Abstract
Study Design: A randomized, double-blind, controlled trial.
Objective: To determine the clinical effectiveness of therapeutic lumbar facet joint nerve blocks with or without steroids in managing chronic low back pain of facet joint origin.
Summary of Background Data: Lumbar facet joints have been shown as the source of chronic pain in 21% to 41% of low back patients with an average prevalence of 31% utilizing controlled comparative local anesthetic blocks. Intraarticular injections, medial branch blocks, and radiofrequency neurotomy of lumbar facet joint nerves have been described in the alleviation of chronic low back pain of facet joint origin.
Methods: The study included 120 patients with 60 patients in each group with local anesthetic alone or local anesthetic and steroids. The inclusion criteria was based upon a positive response to diagnostic controlled, comparative local anesthetic lumbar facet joint blocks.
Outcome measures included the numeric rating scale (NRS), Oswestry Disability Index (ODI), opioid intake, and work status, at baseline, 3, 6, 12, 18, and 24 months.
Results: Significant improvement with significant pain relief of ≥ 50% and functional improvement of ≥ 40% were observed in 85% in Group 1, and 90% in Group II, at 2-year follow-up.
The patients in the study experienced significant pain relief for 82 to 84 weeks of 104 weeks, requiring approximately 5 to 6 treatments with an average relief of 19 weeks per episode of treatment.
Conclusions: Therapeutic lumbar facet joint nerve blocks, with or without steroids, may provide a management option for chronic function-limiting low back pain of facet joint origin.
Keywords: Chronic low back pain, lumbar facet or zygapophysial joint pain, facet joint nerve or medial branch blocks, comparative controlled local anesthetic blocks, therapeutic lumbar facet joint nerve blocks
Introduction
Recent investigations1 have reported the rising prevalence of chronic low back pain. Freburger et al1 showed an increasing prevalence of chronic impairing low back pain over a 14-year interval from 3.9% in 1992 to 10.2% in 2006 - an overall increase in the prevalence of low back pain of 162% with an annual increase of 11.6%. The widely held belief that most of the episodes of low back pain will be short-lived, with 80% to 90% of these attacks resolving in about 6 weeks,2,3 has been questioned.1,4-8
Multiple structures in the lumbar spine including discs, facet joints, and sacroiliac joints have been considered the major sources of pain in the low back and/or lower extremities. Lumbar facet joints have been implicated as the source of chronic pain in 21% to 41% (with an overall prevalence of 31%) in a heterogenous population with chronic low back pain9-18 utilizing controlled comparative local anesthetic blocks with 80% pain relief and the ability to perform previously painful movements as the criterion standard. Further, based on the responses to controlled diagnostic blocks, false-positive rates of 17% to 19% have been established with an overall false-positive rate of 30%.9-14,16-18 Datta et al9 established Level I or II-1 evidence for the diagnostic accuracy of controlled facet joint nerve blocks based on the United States Preventive Services Task Force (USPSTF) criteria.19 In addition, Rubinstein and van Tulder20 concluded that there is strong evidence for the diagnostic accuracy of lumbar facet joint blocks in evaluating low back pain.
Significant controversy surrounds the appropriate management of lumbar facet joint pain, with multiple therapeutic techniques established in managing chronic low back pain.9,10,21-23 The systematic review by Datta et al9 provided Level III (limited) evidence for lumbar intraarticular injections,24,25 Level II-1 evidence for lumbar facet joint nerve blocks,26-28 and Level II-2 evidence for lumbar radiofrequency neurotomy.29-31 The exact mechanism of the therapeutic effect of lumbar facet joint nerve blocks is not known, whereas radiofrequency neurotomy causes denaturing of the nerves. Consequently, with radiofrequency the pain returns when the axons regenerate requiring repetition of the radiofrequency procedure. Similarly, lumbar facet joint nerve blocks may be repeated to reinstate pain relief without any deleterious effects. The basis for intraarticular injections has been the inflammation of the joint.
This report consists of the 2-year results of the comparativeness of lumbar facet joint nerve blocks with or without steroids evaluated in a randomized, double-blind, controlled trial in patients with a confirmed diagnosis of lumbar facet joint pain by means of comparative, controlled, local anesthetic blocks based on modified International Association for the Study of Pain (IASP) criteria, with 80% pain relief and ability to perform previously painful movements.9-11,32
Materials and Methods
The study was conducted at an interventional pain management practice, a specialty referral center, in a private practice setting in the United States. The study was performed based on Consolidated Standards of Reporting Trials (CONSORT) guidelines.33,34 The study protocol was approved by the Institutional Review Board (IRB) and the study has been registered with the clinical trial registry as NCT00355914.
