, the small molecule halofuginone (HF) selectively inhibits mouse and human Th17 cell differentiation by activating a cytoprotective signaling pathway, the amino acid starvation response(88). Moreover, IL-1 receptor I (IL-1RI)+ memory Th17 cells have increased gene expression of IL17, RORC, and IRF4 even before T-cell antigen receptor triggering, indicating that the effect of IL-1β is programmed in these cells via IL-1RI. Although Th17 cells from human umbilical cord blood did not express IL-1RI, the cytokines IL-7, IL-15, and TGF-β up-regulated IL-1RI expression on naive Th17 cells, suggesting that IL-1RI+ naive Th17 cells develop in periphery(89).
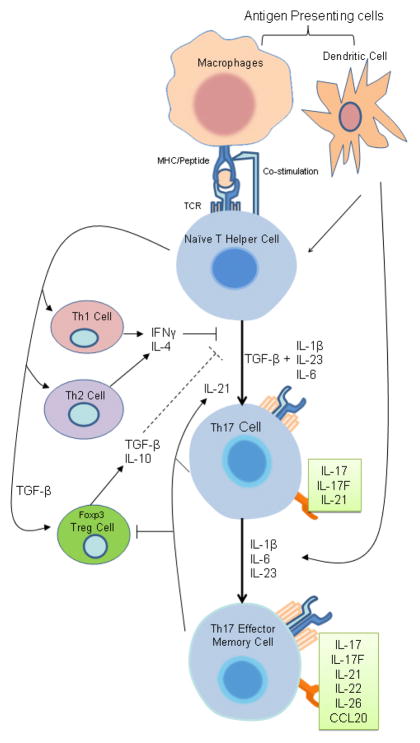
Figure 1.
Th17 Cell Differentiation. Upon activation by antigen presenting cells, naïve T helper cells can differentiate into different T helper subsets. In the presence of TGF-β and pro-inflammatory cytokines such as IL-6, IL-23, or IL-1β, naïve T cell differentiates into Th17 cells. The differentiation of Th17 is inhibited by hallmark cytokines of other T helper subsets. Pro-inflammatory cytokines can induce further effector molecules in committed Th17 cells to establish their terminally differentiated effector phenotype.
6. Effects of IL-17 on Various Cell Types and Tissues
IL-17 cytokines have numerous effects on a wide array of cell types in terms of inducing chemokines and cytokines production. The cytokine profile varies from cell types and the IL-17 cytokine(s) involved. Here we will discuss some examples.
6.1. Synovial Stromal Cells
Initially, the study of IL-17 shows that it induces IL-6 and IL-8 production in a dose-dependent manner in human foreskin fibroblast. The incubation with IL-17 increases the surface expression of intracellular adhesion molecule-1 (ICAM-1)(46). In primary culture of synovial fluid, a similar effect is observed. IL-17 induces secretion of IL-6, IL-8, prostaglandin E2 (PGE2), and granulocyte colony-stimulating factor (G-CSF) by stromal cells, and leading to the hematopoiesis of neutrophils. The recruitment of neutrophils by IL-6 and IL-8 allows for immune response to fight off infections(90).
6.2. Fibroblasts
IL-17F stimulates the production of cytokines such as IL-6 and IL-8 and G-CSF, and it has a role in cartilage matrix turn over via induction of matrix metalloproteinases (MMPs)(47). In fibroblastoid L929 cell line, IL-17 induces the mRNA expressions of monocyte chemotactic protein-1 (MCP-1), macrophage-inflammatory protein-2 (MIP-2) and tissue inhibitor of metalloproteinases (TIMP). In mouse embryonic fibroblast (MEF), granulocyte chemotatic protein-2 (GCP-2), MMP-3, MMP-9, and MMP-13 mRNAs are induced with IL-17 stimulation(91). In conjunction to several chemokines and cytokines, IL-17 also induces the production of various matrix metalloproteinases such as MMP-1, MMP-3, MMP-9, MMP-13, and TIMP-1 in fibroblasts, which are important in tissue remodeling in diseases such as inflammatory bowel disease, rheumatoid arthritis, and myocardial infarction(92–96).
6.3. Endothelial Cells
IL -17F has a negative effect on angiogenesis in human endothelial cells and it induces endothelial cells to produce TGF-β, IL-2, MCP-1, and lymphotoxin-β(48). In contrast, IL-17 is shown to promote angiogenesis and tumor growth as seen in vitro study with human umbilical venous cord endothelial cell (HUVECs), human dermal microvascular endothelial cell (HMVECs), tumor cell lines and mouse fibroblasts. IL-17 promotes angiogenesis via up-regulating the production of various proangiogenic factors in fibroblasts and tumor cells including vascular endothelial growth factor (VEGF), PGE1, PGE2, keratinocyte-derived cytokines and MIP-2(97). In human endothelial cell culture, IL-17 is found to enhance the effects of various growth factors, such as basic fibroblast growth factor (bFGF), human growth factor (HGF), VEGF, to induce proliferation of vascular endothelial cells(98). In addition, the expressions of IL-6, IL-10, IL-12, and PGE2 are also induced(99). IL-17 induces IL-6 production in endothelial cells, melanoma cells, MEF, splenic CD11c+ cells and CD11b+ cells, and tumor cell growth in part via a STAT3 pathway(100). IL-25 (also known as IL-17E) is produced by brain capillary endothelial cells, and IL-25 protects against pro-inflammatory cytokine-induced excessive blood-brain barrier collapse through a protein kinase Cε-dependent pathway(101). Reversibly, endothelial cells also modulate Th17 differentiation. The C-type lectin-like receptors (CLECs) play crucial roles in immunity and homeostasis by recognizing pathogens as well as self-antigens. CLEC-1 is expressed at low levels by endothelial cells, which can be up-regulated by Treg cells and immunosuppressive cytokines IL-10 or TGF-β, and down-regulated by inflammatory stimuli. CLEC-1 expression increases the differentiation of Th17 cells and decreases the differentiation of Tregs(102). In addition, VEGF induced by lipopolysaccharides (LPS) plays a key role in activation of naïve T cells and subsequent polarization to Th1 and Th17 cells in the airway(103).
6.4. Vascular Smooth Muscle Cells (VSMCs)
In cultured cells, IL-17 acts preferentially on VSMCs rather than endothelial cells to enhance production of pro-inflammatory mediators, including IL-6, and chemokines CXCL8 (IL-8) and CCL20(6). IL-17 stimulates C-reactive protein expression in hepatocytes and VSMCs via p38 Mitogen Activated Protein Kinase (MAPK) and extracellular signal–regulated kinases 1 and 2 (ERK1/2)-dependent nuclear factor-κB (NF-κB) and CCAAT enhancer binding protein-β (C/EBPβ) activation(104). In addition, IL-17-induces the migration of VSMCs in a MMP-9-dependent manner. IL-17-induces MMP-9 expression via similar pathways(105).
6.5. Monocytic Cells and Macrophages
While the effects of IL-17 and IL-17F have been well studied, the characterization of the effects of other cytokines in the IL-17 family has been limited. However, IL-17B and IL-17C are found to induce the release of TNF-α and IL-1β from THP-1 cells (human acute monocytic leukemia cell line), a monocytic cell line, in a time and dose-dependent manner, but IL-17 was ineffective in the same condition. The different biological effects among IL-17 family members suggest that they function through different receptor-linked signal pathways. It was shown that IL-17B and IL-17C do not bind to IL-17 receptor extracellular domain(49). In addition, IL-17 stimulates the production and expression of pro-inflammatory cytokines IL-1β and TNF-α by human macrophages, which is completely suppressed by IL-4 and IL-10 but is partially inhibited by IL-13 and TGF-β(99).
6.6. Structural Lung Cells
IL-17 induces hyperresponsive IL8 and IL-6 production to TNF-α in structural lung cells(106). IL-17 is a weak stimulus of IL-8 and IL-6production, but markedly enhances IL-8 and IL-6 responses toother stimuli, such as TNF-α. This modulatory effect of IL-17is associated with a reduced IL-8 and IL-6 mRNA degradation and is not associated with its effect on IL-8 and IL-6 gene transcription.
7. IL-17 Receptors and Signaling Pathways
7.1. IL-17 Receptor Family (Figure 2)
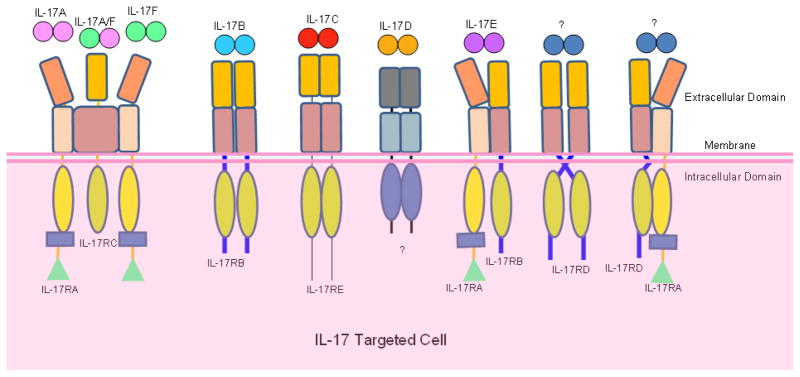
Figure 2.