Participants
One hundred twenty patients were assigned to one of the 2 groups consisting of either a non-steroid group (Group I) or a steroid group (Group II). Both groups were also divided into 2 categories each with the addition of Sarapin. Both groups received bupivacaine with or without steroid, however, category B patients also received Sarapin in both groups. All mixtures consisted of clear solutions. Bupivacaine and Sarapin were mixed in equal volumes, and 0.15 mg of non-particulate betamethasone was added per mL of solution.
Inclusion and Exclusion Criteria
Inclusion criteria consisted of those patients with a history of chronic function-limiting low back pain of at least 6 months duration, 18 years of age, who were able to provide voluntary informed consent, willing to participate in the study as well as the follow-up, with positive results to controlled diagnostic lumbar facet joint nerve blocks with at least 80% concordant pain relief and the ability to perform previously painful movements.
For diagnostic lumbar facet joint interventions the exclusion criteria included radicular pain, surgical interventions of the lumbar spine within the last 3 months, uncontrolled major depression or psychiatric disorders, heavy opioid usage (morphine equivalent of 300 mg), acute or uncontrolled medical illness, chronic severe conditions that could interfere with the interpretations of the outcome assessments, women who were pregnant or lactating, patients unable to be positioned in the prone position, and patients with a history of adverse reactions to local anesthetic, Sarapin, or steroids.
Interventions
All of the patients were provided with the informed consent and protocol approved by the IRB, which described the details of the trial including side effects and the mechanisms of withdrawal from the study.
Diagnostic Lumbar Facet Joint Nerve Blocks
All patients included in the study underwent controlled comparative local anesthetic blocks with 0.5 mL of 1% preservative-free lidocaine, followed by 0.5 mL of 0.25% bupivacaine on a separate occasion, usually 3 to 4 weeks after the first injection, if the results of the lidocaine block were positive. All of the procedures were performed in a sterile operating room, with intermittent fluoroscopic visualization, with intravenous access, and light sedation with midazolam being offered to all patients. A response was considered positive if there was 80% pain relief of at least 2 hours for lidocaine and 3 hours for bupivacaine and greater than the duration of relief with lidocaine, and the ability to perform multiple maneuvers which were painful prior to the diagnostic facet joint blocks. All other types of responses were considered negative; however, the diagnostic phase was not part of the study.
Therapeutic Lumbar Facet Joint Nerve Blocks
In the therapeutic phase, patients were treated with lumbar facet joint nerve blocks under fluoroscopy in a sterile operating room with the injection of a 0.5 to 1.5 mL mixture at each level as assigned by grouping.
Additional Interventions
Patients were followed at 3-month intervals unless otherwise indicated and lumbar facet joint nerve blocks were repeated based on the response to the prior interventions with improvement in physical and functional status. Lumbar facet joint nerve blocks were repeated only when the reported pain levels deteriorated to below 50%, with initial report of significant pain relief of 50% or more after the previous block. The non-responsive patients receiving other types of treatments after stopping therapeutic lumbar facet joint nerve blocks were considered to be withdrawn from the study, and no subsequent data were collected.
Co-Interventions
All patients were provided with the same co-interventions as needed with opioid and non-opioid analgesics, adjuvant analgesics, and previously directed exercise programs prior to enrollment in the study. The adjustments in medical therapy were carried out based on the response to injection therapy and physical and functional needs. However, no specific co-interventions such as physical therapy or occupational therapy were provided.
Objective
The objective of this randomized, double-blind, controlled trial is to determine the clinical effectiveness of therapeutic lumbar facet joint nerve blocks with local anesthetic with or without steroids in managing chronic low back pain of facet joint origin.
Outcomes
Outcome measures included the numeric rating scale (NRS), Oswestry Disability Index 2.0 (ODI), employment status, and opioid intake, with assessment at 3, 6, 12, 18, and 24 months post-treatment.
NRS represented 0 with no pain and 10 with the worst pain imaginable. The ODI was utilized for functional assessment. Value, validity, and frequent usage have been reported.33,35-42
Significant pain relief was described as a 50% or more reduction in the NRS score, and significant improvement in function was described as at least a 40% reduction in ODI.
Opioid intake was evaluated based on the dosage frequency and schedule of the drug, with conversion of opioid intake into morphine equivalents.43
Patients unemployed or employed on a part-time basis with limited or no employment due to pain were classified as employable. Patients who chose not to work, were retired, or were homemakers (not working, but not due to pain) were not considered in the employment pool.
Sample Size
For this evaluation, a sample size of 60 patients for each group was chosen. There were no randomized trials available to base the calculation of sample size. Further, the sample size was much smaller in previous studies of lumbar44 and cervical45 medial branch neurotomies. The literature evaluating the quality of individual articles has shown a sample size of 50 patients in the smallest group as acceptable.46
Randomization
Sixty patients were randomly assigned into each group from a total of 120 patients. Among each group, 30 patients were assigned to each category for Sarapin.
Sequence Generation
Randomization was carried out in blocks of 20 patients by a computer-generated random allocations sequence.
Allocation Concealment
Patients were randomized and the drugs were prepared appropriately by the operating room nurse assisting with the procedure. All drug mixtures appeared to be identical.
Implementation
After the patients had met the inclusion criteria one of the 3 nurses assigned as coordinators of the study enrolled the participants and assigned participants to their respective groups. All the patients were invited to enroll in the study if they met inclusion criteria.
Blinding
The random allocation was not revealed to personnel in the recovery room or to the physician performing the procedure. Study patients were mixed with other patients with no specific indication that patients were participating in the study.
Patients were unblinded if they requested to be unblinded or after completing 24 months of the study. Patients were provided with an opportunity to discontinue or withdraw from the study for lack of pain relief or for any other reason. All the patients with loss of follow-up were considered to be withdrawn.
Statistical Methods
Chi-squared statistic, Fisher's exact test, paired t-test, and one-way analysis of variance were used to analyze the data.
Chi-squared statistic was used to test the differences in proportions. Fisher's exact test was used wherever the expected value was less than 5; a paired t-test was used to compare the pre- and post-treatment results of average pain scores and the ODI measurements at baseline versus 3, 6, 12, 18, and 24 months. The t-test was performed for comparison of mean scores between groups. One-way analysis of variance was used for comparison of means among groups.
Initially, categories with or without Sarapin in each group were analyzed by comparing them to each other. Subsequently, local anesthetic and steroid group were compared if there were no differences.
Intent-to-Treat-Analysis
An intent-to-treat-analysis was performed on all patients utilizing the last follow-up data. Initial data were utilized in the patients who dropped out of the study without further follow-up after the first treatment. Sensitivity analysis was performed utilizing best case, worst case, and last follow-up scores scenarios.
Results
Participant Flow
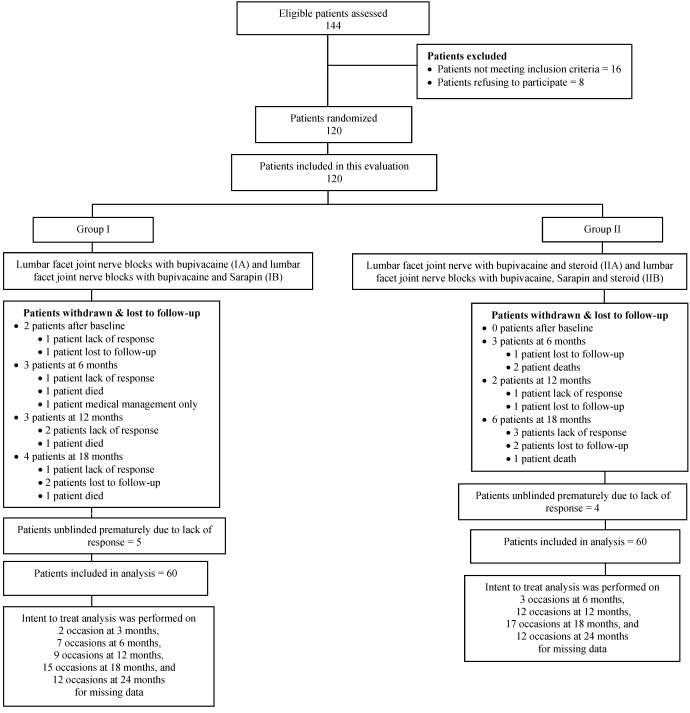
Figure 1 illustrates the participant flow.
Fig 1.
Schematic presentation of patient flow at 2-years follow-up.
Recruitment
The recruitment period lasted from November 2003 to July 2006.
Baseline Data
Demographic characteristics are illustrated in Table 1. There were significant differences between Group I and II with respect to height with Group II patients taller than Group I patients. This is not expected to change the outcomes.
Table 1.