IL-17 Cytokine-Receptor Relationships. The IL-17 cytokine family has six members, IL-17A, IL-17B, IL-17C, IL-17D, IL-17E and IL-17F. The IL-17 receptor family is made of five distinct receptors, IL-17RA, IL-17RB, IL-17RC, IL-17RD and IL-17RE. The IL-17 receptor complexes through which IL-17 cytokines induce signaling are shown. The exact stoichiometry of each IL-17 receptor complex has not been completely elucidated. Ligands for the IL-17RD or IL-17RA-IL-17RD complex are yet to be identified. The receptor through which IL-17D induces signaling needs to be verified.
IL-17 receptors make up a family of unique cytokine receptors. This cytokine receptor family consists of five members including IL-17RA, IL-17RB, IL-17RC, IL-17RD and IL-17RE(47). The original IL-17 receptor, IL-17RA (IL-17R), was first characterized as a type I transmembrane protein of 866 amino acids with three domains including a 293 amino acid extracellular domain, a transmembrane domain of 21 amino acids and cytoplasmic tail of 525 amino acids long(107). The mRNA of IL-17RA is found to be expressed extensively in various tissues and cell types including kidneys, liver, lungs, spleen, fibroblast cells, epithelial cells, T lymphocytes and bone marrow stromal cells(107, 108). Furthermore, in humans, the IL-17RA protein is detected in peripheral blood T lymphocytes and in vascular endothelial cells(109). IL-17RA and IL-17RC are the well studied members of the IL-17 receptor family. IL-17RA is known as the receptor for IL-17A and IL-17RC for IL-17F(110). Although IL-17RA binds preferentially to IL-17A, it also has affinity for IL-17F(107, 111). IL-17RC also has shown to have affinity for IL-17A in humans, whereas in mouse IL-17RC is specific only for IL-17F(110). Using fluorescence resonance energy transfer (FRET) microscopy, IL-17RA is shown to form pre-assembled complexes in the plasma membrane(112). Although each cytokine preferentially binds to one receptor over another, the biological activities of IL-17, IL-17F, and IL-17A/F require both receptors IL-17RA and IL-17RC. The IL-17 receptors may exist as heteordimers or trimers forming an IL-17RA/RC functional receptor complex or other combination of complexes although the exact stoichiometry is still unknown(113–115).
7.2. IL-17 Receptor Complex Components (Figure 3)
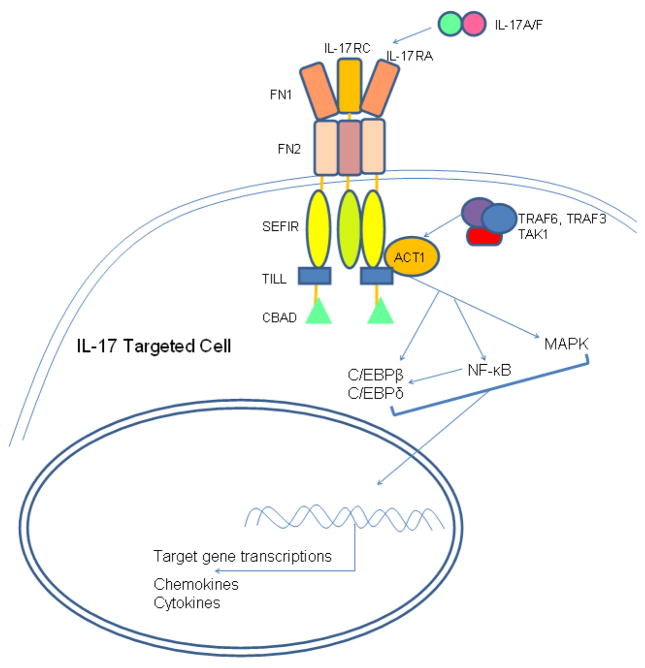
Figure 3.
Main Structural Features of IL-17 Receptor and IL-17 Signaling Pathways. Extracellular domain of the IL-17 receptor has two fibronectin III-like domains (FN), which mediate ligand binding. Intracellular domain of IL-17 receptor contains a SEFIR domain, which defines IL-17 receptors as members of the “similar expression to fibroblast growth factor genes, IL-17 receptors and Toll-like receptors-IL-1R” (SEFIR) family. A TIR-like loop (TILL) and a C/EBPβ-activation domain (CBAD) are found in IL-17RA intracellular domain. ACT1 is a critical adaptor protein in IL-17 signaling pathways. ACT1 associates with TRAF6, TRAF3 and TAK1 in mediating signals downstream of IL-17 receptor. IL-17 receptor pathways activates NF-κB, MAPK, C/EBPβ and C/EBPδ, which lead to target gene transcriptions of pro-inflammatory chemokines and cytokines.
The extracellular domain of IL-17RA contains two fibronectin III-like (FN) domains. FN domain is a common feature of type 1 cytokine receptors and it is important in mediating protein-protein interaction and ligand binding(116, 117). IL-17RA and IL-17RC are members of the “similar expression to fibroblast growth factor genes, IL-17 receptors and Toll-like receptors-IL-1R” (SEFIR) protein family defined by their cytoplasmic SEFIR domain, which share homology with the TLRs/IL-R (TIR) domain. This structural homology suggests that IL-17R may share signaling pathways with Toll/IL-1 receptor. Indeed, activations of Toll/IL-1 receptor and IL-17 receptor pathways result in NF-κB activation and induction of inflammatory cytokines(118), but the signaling pathways upstream of each receptor are different. The SEFIR domain in IL-17R does not have the TIR box3 subdomain and the BB-loop, the structural elements found in the prototypical TIR domains(116). The IL-17RA does have a sequence homologous to the BB-loops at the carboxy-terminal side of the SEFIR domain, which is also named TIR-like loop (TILL). The TILL domain is unique to the IL-17RA, whose functions and interactions in IL-17R signaling remain to be examined(119, 120). Another molecule found to be unique in IL-17R signaling is ACT1 (NF-κB activator 1, also known as TRAF3 interacting protein 2). Like IL-17 receptors, ACT1 is also part of the SEFIR protein family. ACT1 contains a SEFIR domain in its C terminus(121, 122). ACT1 is also found to be a negative regulator of B cell survival via its interaction with TRAF (TNF Receptor Associated Factor) 3 and CD40 and B-cell activating factor (BAFF) signaling(123), but in IL-17 signaling pathway ACT1 is a positive component and indispensable for IL-17-mediated biological activities. Since IL-17 receptors and ACT1 both contain SEFIR domain, it is believed that the interaction of IL-17 receptor with ACT1 is mediated by SEFIR domain. In addition to ACT1, TRAF6 has importance in IL-17 signaling. Because ACT1 contains a TRAF6 binding motif, ACT1 can bind to TRAF6, TRAF3, and TGF-β-activated Kinase 1 (TAK1), thus, TRAF family proteins are critical in NF-κB activation(122). IL-17A/F has synergistic effect with TNF-α to increase CXCL1 expression in macrophages from mice. CXCL1 expression is impaired when TRAF6 is absent in knock-out model, indicating that the receptor for IL-17A/F uses TRAF6 for its signaling(52). In addition to TRAF6, TRAF3 and TAK1 are also involved in IL-17 signaling since the TRAF6 binding motif of ACT1 can bind to TRAF3 as well as TAK1(122). TAK1 is also involved in Toll/IL-1R activation of NF-κB and c-Jun N-terminal kinase (JNK)(124), suggesting that TAK1 may play a role in IL-17-induced NF-κB and JNK activation. Additionally, a domain called C/EBPβ-activation domain (CBAD) has been identified in the C-terminal of IL-17RA. So far this domain is only seen in IL-17RA, which is required for IL-17A-mediated glycogen synthase kinase 3β (GSK3 β) phosphorylation of C/EBPβ(119, 120).
7.3. IL-17 Receptor Signaling (Figure 3)
Although IL-17 cytokines have been shown to induce various cytokines and chemokines in numerous cell types via the interaction with their receptors, the detailed mechanism of the IL-17 signaling pathway remains unclear. Published studies show that IL-17 ultimately activates NF-κB and MAPK pathways in its signaling pathway to induce pro-inflammatory cytokines(94, 104, 125, 126). IL-17 has been shown, in gel shift analyses, to activate NF-κB via p50 and p65, which are the key components of the canonical NF-κB pathway(127). The involvement of IL-17 in the non-canonical NF-κB has not been defined, but NF-κB-inducing kinase (NIK) that is a component of the non-canonical NF-κB is shown to be induced in response IL-17A(125). IL-17 is found to up-regulate the expression of CCAAT/enhancer binding proteins, C/EBP-δ and C/EBP-β, which are important regulators in IL-6 transcription(127). Activation of MAPK pathway in IL-17 signaling is important in controlling the stability of mRNA transcripts of pro-inflammatory cytokines via interactions with the AREs (AU-rich elements) of the 3′ untranslated region of the transcripts(128). IL-17 activates MAPK pathway is shown to activate activator protein 1 (AP1)(129), but the exact mechanism by which IL-17 regulates mRNA stability needs to be clarified.