Demographic characteristics.
| Group I (local anesthetic without steroids) (N = 60) | Group II (local anesthetic with steroids) (N = 60) | ||
|---|---|---|---|
| Gender | Male | 35% (21) | 45% (27) |
| Female | 65% (39) | 55% (33) | |
| Age | Mean ± SD | 48 ± 15 | 46 ± 17 |
| Height (inches) | Mean ± SD | 66 ± 3.8 | 68* ± 4.1 |
| Weight (pounds) | Mean ± SD | 183 ± 48 | 189 ± 50 |
| Duration of pain (months) | Mean ± SD | 108 ± 102 | 108 ± 94 |
| Mode of onset of pain | Gradual | 52% (31) | 62% (37) |
| Sudden | 16% (10) | 5% (3) | |
| WC/MVA | 32% (19) | 33% (20) | |
| H/O of previous lumbar surgery | 20% (112) | 13% (8) |
Group I = bupivacaine with or without Sarapin
Group II = bupivacaine and steroids with or without Sarapin
WC = Workers compensation
MVA = Motor vehicle injury
The number of joints involved was as follows: 2 joints were involved in 70% of the patients and 3 joints were involved in 30% of the patients. Bilateral involvement was seen in 79% of the patients.
Analysis of Data
Numbers Analyzed
Data were analyzed for both categories in each group to evaluate the influence of Sarapin. There were no significant differences. Thus, descriptions are provided for the 2 groups with local anesthetic with or without steroid.
Figure 1 illustrates details of patient follow-up and intent-to-treat analysis.
Missing Data
A sensitivity analysis with changes in numeric pain scale was performed utilizing last follow-up score, best case scenario, and worst case scenario as shown in Table 2. There were no significant differences; hence, intention-to-treat analysis with last follow-up visit was utilized.
Table 2.
Sensitivity analysis of numeric pain rating scores (NRS).
| Last visit Values | Best Case Values | Worst Case Values | Average Values | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group I | Group II | Diff. | P value | Group I | Group II | Diff. | P value | Group I | Group II | Diff. | P value | Group I | Group II | Dff. | P value | |
| Baseline | 8.22 ± 0.78 | 7.93 ± 0.99 | 0.28 ± 0.16 | 0.085 | 8.22 ± 0.78 | 7.93 ± 0.99 | 0.28 ± 0.16 | 0.085 | 8.22 ± 0.78 | 7.93 ± 0.99 | 0.28 ± 0.16 | 0.085 | 8.22 ± 0.78 | 7.93 ± 0.99 | 0.28 ± 0.16 | 0.085 |
| 3 months | 3.83* ± 1.29 | 3.52* ± 1.11 | 0.32 ± 0.22 | 0.153 | 3.83* ± 1.29 | 3.52* ± 1.11 | 0.32 ± 0.22 | 0.153 | 3.83* ± 1.29 | 3.52* ± 1.11 | 0.32 ± 0.22 | 0.153 | 3.83* ± 1.29 | 3.52* ± 1.11 | 0.32 ± 0.22 | 0.153 |
| 6 months | 3.55* ± 1.38 | 3.28* ± 0.85 | 0.27 ± 0.21 | 0.205 | 3.53* ± 1.42 | 3.28* ± 0.85 | 0.25 ± 0.21 | 0.244 | 3.97* ± 1.83 | 3.52* ± 1.32 | 0.45 ± 0.29 | 0.125 | 3.48* ± 1.36 | 3.28* ± 0.83 | 0.20 ± 0.21 | 0.332 |
| 12 months | 3.57* ± 1.45 | 3.40* ± 1.08 | 0.17 ± 0.23 | 0.477 | 3.57* ± 1.45 | 3.40* ± 1.08 | 0.17 ± 0.23 | 0.477 | 4.05* ± 2.10 | 4.07* ± 1.91 | -0.02 ± 0.37 | 0.964 | 3.25* ± 1.05 | 3.40* ± 1.03 | -0.15 ± 0.19 | 0.431 |
| 18 months | 3.47* ± 1.47 | 3.33* ± 1.0 | 0.13 ± 0.23 | 0.562 | 3.35* ± 1.42 | 3.25* ± 1.02 | 0.10 ± 0.23 | 0.659 | 4.45* ± 2.32 | 4.50* ± 2.07 | -0.05 ± 0.40 | 0.901 | 3.15* ± 0.73 | 3.28* ± 0.85 | -0.13 ± 0.16 | 0.358 |
| 24 months | 3.45* ± 1.48 | 3.22* ± 0.9 | 0.23 ± 0.22 | 0.299 | 3.37* ± 1.43 | 3.15* ± 0.92 | 0.22 ± 0.22 | 0.324 | 4.12* ± 2.34 | 3.78* ± 1.76 | 0.33 ± 0.88 | 0.376 | 3.05* ± 0.95 | 3.10* ± 0.82 | -0.05 ± 0.16 | 0.757 |
* indicates significant difference with baseline values
Outcomes
Pain Relief
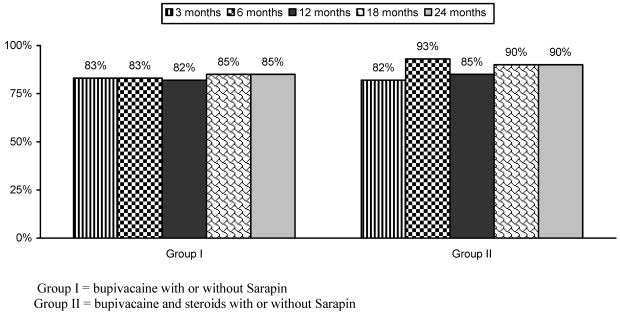
Numeric pain scale scores are illustrated in Table 3. There were significant changes in pain scores from baseline, at 3, 6, 12, 18, and 24 months in all the groups, with no differences among Groups I and II. At 2 years, the proportion of patients with significant pain relief was 85% and 90%, in Groups I and II respectively (Fig. 2).
Table 3.
Pain relief characteristics.
| Group I (local anesthetic without steroids) (N = 60) | Group II (local anesthetic with steroids) (N = 60) | ||
|---|---|---|---|
| Average Pain Scores (Mean ± SD) | Baseline | 8.2 ± 0.8 | 7.9 ± 1.0 |
| 3 months | 3.8* ± 1.3 | 3.5* ± 1.1 | |
| 6 months | 3.6* ± 1.5 | 3.3* ± 0.8 | |
| 12 months | 3.7* ± 1.7 | 3.5* ± 1.1 | |
| 18 months | 3.5* ± 1.5 | 3.3* ± 1.0 | |
| 24 months | 3.5* ± 1.5 | 3.2* ± 0.9 |
* indicates significant difference with baseline values
Group I = bupivacaine with or without Sarapin
Group II = bupivacaine and steroids with or without Sarapin
Fig 2.
Proportion of patients with significant relief of ≥ 50%.
Table 4 illustrates the therapeutic procedural characteristics with average weeks of pain relief per procedure over a period of 2 years.
Table 4.
Therapeutic procedural characteristics over a period of 2 years with average relief per procedure in weeks.
| Group I (local anesthetic without steroids) (N = 60) | Group II (local anesthetic with steroids) (N = 60) | |||
|---|---|---|---|---|
| Number of Procedures | Average relief per procedure | Average total relief with sequential procedures | Average relief per procedure | Average total relief with sequential procedures |
| One | 42 ± 47.1 (7) | 42 ± 47.1 (7) | 59 ± 51.7 (4) | 59 ± 51.7 (4) |
| Two | 39 ± 25.5 (4) | 79 ± 51.0 (4) | 29 ± 21.3 (6) | 58 ± 42.6 (6) |
| Three | 21 ± 12.6 (8) | 63 ± 37.8 (8) | 21 ± 10.9 (4) | 63 ± 32.6 (4) |
| Four | 18 ± 11.8 (2) | 71 ± 47.4 (2) | 18 ± 6.9 (8) | 71 ± 27.7 (8) |
| Five | 16 ± 5.8 (3) | 81 ± 28.5 (3) | 18 ± 2.9 (5) | 89 ± 14.4 (5) |
| Six | 13 ± 3.8 (5) | 80 ± 20.3 (5) | 15 ± 2.9 (5) | 88 ± 17.6 (5) |
| Seven | 13 ± 0.7 (10) | 93 ± 4.8 (10) | 13 ± 2.1 (6) | 91 ± 14.5 (6) |
| Eight | 13 ± 0.6 (18) | 100 ± 5.1 (18) | 12 ± 0.6 (20) | 99 ± 4.8 (20) |
| Nine | 11 ± 0.4 (3) | 99 ± 3.8 (3) | 11 ± 0.1 (2) | 103 ± 0.7 (2) |
| Average relief per procedure | 19 ± 19.9 (60) | 82 ± 31.8 (60) | 19 ± 18.2 (60) | 84 ± 27.5 (60) |
Group I = bupivacaine with or without Sarapin
Group II = bupivacaine and steroids with or without Sarapin
Functional Assessment
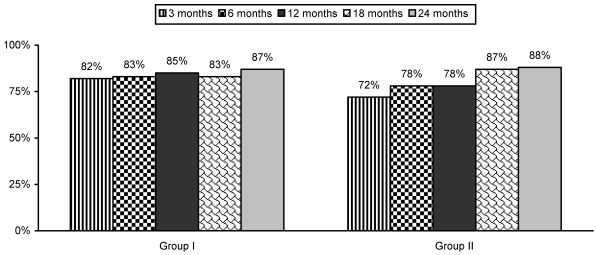
Table 5 and Figure 3 illustrate functional assessment characteristics evaluated by ODI.
Table 5.