8. Th17-Mediated Inflammations
8.1. Experimental Autoimmune Encephalomyelitis (EAE)/Multiple Sclerosis
The multiple sclerosis/EAE has pathological conditions, which cannot be explained by the Th1-Th2 paradigm and lead to the discovery of two other T helper cell subsets, Tregs and Th17. In the EAE models, the concept that Th17 cells are responsible for driving autoimmune inflammations is exemplified. EAE is induced by passive transfer of IL-17-producing memory activated T cells(61). Th1 cytokines IL-12 and IFN-γ, initially thought to induce EAE, suppress IL-17 production, and are shown to have protective mechanism in EAE. The disease is exacerbated when there is a lack of IL-17 suppression in IL-12 or IFN-γ-deficient mouse models(61, 130, 131).
8.2. Inflammatory Skin Disease in Mice and Psoriasis in Humans
Evidences suggest that Th17 cells, but not Th1 cells, play an important role in the pathogenesis of inflammatory skin disease: a) Higher frequencies of Th17 cells, IL-17A+, IL-17A/TNF-α+ and IL-17A/IFN-γ+ in psoriatic lesions but not Th1 cells; b) Anti-TNF-α therapy in psoriasis work by decreasing Th17 cells; and c) IL-15 triggers IL-17 from human T cell blasts and IL-15 gene variants (single nucleotide polymorphisms, SNPs) are associated with psoriasis(1).
8.3. Inflammatory Bowel Disease (IBD)(1)
Th17 cells are more potent in transferring IBD than Th1 cells(132). IL-17F increases dextran sulphate sodium (DSS)-induced colitis, while IL-17F knock-out mice are relatively protected. However, controversially, IL-17A is protective in DSS-induced colitis since IL-17A knock-out mice developed more severe disease(133). Anti-IL-17A monoclonal antibody treatment, presumably neutralizing IL-17A, aggravates DSS-induced colitis(13). Future work is warranted to solve this puzzle.
8.4. Experimental Arthritis/Rheumatoid Arthritis(1)
IL-17 knock-out mice have reduced collagen-induced arthritis(134). Th17 cells through secretion of IL-17A act on osteroblastic cells via inducing the expression of receptor activator for nuclear factor-κB ligand (RANKL). Interaction between RANKL on osteroclast precursors and RANKL results in the differentiation of osteroclasts and bone resorption(135). These results suggest that IL-17/Th17 cells play an important role in promoting the pathogenesis of arthritis. In patients with arthritis, higher concentration of IL-17 is found in the joints when compared to the controls. Up-regulations of MMP-1, -2, -3, -13 by IL-17 are seen in whole synovial tissue explant from RA patients, primary synovial fibroblasts, human cartilage and chondrocyte cultures. Mild cartilage proteoglycan degradation is seen with IL-17 stimulation and the effect on proteoglycan is exacerbated with TNF-α or Oncostatin M co-stimulation(136).
8.5. Atherosclerosis
Th17 cells may also play an important role in modulating autoimmune responses in atherosclerosis(137–139). More broadly, in pathological states such as obesity, hypertension, diabetes, metabolic syndrome and other cardiovascular disorders, perivascular tissue becomes dysfunctional. Production of protective factors diminishes while detrimental adipocytokines such as leptin, resistin, IL-6, TNF-α or IL-17 increase(140). Induction of atherosclerosis by the Western-type diet in low density lipoprotein receptor deficient (LDLR−/−) mice with IL-17R−/− bone marrow transplantation (BMT) induces a 46% reduction in lesion size in the aortic root. The atherosclerotic plaque composition reveals no significant changes in collagen content and neutrophil counts, but a reduction in mast cell numbers and an increase in macrophage numbers. In addition, upon IL-17R−/− BMT the investigators observe a decrease in anti-oxLDL antibodies of the IgG class, a reduced IL-6 production and an increased IL-10 production, suggesting that signaling via the IL-17 receptor in bone marrow derived cells enhances the process of atherosclerosis(141). Similar to that found in LDLR−/− mice, ApoE−/− mice, another atherosclerosis mouse model(36), reveal (a) significantly increased secretion of Th17 related cytokines, IL-17 and IL-6 and expression of RORγt; and (b) obviously decreased numbers in Treg cells, secretion of Treg related cytokine TGF-β1 and expression of Foxp3 as compared with age-matched wild-type C57BL/6J mice. Meanwhile, the expressions of Treg-related mediators are much lower in ApoE-/- mice than that in their age-matched wild-type littermates. These results suggest a potential role of Th17/Treg imbalance in the formation and progression of atherosclerosis(139). In supporting these findings, Li and the colleagues showed that the IL-1 receptor associated kinase 1 deficient (IRAK-1−/−) mice have constitutively higher populations of Treg cells. In contrast, when stimulated with T cell antigen receptor (TCR) agonists together with IL-6 and TGF-β, IRAK-1−/− CD4 Th cells exhibit attenuated STAT3 Ser727 phosphorylation and reduced expression of IL-17 and RORγt compared with that in wild-type cells. These results demonstrate that IRAK-1 deletion results in decreased IL-17 expression and dampened inflammatory responses in acute and chronic inflammatory mice including atherosclerosis(142). Controversially, Mallat and the colleagues found that loss of suppressor of cytokine signaling 3 (SOCS3) in T cells increases both IL-17 and IL-10 production, induces an anti-inflammatory macrophage phenotype, and leads to unexpected IL-17-dependent reduction in lesion development and vascular inflammation. In vivo administration of IL-17 reduces endothelial vascular cell adhesion molecule-1 expression and vascular T cell infiltration, and significantly limits atherosclerotic lesion development. These results identify novel SOCS3-controlled IL-17 regulatory pathways in atherosclerosis and may have important implications for the understanding of the increased susceptibility to vascular inflammation in patients with dominant-negative STAT3 mutations and defective Th17 cell differentiation(138). However, the issue of how pro-inflammatory cytokine IL-17 plays a suppressive role in inflammatory autoimmune atherosclerosis in SOCS3−/− mice remains a mystery. Interestingly, adult SOCS3-null mice on a leukemia-inhibitory factor (LIF)-null background succumb to a spontaneous fatal inflammatory disease characterized by neutrophilia and inflammatory-cell tissue infiltrates, suggesting that genetic reduction of embryonic LIF production rescues placentation in SOCS3−/− embryos but does not prevent inflammatory disease(143, 144). Obviously, the future work is needed to solve this controversy.
8.6. Diabetes
IL-17/Th17 cells play an important role in promoting diabetes. The dendritic cell (DC)-dependent loss in Tregs leads to an increase in the number of T cells producing inflammatory cytokines, such as IFN-γ (Th1 cells) and IL-17 (Th17 cells). Loss of DCs leads to a loss of Tregs, and that the remaining Tregs exhibit decreased Foxp3 expression. The increase in Tregs induced by DC expansion is sufficient to prevent type 1 autoimmune diabetes(145). Treatment with either neutralizing anti-IL-17 or recombinant IL-25 (IL-17E) has no effect on diabetes development in young (<5 weeks) non-obese diabetic (NOD) mice, a model of spontaneous autoimmune diabetes. However, either intervention prevents diabetes when treatment is started at 10 weeks of age (P < 0.001). Insulitis scoring and immunofluorescence staining reveal that both anti-IL-17 and IL-25 significantly reduce peri-islet T cell infiltrates and decrease GAD65 autoantibody levels. In addition, Berberine, an alkaloid derivative from Berberis vulgaris L., inhibits Th17 differentiation by activating extracellular signal-regulated kinases 1 and 2 (ERK1/2) and inhibits Th1 differentiation by inhibiting p38 mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) activation. Berberine down-regulates the activity of STAT1 and STAT4 through the suppression of p38 MAPK and JNK activation. Berberine controls the stability of STAT4 through the ubiquitin-proteasome pathway and prevents the progression of type 1 diabetes in NOD mice(146). Similarly, innocuous IFN-γ induced by adjuvant-free antigen suppresses diabetes and restores normoglycemia in NOD mice through inhibition of IL-17 production(147). In addition to the studies performed in mouse models of diabetes, the production of pro-inflammatory cytokines IL-17, TNF-α, and secretion of IL-6 by peripheral blood lymphocytes from patients with type 1 diabetes mellitus (T1D) is reduced, when the autoantigen-specific T cell clones are suppressed(148). Another study also suggests that there is a significant, although modest, increase in the frequencyof IL-17-secreting cells in lymphocytes from long-term patients with T1D compared with healthy controls(149). These studies suggest that Th17 cells are involved in the pathogenesis of autoimmune diabetes and that IL-25 treatment results in a T-cell-mediated dominant protective effect against autoimmunity(14).
9. Protective Mechanisms of IL-17 in Infections
Th17 cells are powerful tissue inflammation inducers associated with various experimental and human autoimmune diseases. However, the extreme importance of Th17 cells appears to be in host defense especially in clearing of invaded pathogens, which are not sufficiently handled by Th1 or Th2 cells. IL-17 is found to be important in the defense against Klebsiella pneumoniae as seen in IL-23p19 knock-out (gene deficient, KO) mice and IL-17R KO models. IL-17KO mice are highly susceptible to infection of K. pneumoniae because IL-17 is important in the recruitment of neutrophils to the K. pneumoniae infection site(150, 151). In addition, IL-17 and IL-17F also induce anti-infection neutrophil infiltration into lung in the clearance of mycoplasma pneumonia in mouse model of acute respiratory tract infection(152). In another knock-out model, IL-17RA KO mice are more prone to Candida albicans infection in comparison to wild type mice. Also, when wild type mice are given IL-17 injection, they are protected from lethal doses of the pathogen(153). IL-17 or IL-17 in conjunction with IL-22 also up-regulates the expression of antimicrobial molecules such as β-defensins in keratinocytes. Of note, defensins are antibiotics found in the skin, lungs, and gastrointestinal tract(53, 154). IL-17 also enhances the production of mucin in the airways, which traps pathogens in the lung mucosa(85).
10. Th17 Cells Interplay with Tregs
10.1. Updates of Tregs
The findings that the lack of Foxp3 expression, and consequently the absence of Tregs lead to both human and mouse autoimmune diseases have rapidly expanded knowledge of Tregs development and function during the past 5 years. In the previous sections, 3.2, 5, 8.5, 8.6, we have discussed some recent progress in characterizing Tregs. In the following section, we will highlight some additional progresses: (1) Human blood, isolated from an outbred population in a pathogenic environment, contains up to 30% CD4+CD25+ cells. Only the 2–4% of the cells with the highest CD25 expression can be considered regulatory. In addition to CD25, the best-accepted alternative is the lack of cell surface CD127 (IL-7 receptor). In addition, HLA-DR expression identifies a functionally distinct population of what appear to be terminally differentiated human Tregs(155); (2) Tregs recognize a wide range of antigenic specificities with increased reactivity to self antigens. The Treg repertoire is highly diverse with a distinct set of T-cell receptors (TCRs), and yet is overlapping to some extent with the repertoire of conventional T cells(156); (3) TGF-β signaling is not required for thymic expression of Foxp3, nor is TGF-β signaling or responsiveness required for in vitro suppressive activity of Foxp3+ Tregs. TGF-β treatment is capable of inducing both Foxp3 and RORγt expression, which leads exclusively to Treg differentiation. These data suggested that there is an alternative, extrathymic pathway for Treg differentiation(157); (4) Foxp3+CD4+CD25+ Treg subsets that maintain immunologic homeostasis have been considered to be a homogeneous population of naturally occurring, thymus-derived CD4+CD25+ T cells (nTregs). However, similar Foxp3+ Tregs can be induced from CD25− precursors in vivo, and ex vivo with IL-2 and TGF-β (adaptive Tregs, inducible Tregs, or iTregs). These two subsets differ in their principal antigen specificities and in the T-cell receptor signal strength and co-stimulatory requirements needed for their generation. Although IL-6 can convert nTregs to Th17 cells, iTregs induced by IL-2 and TGF-β are resistant to this cytokine and thereby might retain suppressive function at inflammatory sites. Thus, nTregs and iTregs may have different roles in the adaptive immune response(158); (5) Tregs consist of lymphocytes with several phenotypic markers that share the ability to suppress, by various mechanisms, inflammatory responses. These Tregs consist of subsets such as IL-10 secreting type I Tregs, type 3 Tregs that produce TGF-β, as well as iTregs and nTregs(159); (6) In autoimmunity, chronically activated APCs under the influence of intracellular signaling pathways, such as phosphatidylinositol-3 kinase (PI3K), JAK-STAT, MAPK, and NF-κB pathways, can escape surveillance by Tregs, leading to the activation of T cells that are refractory to suppression by Tregs. Moreover, APCs and APC-derived inflammatory cytokines such as TNF-α, IL-6, IL-1β, and IL-23 can render Tregs defective and can also reciprocally enhance the activity of the IL-17-producing pathogenic Th17 T cell subset. These results suggest that APC-Treg interactions play an important role in maintaining immune tolerance(160); (7) Treatment with nTregs resolves colitis (a CD45RBhighCD4+ T cell transfer model of colitis), but only when iTregs are also present. Although iTregs require Foxp3 for suppressive activity and phenotypic stability, their gene expression profile is distinct from the established nTregs’ “genetic signature,” indicating that these two groups of Tregs have developmental and possibly mechanistic differences. These results have identified a functional role for iTregs in vivo and demonstrated that both iTregs and nTregs can act in concert to maintain tolerance(161, 162); (8) Epigenetics is defined by regulation of gene expression without altering nucleotide sequence in the genome. Several epigenetic markers, such as histone acetylation and methylation, and cytosine residue methylation in CpG dinucleotides, have been reported at the Foxp3 locus. In particular, CpG dinucleotides at the Foxp3 locus are methylated in naive CD4+CD25− T cells, activated CD4+ T cells, and TGF-β-induced iTregs, whereas they are completely demethylated in nTregs. The DNA methyltransferases DNMT1 and DNMT3b are associated with the Foxp3 locus in CD4+ T cells. Methylation of CpG residues represses Foxp3 expression, whereas complete demethylation is required for stable Foxp3 expression(163); (9) The composition of the extracellular matrix provides contextual cues to leukocytes in inflamed and healing tissues. One example of this is hyaluronate (HA); Low molecular weight hyaluronate (LMW-HA) generated during active inflammation is a TLR ligand and an endogenous “danger signal,” while high molecular weight-HA (HMW-HA) is predominantly in healing or intact tissues and functions in an inverse manner. HMW-HA actively promotes immune tolerance by augmenting Treg function, but LMW-HA does not. In a human iT(Reg) model, HMW-HA but not LMW-HA provides a co-stimulatory signal through cross-linking CD44, which promotes Foxp3 expression. Thus, HMW-HA contributes to the maintenance of immune homeostasis in uninjured tissue and effectively communicates an “all-clear” signal to down-regulate the adaptive immune system through Tregs after tissue matrix integrity has been restored(164); (10) Using in vitro functional assays and phenotypic analysis, Tregs isolated from patients with a variety of autoimmune diseases have been demonstrated to exhibit reduced regulatory function as compared with those isolated from healthy controls(165). Our laboratory among others has shown that loss of Treg function results in autoimmune responses(166, 167). We have characterized a Treg-specific, IL-2-dependent apoptotic pathway and demonstrated that Treg apoptotic/survival pathways are therapeutic targets for Treg-based immunotherapy (see our invited review)(21); and (11) Bluestone and the colleagues recently reported that substantial percentages of cells have transient or unstable expression of the transcription factor Foxp3. These ‘exFoxp3’ T cells have an activated-memory T cell phenotype and produce pro-inflammatory cytokines. Moreover, exFoxp3 cell numbers are higher in inflamed tissues in autoimmune conditions. Adoptive transfer of autoreactive exFoxp3 cells leads to the rapid onset of diabetes. Finally, analysis of the T cell receptor repertoire suggests that exFoxp3 cells can be developed from both natural and adaptive Tregs. Thus, the generation of potentially autoreactive effector T cells as a consequence of Foxp3 instability has important implications for understanding autoimmune disease pathogenesis(168).
10.2. Reciprocally interconnected Th17 and Tregs (Figure 4)
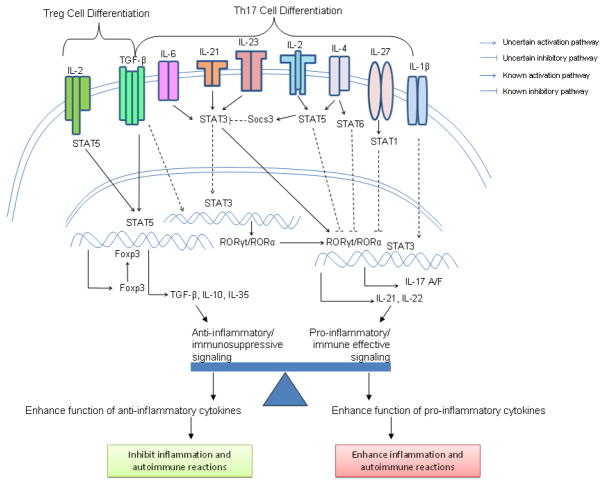
Figure 4.