Functional assessment evaluated by Oswestry Disability Index scores (Mean ± SD).
| Group I (local anesthetic without steroids) (N = 60) | Group II (local anesthetic with steroids) (N = 60) | |
|---|---|---|
| Baseline | 26.6 ± 4.6 | 25.9 ± 5.0 |
| 3 months | 12.7* ± 4.7 | 13.5* ± 5.6 |
| 6 months | 12.7* ± 4.7 | 12.2* ± 5.0 |
| 12 months | 12.3* ± 4.8 | 12.0* ± 5.4 |
| 18 months | 12.1* ± 5.0 | 11.2* ± 4.9 |
| 24 months | 12.0* ± 4.9 | 11.0* ± 4.8 |
* indicates significant difference with baseline values
Group I = bupivacaine with or without Sarapin
Group II = bupivacaine and steroids with or without Sarapin
Fig 3.
Proportion of patients with significant functional status improvement (≥ 40%) as measured by Oswestry Disability Index (ODI).
Opioid Intake
Table 6 illustrates opioid intake with no significant change.
Table 6.
Daily opioid intake in mg of morphine equivalents.
| Group I | Group II | P value | |
|---|---|---|---|
| Baseline | 31 ± 25.2 | 37 ± 40.4 | 0.294 |
| 12 months | 29 ± 25.6 | 33 ± 31.1 | 0.410 |
| 24 months | 27 ± 23.8 | 30 ± 27.1 | 0.549 |
Employment Characteristics
Table 7 illustrates the summary of employment characteristics in both groups. Among the patients eligible for employment, total employed changed from 10 at baseline to 16 at the end of 24 months in Group I, and it changed from 17 to 22 in Group II.
Table 7.
Employment characteristics.
| Employment status | Group I (local anesthetic without steroids) (N = 60) | Group II (local anesthetic with steroids) (N = 60) | ||||
|---|---|---|---|---|---|---|
| Baseline | 12 months | 24 months | Baseline | 12 months | 24 months | |
| Employed part-time | 4 | 4 | 4 | 4 | 2 | 0 |
| Employed full-time | 6 | 12 | 12 | 13 | 20 | 22 |
| Total Employed | 10 | 16 | 16 | 17 | 22 | 22 |
| Unemployed | 6 | 1 | 2 | 6 | 3 | 2 |
| Housewife | 7 | 4 | 3 | 3 | 2 | 3 |
| Disabled | 29 | 30 | 30 | 25 | 24 | 25 |
| Over 65 year of age | 8 | 9 | 9 | 9 | 9 | 8* |
| Total not working | 50 | 44 | 44 | 43 | 38 | 38 |
| Total Number of Patients | 60 | 60 | 60 | 60 | 60 | 60 |
* 1 patient over age of 65 returned to work
Group I = bupivacaine with or without Sarapin
Group II = bupivacaine and steroids with or without Sarapin
Adverse Events
There were no adverse events reported during this study.
Discussion
This randomized, double-blind, controlled trial comprised 120 patients with chronic function-limiting low back pain of facet joint origin who were treated with therapeutic lumbar facet joint nerve blocks. Significant pain relief was shown in 85% of Group I and 90% of Group II at the end of the 2 year study period. No significant differences were noted whether patients received treatment with local anesthetic only or local anesthetic and steroids. In addition, functional assessment as measured by ODI also showed significant improvement, with at least a 40% reduction in disability scores in 87% of patients in Group I and 88% of patients in Group II. Over the 2 year period, the average pain relief per procedure was 19 weeks; the average number of procedures was 5-6; total relief lasted 82 to 84 weeks. The results of employment status and opioid reduction were not significant. Pain relief and improvement in functional status were significant. Strict criteria were used for diagnosing facet joint pain; controlled, comparative local anesthetic blocks were used, thus avoiding criticism of including patients without facet joint pain in the study. Overall the results of the current study are similar to previous studies.26-28 There are no other studies available, either observational or randomized, evaluating the therapeutic role of lumbar facet joint nerve blocks with a long-term follow-up of at least 2 years.
In this randomized, double-blind, controlled trial we found that the 2 drugs used in combination with local anesthetic, namely Sarapin and steroid, did not differ significantly in their response. The small differences between the 2 treatments are unlikely to be of clinical importance even in larger studies.
The lack of placebo control could be criticized as a drawback. Placebo control in any neural blockade is an extremely difficult task. In the United States, it also adds ethical issues and difficulty with recruitment. What has been described as placebo control has been met with design flaws. The effect of any solution injected into a closed space such as an intraarticular space or epidural space or over a nerve has not been appropriately evaluated. Carette et al24 showed that patients responded similarly to an intraarticular injection whether it contained a sodium chloride solution or local anesthetic with steroid; however, the response was low in both groups. Thus, their study shows that sodium chloride solution injected into an intraarticular space has similar effects as local anesthetic with steroids; the conclusion is that intraarticular steroids are not an effective therapy. The issue is also exemplified by the fact that Birkenmaier et al47, utilizing either pericapsular injections or medial branch blocks, went on to perform cryoneurolysis. Not surprisingly, the results were superior in patients who were diagnosed using medial branch blocks rather than pericapsular injections of local anesthetic. This study was the basis for Chou et al48 to discard the value of diagnostic lumbar facet joint nerve blocks. In addition, the literature shows differing effects with injections of various solutions such as local anesthetic, normal saline, or dextrose and also shows differing effects by injection into either the disc, facet joint, or multifidus muscle.49-55 It has been shown that a small volume of local anesthetic or normal saline abolishes muscle twitch induced by a low current 0.5 (mA) during electrolocation.49-51 Further, there is direct evidence for spinal cord involvement in placebo analgesia.52
The difference between 2 placebo injections of a sodium chloride solution and dextrose has been shown.49 The experimental and clinical findings from investigation of the electrophysiological effects of 0.9% sodium chloride and dextrose 5% in water solution have added new knowledge and controversy to multiple aspects of neural stimulation used in regional anesthesia. The potential inaccuracy created by 0.9% sodium chloride solution versus 5% dextrose has been described.49,55 Further, the evidence also has shown differing effects of sodium chloride solution when injected into the disc, the facet joint, or paraspinal muscles.53,54 Indahl et al53,54 studied the electromyographic response of the porcine multifidus musculature after nerve stimulation,54 and interaction among the porcine lumbar intervertebral disc, zygapophysial joints, and paraspinal muscles.53 They showed that stimulation of the disc and the facet joint capsule produced contractions in the multifidus fascicles.54 They also demonstrated that the introduction of lidocaine into the facet joint resulted in a significantly reduced electromyographic response, with the most drastic reduction seen when stimulating the facet joint capsule. Surprisingly, they53 also showed that the introduction of physiologic saline into the zygapophysial joint reduced the stimulation pathway from the intervertebral disc to the paraspinal musculature. Consequently, they hypothesized that the paraspinal muscle activation caused by nerve stimulation in the annulus fibrosus of a lumbar intervertebral disc could be altered by saline injection into the zygapophysial joint.
The evidence cited above leads to the conclusion that the effect of local anesthetic on lumbar facet joint nerve blocks cannot be attributed to placebo effect, even though it has been misinterpreted by some.56 Recent articles concerning vertebroplasty57,58 have generated further interest in placebo control trials. However, in neither group, even though they were randomized, were the results long lasting; this is in addition to other criticisms of the design, etc.59-63 Consequently, placebo effects are not expected to be seen in a high proportion of patients nor are they expected to be long lasting with repeat interventions over a period of 2 years. However, the limitations of the lack of placebo must not be underestimated. If feasible, a placebo-controlled study with appropriate design that includes not injecting the placebo solution over the facet joint nerves, and the subsequent results, would be highly valid and provide conclusive knowledge on the issue of placebo controlled blocks.
The present study resolves the issue of adding Sarapin and steroid to local anesthetic for therapeutic lumbar facet joint nerve blocks. In the past, conflicting results have been reported.64,65 The basis for intraarticular injections has always been that inflammation is present, and that steroids should be used to treat the inflammation. However, with lumbar facet joint nerve blocks, no such claims have been made either about the presence or reduction of inflammation with the blockade. The present study shows equal effectiveness for local anesthetics with or without steroid, indicating a lack of support for the proposition of inflammation. The literature is replete with descriptions of epidural corticosteroid injections providing a certain level of efficacy by their anti-inflammatory, immuno-suppressive, anti-edema effects, as well as the inhibition of neurotransmission within the C-fibers.66-69 At the same time, local anesthetics also have been described as providing long-term symptomatic relief, even though the mechanism of action continues to be an enigma.69-71 Local anesthetics have been postulated to provide relief by various mechanisms including suppression of nociceptive discharge,70 the blockade of the axonal transport,72,73 the block of the sympathetic reflex arc and sensitization,74,75 and anti-inflammatory effects.76 The long-term effectiveness of local anesthetics has been shown in a host of previous studies as a result of local anesthetic nerve blocks or epidural injections.36,38-42,77
A review of the literature shows that the present study is the largest to evaluate the effectiveness of lumbar facet joint nerve blocks in a randomized controlled trial (though not placebo controlled) with a 2-year follow-up. The argument that the same drugs are used for diagnostic and therapeutic blocks has no relevance. This is similar to transforaminal epidural injections wherein the same local anesthetic is utilized for both diagnosis and therapy.