Interplay between Tregs and Th17 Lineages. Differentiation of CD4+ T Cell is important for immune regulation and host defense. Signaling pathways in Tregs and Th17 differentiation show specific cytokines, receptors, and transcription factors involved in each lineage. TGF-β is a common factor in the differentiation of two lineages with opposing effects. Effector cytokines of Tregs enhance anti-inflammatory/immunosuppressive effects and thus inhibit inflammation and autoimmune reactions. Effector cytokines of Th17 are pro-inflammatory and enhance inflammation and autoimmune reactions.
Regulatory T cells are the subset of T helper cells important in immune suppression and prevention of autoimmune diseases(169). It has been shown that IFN-γ and IL-4, produced by Th1 and Th2 respectively, inhibit the differentiation of Th17. Tregs has been shown clearly to suppress Th1 and Th2 cell immune responses(170). However, the IL-17 production or Th17 cell development has not been shown to be down-regulated by Treg in vitro(37, 171) (see next section for the new results). The developmental programs of Th17 cells and adaptive Tregs are reciprocally interconnected(1, 172). Upon TCR stimulation, a naïve T cell can be driven to express Foxp3 and become a Treg cell in the presence of TGF-β. Immunosuppressive cytokine TGF-β has a broad inhibitory effect on the immune system. TGF-β alone induces Foxp3, which is the transcription factor required for Treg cell induction and maintenance. RORγt is the transcription factor crucial in Th17 development in naïve T cell. When T helper cells are differentiated into Tregs, RORγt expression is progressively extinguished and the opposite is seen in Th17 development when IL-6 is present(173, 174) (1). During an immune response, antigen presenting cells (APCs) such as dendritic cells in responses to activation by microbial antigens produces IL-6. IL-6 acts in concert with TGF-β to inhibit adaptive Treg generation and to induce Th17 cell differentiation(63, 65, 67). The balance of TGF-β and IL-6 in adaptive Treg and Th17 differentiation shows the reciprocal relationship between adaptive Treg and Th17 cells. In the absence of inflammatory stimuli, TGF-β-induced Treg keeps the immune system in balance by suppressing autoimmunity. In the presence of inflammatory assault, adaptive Treg generation is suppressed and Th17-induced pro-inflammatory responses are activated. In IL-6 KO (knock out) mouse model, the generation of Th17 is defective while there is an increase in the numbers of adaptive Treg generated(63, 64). Thus, IL-6 and IL-21 act as switch factors via controlling the Foxp3/RORγt balance (174). Both Th17-specific transcription factors RORγt and RORα physically interact with Treg-specific transcription factor Foxp3 to antagonize each other’s functions(174) (175). The results from IL-6−/− deficient mice show a severe defect in the generation of Th17 cells and increased numbers of Tregs in peripheral T cell repertoire(60, 63). Additionally, IL-2, which is a growth factor to promote Treg generation and survival, inhibits Th17 generation. IL-2 receptor signaling promotes the generation of Tregs but inhibits the production of Th17 cells in the periphery(176) (177). IL-2 deficient mice have decreased the numbers of Tregs while enhanced Th17 cell generation(176). These common γ-chain cytokines, IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21, activate STAT5, and the absence of STAT5 abrogates Treg differentiation. Both STAT5 deficient and IL-2 deficient mice have elevated serum levels of IL-17. The action of STAT5 is working through binding the Foxp3 gene and inhibiting Th17 differentiation(178).
10.3. Inhibition of Th17 by Tregs
Evidence to date has indicated that Th17 cells are resistant to suppression by human Foxp3+ Tregs. It has been recently demonstrated that CD39, an ectonucleotidase which hydrolyzes ATP, is expressed on a subset of human natural Tregs. Although both CD4+CD25highCD39+ and CD4+CD25highCD39− T cells suppress proliferation and IFN-γ production by responder T cells, only the CD4+CD25highCD39+, which are predominantly FoxP3+, suppress IL-17 production, whereas CD4+CD25highCD39− T cells produce IL-17. These findings suggest that CD4+CD25highFoxp3+CD39+ Tregs play an important role in constraining pathogenic Th17 cells in patients with autoimmune inflammation(179).
11. Conclusions
The identification of Th17 CD4+ T helper subset and Tregs as well as Th9 cells and Tfh cells significantly add to the old Th1-Th2 paradigm and substantially improve our understanding of the roles of T helper cell subsets in the processes of innate immunity and adaptive immunity. Although much progress has been made in the characterization of Th17 cells and Tregs, the interactions of these two T helper subsets in various inflammatory and autoimmune disease models need to be further defined. Future works to better characterize Th17 and Treg interplay in inflammation and autoimmune diseases(180) would eventually lead to development of therapeutics for diseases such as atherosclerosis, diabetes, and arthritis.
Acknowledgments
This work was partially supported by the National Institutes of Health Grants HL094451, HL67033, HL82774, and HL77288. We are grateful to Dr. Fang Liu for critical reading.
References
- 1.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 2.Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–83. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. Faseb J. 2003;17:1183–5. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- 5.de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, Idu MM, van Maldegem F, Aten J, van der Wal AC. Differential expression of interleukin-17 family cytokines in intact and complicated human atherosclerotic plaques. J pathol. 2009 doi: 10.1002/path.2667. [DOI] [PubMed] [Google Scholar]
- 6.Rao DA, Eid RE, Qin L, Yi T, Kirkiles-Smith NC, Tellides G, Pober JS. Interleukin (IL)-1 promotes allogeneic T cell intimal infiltration and IL-17 production in a model of human artery rejection. J Exp Med. 2008;205:3145–58. doi: 10.1084/jem.20081661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones CE, Chan K. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am J Respir Cell Mol Biol. 2002;26:748–53. doi: 10.1165/ajrcmb.26.6.4757. [DOI] [PubMed] [Google Scholar]
- 8.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–8. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 9.Kotake S, Kamatani N. The Role of IL-17 in Joint Destruction. Drug News Perspect. 2002;15:17–23. doi: 10.1358/dnp.2002.15.1.660504. [DOI] [PubMed] [Google Scholar]
- 10.Paradowska A, Masliniski W, Grzybowska-Kowalczyk A, Lacki J. The function of interleukin 17 in the pathogenesis of rheumatoid arthritis. Arch Immunol Ther Exp (Warsz) 2007;55:329–34. doi: 10.1007/s00005-007-0032-8. [DOI] [PubMed] [Google Scholar]
- 11.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–73. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:382–8. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Emamaullee JA, Davis J, Merani S, Toso C, Elliott JF, Thiesen A, Shapiro AM. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes. 2009;58:1302–11. doi: 10.2337/db08-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009 doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–35. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King C. New insights into the differentiation and function of T follicular helper cells. Nat Rev Immunol. 2009;9:757–66. doi: 10.1038/nri2644. [DOI] [PubMed] [Google Scholar]
- 18.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–6. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 19.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, Khoury S, Oukka M, Kuchroo VK. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol. 2008;9:1347–55. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soroosh P, Doherty TA. Th9 and allergic disease. Immunology. 2009;127:450–8. doi: 10.1111/j.1365-2567.2009.03114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang XF. Factors regulating apoptosis and homeostasis of CD4+CD25highFOXP3+ regulatory T cells are new therapeutic targets. Front Biosci. 2008;13:1472–99. doi: 10.2741/2775. [DOI] [PubMed] [Google Scholar]
- 22.Ke XY, Wang J, Li L, Chen IH, Wang H, Yang XF. Roles of CD4+CD25high FOXP3+ Tregs in lymphomas and tumors are complex. Front Biosci. 2008;13:3986–4001. doi: 10.2741/2986. [DOI] [PubMed] [Google Scholar]
- 23.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 24.Basso AS, Cheroutre H, Mucida D. More stories on Th17 cells. Cell Res. 2009;19:399–411. doi: 10.1038/cr.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–7. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 26.Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–34. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lohmann T, Laue S, Nietzschmann U, Kapellen TM, Lehmann I, Schroeder S, Paschke R, Kiess W. Reduced expression of Th1-associated chemokine receptors on peripheral blood lymphocytes at diagnosis of type 1 diabetes. Diabetes. 2002;51:2474–80. doi: 10.2337/diabetes.51.8.2474. [DOI] [PubMed] [Google Scholar]
- 28.Okazaki H, Kakurai M, Hirata D, Sato H, Kamimura T, Onai N, Matsushima K, Nakagawa H, Kano S, Minota S. Characterization of chemokine receptor expression and cytokine production in circulating CD4+ T cells from patients with atopic dermatitis: up-regulation of C-C chemokine receptor 4 in atopic dermatitis. Clin Exp Allergy. 2002;32:1236–42. doi: 10.1046/j.1365-2745.2002.01383.x. [DOI] [PubMed] [Google Scholar]
- 29.Anthony RM, Rutitzky LI, Urban JF, Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–87. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krakowski M, Owens T. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur J Immunol. 1996;26:1641–6. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- 31.Tran EH, Prince EN, Owens T. IFN-gamma shapes immune invasion of the central nervous system via regulation of chemokines. J Immunol. 2000;164:2759–68. doi: 10.4049/jimmunol.164.5.2759. [DOI] [PubMed] [Google Scholar]
- 32.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activitiessimilar as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 33.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 34.Sakaguchi S, Powrie F. Emerging challenges in regulatory T cell function and biology. Science. 2007;317:627–9. doi: 10.1126/science.1142331. [DOI] [PubMed] [Google Scholar]
- 35.Shevach EM, DiPaolo RA, Andersson J, Zhao DM, Stephens GL, Thornton AM. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 36.Yang XF, Yin Y, Wang H. VASCULAR INFLAMMATION AND ATHEROGENESIS ARE ACTIVATED VIA RECEPTORS FOR PAMPs AND SUPPRESSED BY REGULATORY T CELLS. Drug Discov Today Ther Strateg. 2008;5:125–142. doi: 10.1016/j.ddstr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–61. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, Berrino L, Fambrini M, Caproni M, Tonelli F, Lazzeri E, Parronchi P, Liotta F, Maggi E, Romagnani S, Annunziato F. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–16. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–46. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 40.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–92. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 41.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–49. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eddahri F, Denanglaire S, Bureau F, Spolski R, Leonard WJ, Leo O, Andris F. Interleukin-6/STAT3 signaling regulates the ability of naive T cells to acquire B-cell help capacities. Blood. 2009;113:2426–33. doi: 10.1182/blood-2008-04-154682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J, Liu XS. Development and function of IL-10 IFN-gamma-secreting CD4(+) T cells. J Leukoc Biol. 2009;86:1305–10. doi: 10.1189/jlb.0609406. [DOI] [PubMed] [Google Scholar]
- 44.Dumitriu IE, Araguas ET, Baboonian C, Kaski JC. CD4+ CD28 null T cells in coronary artery disease: when helpers become killers. Cardiovasc Res. 2009;81:11–9. doi: 10.1093/cvr/cvn248. [DOI] [PubMed] [Google Scholar]
- 45.Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150:5445–56. [PubMed] [Google Scholar]
- 46.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–6. [PubMed] [Google Scholar]
- 47.Hymowitz SG, Filvaroff EH, Yin JP, Lee J, Cai L, Risser P, Maruoka M, Mao W, Foster J, Kelley RF, Pan G, Gurney AL, de Vos AM, Starovasnik MA. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. Embo J. 2001;20:5332–41. doi: 10.1093/emboj/20.19.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Starnes T, Robertson MJ, Sledge G, Kelich S, Nakshatri H, Broxmeyer HE, Hromas R. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J Immunol. 2001;167:4137–40. doi: 10.4049/jimmunol.167.8.4137. [DOI] [PubMed] [Google Scholar]
- 49.Allison J, Thomas H, Beck D, Brady JL, Lew AM, Elefanty A, Kosaka H, Kay TW, Huang DC, Strasser A. Transgenic overexpression of human Bcl-2 in islet beta cells inhibits apoptosis but does not prevent autoimmune destruction. Int Immunol. 2000;12:9–17. doi: 10.1093/intimm/12.1.9. [DOI] [PubMed] [Google Scholar]
- 50.Lee J, Ho WH, Maruoka M, Corpuz RT, Baldwin DT, Foster JS, Goddard AD, Yansura DG, Vandlen RL, Wood WI, Gurney AL. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J Biol Chem. 2001;276:1660–4. doi: 10.1074/jbc.M008289200. [DOI] [PubMed] [Google Scholar]
- 51.Wright JF, Guo Y, Quazi A, Luxenberg DP, Bennett F, Ross JF, Qiu Y, Whitters MJ, Tomkinson KN, Dunussi-Joannopoulos K, Carreno BM, Collins M, Wolfman NM. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J Biol Chem. 2007;282:13447–55. doi: 10.1074/jbc.M700499200. [DOI] [PubMed] [Google Scholar]
- 52.Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;17:435–40. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- 53.Liang SC, Long AJ, Bennett F, Whitters MJ, Karim R, Collins M, Goldman SJ, Dunussi-Joannopoulos K, Williams CM, Wright JF, Fouser LA. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179:7791–9. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- 54.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003;170:2106–12. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- 55.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–9. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 56.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–4. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 57.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 59.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–15. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 60.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 64.Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells: effector T cells with inflammatory properties. Semin Immunol. 2007;19:362–71. doi: 10.1016/j.smim.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 66.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–3. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 67.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 68.Zhou L, Ivanov, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–74. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 69.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–58. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 70.Glimcher LH, Murphy KM. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 2000;14:1693–711. [PubMed] [Google Scholar]
- 71.Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, Hunter CA, Kastelein RA, Cua DJ. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–26. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–7. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 73.Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–66. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 74.Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, Sahota G, Sim J, Mukasa R, Cemerski S, Hatton RD, Stormo GD, Weaver CT, Russell JH, Murphy TL, Murphy KM. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460:405–9. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harris TJ, Grosso JF, Yen HR, Xin H, Kortylewski M, Albesiano E, Hipkiss EL, Getnet D, Goldberg MV, Maris CH, Housseau F, Yu H, Pardoll DM, Drake CG. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol. 2007;179:4313–7. doi: 10.4049/jimmunol.179.7.4313. [DOI] [PubMed] [Google Scholar]
- 76.Ivanov, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 77.Wei L, Laurence A, Elias KM, O’Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–10. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang XF, Fang P, Meng S, Jan M, Xiong X, Yin Y, Wang H. The FOX transcription factors regulate vascular pathology, diabetes and Tregs. Front Biosci (Schol Ed) 2009;1:420–36. doi: 10.2741/s35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1-and Th17-cell differentiation. Immunity. 2007;26:579–91. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 80.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–7. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 81.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–9. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 83.Chen Z, Laurence A, O’Shea JJ. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation. Semin Immunol. 2007;19:400–8. doi: 10.1016/j.smim.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–7. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 85.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem. 2003;278:17036–43. doi: 10.1074/jbc.M210429200. [DOI] [PubMed] [Google Scholar]
- 86.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–24. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–9. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sundrud MS, Koralov SB, Feuerer M, Calado DP, Kozhaya AE, Rhule-Smith A, Lefebvre RE, Unutmaz D, Mazitschek R, Waldner H, Whitman M, Keller T, Rao A. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science. 2009;324:1334–8. doi: 10.1126/science.1172638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee WW, Kang SW, Choi J, Lee SH, Shah K, Eynon EE, Flavell RA, Kang I. Regulating human Th17 cells via differential expression of IL-1 receptor. Blood. 115:530–40. doi: 10.1182/blood-2009-08-236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qiu Z, Dillen C, Hu J, Verbeke H, Struyf S, Van Damme J, Opdenakker G. Interleukin-17 regulates chemokine and gelatinase B expression in fibroblasts to recruit both neutrophils and monocytes. Immunobiology. 2009;214:835–42. doi: 10.1016/j.imbio.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 92.Bamba S, Andoh A, Yasui H, Araki Y, Bamba T, Fujiyama Y. Matrix metalloproteinase-3 secretion from human colonic subepithelial myofibroblasts: role of interleukin-17. J Gastroenterol. 2003;38:548–54. doi: 10.1007/s00535-002-1101-8. [DOI] [PubMed] [Google Scholar]
- 93.Chabaud M, Garnero P, Dayer JM, Guerne PA, Fossiez F, Miossec P. Contribution of interleukin 17 to synovium matrix destruction in rheumatoid arthritis. Cytokine. 2000;12:1092–9. doi: 10.1006/cyto.2000.0681. [DOI] [PubMed] [Google Scholar]
- 94.Cortez DM, Feldman MD, Mummidi S, Valente AJ, Steffensen B, Vincenti M, Barnes JL, Chandrasekar B. IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta, NF-kappaB, and AP-1 activation. Am J Physiol Heart Circ Physiol. 2007;293:H3356–65. doi: 10.1152/ajpheart.00928.2007. [DOI] [PubMed] [Google Scholar]
- 95.Jovanovic DV, Martel-Pelletier J, Di Battista JA, Mineau F, Jolicoeur FC, Benderdour M, Pelletier JP. Stimulation of 92-kd gelatinase (matrix metalloproteinase 9) production by interleukin-17 in human monocyte/macrophages: a possible role in rheumatoid arthritis. Arthritis Rheum. 2000;43:1134–44. doi: 10.1002/1529-0131(200005)43:5<1134::AID-ANR24>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 96.Rifas L, Arackal S. T cells regulate the expression of matrix metalloproteinase in human osteoblasts via a dual mitogen-activated protein kinase mechanism. Arthritis Rheum. 2003;48:993–1001. doi: 10.1002/art.10872. [DOI] [PubMed] [Google Scholar]
- 97.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–7. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 98.Takahashi H, Numasaki M, Lotze MT, Sasaki H. Interleukin-17 enhances bFGF-, HGF- and VEGF-induced growth of vascular endothelial cells. Immunol Lett. 2005;98:189–93. doi: 10.1016/j.imlet.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 99.Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160:3513–21. [PubMed] [Google Scholar]
- 100.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–64. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sonobe Y, Takeuchi H, Kataoka K, Li H, Jin S, Mimuro M, Hashizume Y, Sano Y, Kanda T, Mizuno T, Suzumura A. Interleukin-25 expressed by brain capillary endothelial cells maintains blood-brain barrier function in a protein kinase Cepsilon-dependent manner. J Biol Chem. 2009;284:31834–42. doi: 10.1074/jbc.M109.025940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thebault P, Lhermite N, Tilly G, Le Texier L, Quillard T, Heslan M, Anegon I, Soulillou JP, Brouard S, Charreau B, Cuturi MC, Chiffoleau E. The C-type lectin-like receptor CLEC-1, expressed by myeloid cells and endothelial cells, is up-regulated by immunoregulatory mediators and moderates T cell activation. J Immunol. 2009;183:3099–108. doi: 10.4049/jimmunol.0803767. [DOI] [PubMed] [Google Scholar]
- 103.Kim YS, Hong SW, Choi JP, Shin TS, Moon HG, Choi EJ, Jeon SG, Oh SY, Gho YS, Zhu Z, Kim YK. Vascular endothelial growth factor is a key mediator in the development of T cell priming and its polarization to type 1 and type 17 T helper cells in the airways. J Immunol. 2009;183:5113–20. doi: 10.4049/jimmunol.0901566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Patel DN, King CA, Bailey SR, Holt JW, Venkatachalam K, Agrawal A, Valente AJ, Chandrasekar B. Interleukin-17 stimulates C-reactive protein expression in hepatocytes and smooth muscle cells via p38 MAPK and ERK1/2-dependent NF-kappaB and C/EBPbeta activation. J Biol Chem. 2007;282:27229–38. doi: 10.1074/jbc.M703250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cheng G, Wei L, Xiurong W, Xiangzhen L, Shiguang Z, Songbin F. IL-17 Stimulates Migration of Carotid Artery Vascular Smooth Muscle Cells in an MMP-9 Dependent Manner via p38 MAPK and ERK1/2-Dependent NF-kappaB and AP-1 Activation. Cell Mol Neurobiol. 2009 doi: 10.1007/s10571-009-9409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van den Berg A, Kuiper M, Snoek M, Timens W, Postma DS, Jansen HM, Lutter R. Interleukin-17 induces hyperresponsive interleukin-8 and interleukin-6 production to tumor necrosis factor-alpha in structural lung cells. Am J Respir Cell Mol Biol. 2005;33:97–104. doi: 10.1165/rcmb.2005-0022OC. [DOI] [PubMed] [Google Scholar]
- 107.Yao Z, Spriggs MK, Derry JM, Strockbine L, Park LS, VandenBos T, Zappone JD, Painter SL, Armitage RJ. Molecular characterization of the human interleukin (IL)-17 receptor. Cytokine. 1997;9:794–800. doi: 10.1006/cyto.1997.0240. [DOI] [PubMed] [Google Scholar]
- 108.Silva WA, Jr, Covas DT, Panepucci RA, Proto-Siqueira R, Siufi JL, Zanette DL, Santos AR, Zago MA. The profile of gene expression of human marrow mesenchymal stem cells. Stem Cells. 2003;21:661–9. doi: 10.1634/stemcells.21-6-661. [DOI] [PubMed] [Google Scholar]
- 109.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–74. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 110.Kuestner RE, Taft DW, Haran A, Brandt CS, Brender T, Lum K, Harder B, Okada S, Ostrander CD, Kreindler JL, Aujla SJ, Reardon B, Moore M, Shea P, Schreckhise R, Bukowski TR, Presnell S, Guerra-Lewis P, Parrish-Novak J, Ellsworth JL, Jaspers S, Lewis KE, Appleby M, Kolls JK, Rixon M, West JW, Gao Z, Levin SD. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol. 2007;179:5462–73. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–27. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kramer JM, Yi L, Shen F, Maitra A, Jiao X, Jin T, Gaffen SL. Evidence for ligand-independent multimerization of the IL-17 receptor. J Immunol. 2006;176:711–5. doi: 10.4049/jimmunol.176.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, Tocker J, Peschon J. Cutting edge:interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177:36–9. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 114.Wright JF, Bennett F, Li B, Brooks J, Luxenberg DP, Whitters MJ, Tomkinson KN, Fitz LJ, Wolfman NM, Collins M, Dunussi-Joannopoulos K, Chatterjee-Kishore M, Carreno BM. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J Immunol. 2008;181:2799–805. doi: 10.4049/jimmunol.181.4.2799. [DOI] [PubMed] [Google Scholar]
- 115.Claudio E, Sonder SU, Saret S, Carvalho G, Ramalingam TR, Wynn TA, Chariot A, Garcia-Perganeda A, Leonardi A, Paun A, Chen A, Ren NY, Wang H, Siebenlist U. The adaptor protein CIKS/Act1 is essential for IL-25-mediated allergic airway inflammation. J Immunol. 2009;182:1617–30. doi: 10.4049/jimmunol.182.3.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Novatchkova M, Leibbrandt A, Werzowa J, Neubuser A, Eisenhaber F. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem Sci. 2003;28:226–9. doi: 10.1016/S0968-0004(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 117.Murphy JM, Young IG. IL-3, IL-5, and GM-CSF signaling: crystal structure of the human beta-common receptor. Vitam Horm. 2006;74:1–30. doi: 10.1016/S0083-6729(06)74001-8. [DOI] [PubMed] [Google Scholar]
- 118.Miggin SM, Palsson-McDermott E, Dunne A, Jefferies C, Pinteaux E, Banahan K, Murphy C, Moynagh P, Yamamoto M, Akira S, Rothwell N, Golenbock D, Fitzgerald KA, O’Neill LA. NF-kappaBactivation by the Toll-IL-1 receptor domain protein MyD88 adapter-like is regulated by caspase-1. Proc Natl Acad Sci U S A. 2007;104:3372–7. doi: 10.1073/pnas.0608100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maitra A, Shen F, Hanel W, Mossman K, Tocker J, Swart D, Gaffen SL. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc Natl Acad Sci U S A. 2007;104:7506–11. doi: 10.1073/pnas.0611589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shen F, Li N, Gade P, Kalvakolanu DV, Weibley T, Doble B, Woodgett JR, Wood TD, Gaffen SL. IL-17 receptor signaling inhibits C/EBPbeta by sequential phosphorylation of the regulatory 2 domain. Sci Signal. 2009;2:ra8. doi: 10.1126/scisignal.2000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem. 2006;281:35603–7. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- 122.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, Kordula T, Zhang QW, Vallance B, Swaidani S, Aronica M, Tuohy VK, Hamilton T, Li X. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–56. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 123.Qian Y, Qin J, Cui G, Naramura M, Snow EC, Ware CF, Fairchild RL, Omori SA, Rickert RC, Scott M, Kotzin BL, Li X. Act1, a negative regulator in CD40- and BAFF-mediated B cell survival. Immunity. 2004;21:575–87. doi: 10.1016/j.immuni.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 124.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–95. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 125.Awane M, Andres PG, Li DJ, Reinecker HC. NF-kappa B-inducing kinase is a common mediator of IL-17-, TNF-alpha-, and IL-1 beta-induced chemokine promoter activation in intestinal epithelial cells. J Immunol. 1999;162:5337–44. [PubMed] [Google Scholar]
- 126.Hata K, Andoh A, Shimada M, Fujino S, Bamba S, Araki Y, Okuno T, Fujiyama Y, Bamba T. IL-17 stimulates inflammatory responses via NF-kappaB and MAP kinase pathways in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1035–44. doi: 10.1152/ajpgi.00494.2001. [DOI] [PubMed] [Google Scholar]
- 127.Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, Gaffen SL. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559–67. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 128.Anderson P. Post-transcriptional control of cytokine production. Nat Immunol. 2008;9:353–9. doi: 10.1038/ni1584. [DOI] [PubMed] [Google Scholar]
- 129.Shen MM. Identification of differentially expressed genes in mouse development using differential display and in situ hybridization. Methods. 2001;24:15–27. doi: 10.1006/meth.2001.1152. [DOI] [PubMed] [Google Scholar]
- 130.Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, Kamoun M, Rostami A. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169:7104–10. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 131.Zhang GX, Gran B, Yu S, Li J, Siglienti I, Chen X, Kamoun M, Rostami A. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol. 2003;170:2153–60. doi: 10.4049/jimmunol.170.4.2153. [DOI] [PubMed] [Google Scholar]
- 132.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–70. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 133.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, Dong C. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–75. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–7. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 135.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–82. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moran EM, Mullan R, McCormick J, Connolly M, Sullivan O, Fitzgerald O, Bresnihan B, Veale DJ, Fearon U. Human rheumatoid arthritis tissue production of IL-17A drives matrix and cartilage degradation: synergy with tumour necrosis factor-alpha, Oncostatin M and response to biologic therapies. Arthritis Res Ther. 2009;11:R113. doi: 10.1186/ar2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–32. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Taleb S, Romain M, Ramkhelawon B, Uyttenhove C, Pasterkamp G, Herbin O, Esposito B, Perez N, Yasukawa H, Van Snick J, Yoshimura A, Tedgui A, Mallat Z. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med. 2009;206:2067–77. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xie JJ, Wang J, Tang TT, Chen J, Gao XL, Yuan J, Zhou ZH, Liao MY, Yao R, Yu X, Wang D, Cheng Y, Liao YH, Cheng X. The Th17/Treg functional imbalance during atherogenesis in ApoE(-/-) mice Th17/Treg imbalance in atherogenesis. Cytokine. 2009 doi: 10.1016/j.cyto.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 140.Guzik TJ, Marvar PJ, Czesnikiewicz-Guzik M, Korbut R. Perivascular adipose tissue as a messenger of the brain-vessel axis: role in vascular inflammation and dysfunction. J Physiol Pharmacol. 2007;58:591–610. [PubMed] [Google Scholar]
- 141.van Es T, van Puijvelde GH, Ramos OH, Segers FM, Joosten LA, van den Berg WB, Michon IM, de Vos P, van Berkel TJ, Kuiper J. Attenuated atherosclerosis upon IL-17R signaling disruption in LDLr deficient mice. Biochem Biophys Res Commun. 2009;388:261–5. doi: 10.1016/j.bbrc.2009.07.152. [DOI] [PubMed] [Google Scholar]
- 142.Maitra U, Davis S, Reilly CM, Li L. Differential regulation of Foxp3 and IL-17 expression in CD4 T helper cells by IRAK-1. J Immunol. 2009;182:5763–9. doi: 10.4049/jimmunol.0900124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Robb L, Boyle K, Rakar S, Hartley L, Lochland J, Roberts AW, Alexander WS, Metcalf D. Genetic reduction of embryonic leukemia-inhibitory factor production rescues placentation in SOCS3-null embryos but does not prevent inflammatory disease. Proc Natl Acad Sci U S A. 2005;102:16333–8. doi: 10.1073/pnas.0508023102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O’Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103:8137–42. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Darrasse-Jeze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao KH, Masilamani RF, Dustin ML, Rudensky A, Liu K, Nussenzweig MC. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–62. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cui G, Qin X, Zhang Y, Gong Z, Ge B, Zang YQ. Berberine differentially modulates the activities of ERK, p38 MAPK, and JNK to suppress Th17 and Th1 T cell differentiation in type 1 diabetic mice. J Biol Chem. 2009;284:28420–9. doi: 10.1074/jbc.M109.012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jain R, Tartar DM, Gregg RK, Divekar RD, Bell JJ, Lee HH, Yu P, Ellis JS, Hoeman CM, Franklin CL, Zaghouani H. Innocuous IFNgamma induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J Exp Med. 2008;205:207–18. doi: 10.1084/jem.20071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Boehm BO, Rosinger S, Sauer G, Manfras BJ, Palesch D, Schiekofer S, Kalbacher H, Burster T. Protease-resistant human GAD-derived altered peptide ligands decrease TNF-alpha and IL-17 production in peripheral blood cells from patients with type 1 diabetes mellitus. Mol Immunol. 2009;46:2576–84. doi: 10.1016/j.molimm.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 149.Bradshaw EM, Raddassi K, Elyaman W, Orban T, Gottlieb PA, Kent SC, Hafler DA. Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. J Immunol. 2009;183:4432–9. doi: 10.4049/jimmunol.0900576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–81. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–9. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu Q, Martin RJ, Rino JG, Breed R, Torres RM, Chu HW. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 2007;9:78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–31. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 154.Oppenheim JJ, Biragyn A, Kwak LW, Yang D. Roles of antimicrobial peptides such as defensins in innate and adaptive immunity. Ann Rheum Dis. 2003;62(Suppl 2):ii17–21. doi: 10.1136/ard.62.suppl_2.ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Costantino CM, Baecher-Allan CM, Hafler DA. Human regulatory T cells and autoimmunity. Eur J Immunol. 2008;38:921–4. doi: 10.1002/eji.200738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pacholczyk R, Kern J. The T-cell receptor repertoire of regulatory T cells. Immunology. 2008;125:450–8. doi: 10.1111/j.1365-2567.2008.02992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Buckner JH, Ziegler SF. Functional analysis of FOXP3. Ann N Y Acad Sci. 2008;1143:151–69. doi: 10.1196/annals.1443.014. [DOI] [PubMed] [Google Scholar]
- 158.Horwitz DA, Zheng SG, Gray JD. Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other. Trends Immunol. 2008;29:429–35. doi: 10.1016/j.it.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 159.George J. Mechanisms of disease: the evolving role of regulatory T cells in atherosclerosis. Nat Clin Pract Cardiovasc Med. 2008;5:531–40. doi: 10.1038/ncpcardio1279. [DOI] [PubMed] [Google Scholar]
- 160.Andre S, Tough DF, Lacroix-Desmazes S, Kaveri SV, Bayry J. Surveillance of antigen-presenting cells by CD4+ CD25+ regulatory T cells in autoimmunity: immunopathogenesis and therapeutic implications. Am J Pathol. 2009;174:1575–87. doi: 10.2353/ajpath.2009.080987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Haribhai D, Lin W, Edwards B, Ziegelbauer J, Salzman NH, Carlson MR, Li SH, Simpson PM, Chatila TA, Williams CB. A central role for induced regulatory T cells in tolerance induction in experimental colitis. J Immunol. 2009;182:3461–8. doi: 10.4049/jimmunol.0802535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chatila TA. Regulatory T cells: key players in tolerance and autoimmunity. Endocrinol Metab Clin North Am. 2009;38:265–72. vii. doi: 10.1016/j.ecl.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lal G, Bromberg JS. Epigenetic mechanisms of regulation of Foxp3 expression. Blood. 2009;114:3727–35. doi: 10.1182/blood-2009-05-219584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bollyky PL, Falk BA, Wu RP, Buckner JH, Wight TN, Nepom GT. Intact extracellular matrix and the maintenance of immune tolerance: high molecular weight hyaluronan promotes persistence of induced CD4+CD25+ regulatory T cells. J Leukoc Biol. 2009;86:567–72. doi: 10.1189/jlb.0109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Valencia X, Lipsky PE. CD4+CD25+FoxP3+ regulatory T cells in autoimmune diseases. Nat Clin Pract Rheumatol. 2007;3:619–26. doi: 10.1038/ncprheum0624. [DOI] [PubMed] [Google Scholar]
- 166.von Herrath MG, Harrison LC. Antigen-induced regulatory T cells in autoimmunity. Nat Rev Immunol. 2003;3:223–32. doi: 10.1038/nri1029. [DOI] [PubMed] [Google Scholar]
- 167.Yan Y, Chen Y, Yang F, Chen IH, Xiong Z, Wang J, Lachman LB, Wang H, Yang XF. HLA-A2.1-restricted T cells react to SEREX-defined tumor antigen CML66L and are suppressed by CD4+CD25+ regulatory T cells. Int J Immunopathol Pharmacol. 2007;20:75–89. doi: 10.1177/039463200702000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–7. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 170.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–7. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 171.O’Connor RA, Malpass KH, Anderton SM. The inflamed central nervous system drives the activation and rapid proliferation of Foxp3+ regulatory T cells. J Immunol. 2007;179:958–66. doi: 10.4049/jimmunol.179.2.958. [DOI] [PubMed] [Google Scholar]
- 172.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–7. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–2. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhou L, Lopes JE, Chong MM, Ivanov, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–40. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Du J, Huang C, Zhou B, Ziegler SF. Isoform-specific inhibition of ROR alpha-mediated transcriptional activation by human FOXP3. J Immunol. 2008;180:4785–92. doi: 10.4049/jimmunol.180.7.4785. [DOI] [PubMed] [Google Scholar]
- 176.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O’Shea J. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–81. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 177.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 178.Adamson AS, Collins K, Laurence A, O’Shea JJ. The Current STATus of lymphocyte signaling: new roles for old players. Curr Opin Immunol. 2009;21:161–6. doi: 10.1016/j.coi.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O’Farrelly C, Tubridy N, Mills KH. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183:7602–10. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 180.Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat Rev Immunol. 2009;9:883–9. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]