In summary, the results present a real-world example describing patients in a private interventional pain management practice setting, with results generalizable to similar settings. However, the results are not applicable to the general population unless the same methodology is used for both diagnosis and therapy. The generalizability of the findings of this study might only be feasible if studies are published using large populations in multiple settings.
Conclusion
The evidence in this report demonstrates lumbar facet joint pain diagnosed by controlled, comparative local anesthetic blocks may be treated with lumbar facet joint nerve blocks either with or without steroid.
Acknowledgments
The authors wish to thank Sekar Edem for his assistance in the literature search; and Tonie M. Hatton and Diane E. Neihoff, transcriptionists, for their assistance in the preparation of this manuscript.
References
- 1.Freburger JK, Holmes GM, Agans RP. et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169:251–8. doi: 10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shekelle PG, Markovich M, Louie R. An epidemiologic study of episodes of back pain care. Spine (Phila Pa 1976) 1995;20:1668–73. doi: 10.1097/00007632-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Anderson GBJ, Svensson HO. The intensity of work recovery in low back pain. Spine (Phila Pa 1976) 1983;8:880–7. doi: 10.1097/00007632-198311000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Gureje O, Simon GE, von Korff M. A cross-national study of the course of persistent pain in primary care. Pain. 2001;92:195–200. doi: 10.1016/s0304-3959(00)00483-8. [DOI] [PubMed] [Google Scholar]
- 5.Kadam UT, Thomas E, Croft PR. Is chronic widespread pain a predictor of all-cause morbidity? A 3 year prospective population based study in family practice. J Rheumatol. 2005;32:1341–48. [PubMed] [Google Scholar]
- 6.Cassidy JD, Côté P, Carroll LJ. et al. Incidence and course of low back pain episodes in the general population. Spine (Phila Pa 1976) 2005;30:2817–23. doi: 10.1097/01.brs.0000190448.69091.53. [DOI] [PubMed] [Google Scholar]
- 7.Enthoven P, Skargren E, Oberg B. Clinical course in patients seeking primary care for back or neck pain: A prospective 5-year follow-up of outcome and health care consumption with subgroup analysis. Spine (Phila Pa 1976) 2004;29:2458–65. doi: 10.1097/01.brs.0000143025.84471.79. [DOI] [PubMed] [Google Scholar]
- 8.Miedema HS, Chorus AM, Wevers CW. et al. Chronicity of back problems during working life. Spine (Phila Pa 1976) 1998;23:2021–28. doi: 10.1097/00007632-199809150-00020. [DOI] [PubMed] [Google Scholar]
- 9.Datta S, Lee M, Falco FJE. et al. Systematic assessment of diagnostic accuracy and therapeutic utility of lumbar facet joint interventions. Pain Physician. 2009;12:437–60. [PubMed] [Google Scholar]
- 10.Manchikanti L, Boswell MV, Singh V. et al. Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician. 2009;12:699–802. [PubMed] [Google Scholar]
- 11.Manchikanti L, Boswell MV, Singh V. et al. Comprehensive review of neurophysiologic basis and diagnostic interventions in managing chronic spinal pain. Pain Physician. 2009;12:E71–120. [PubMed] [Google Scholar]
- 12.Manchikanti L, Singh V, Pampati V. et al. Is there correlation of facet joint pain in lumbar and cervical spine? Pain Physician. 2002;5:365–71. [PubMed] [Google Scholar]
- 13.Manchikanti L, Boswell MV, Singh V. et al. Prevalence of facet joint pain in chronic spinal pain of cervical, thoracic, and lumbar regions. BMC Musculoskelet Disord. 2004;5:15. doi: 10.1186/1471-2474-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manchukonda R, Manchikanti KN, Cash KA. et al. Facet joint pain in chronic spinal pain: An evaluation of prevalence and false-positive rate of diagnostic blocks. J Spinal Disord Tech. 2007;20:539–45. doi: 10.1097/BSD.0b013e3180577812. [DOI] [PubMed] [Google Scholar]
- 15.Schwarzer AC, Wang SC, Bogduk N. et al. Prevalence and clinical features of lumbar zygapophysial joint pain: A study in an Australian population with chronic low back pain. Ann Rheum Dis. 1995;54:100–6. doi: 10.1136/ard.54.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manchikanti L, Singh V, Pampati V. et al. Evaluation of the relative contributions of various structures in chronic low back pain. Pain Physician. 2001;4:308–16. [PubMed] [Google Scholar]
- 17.Manchikanti L, Hirsch JA, Pampati V. Chronic low back pain of facet (zygapophysial) joint origin: Is there a difference based on involvement of single or multiple spinal regions? Pain Physician. 2003;6:399–405. [PubMed] [Google Scholar]
- 18.Manchikanti L, Manchukonda R, Pampati V. et al. Prevalence of facet joint pain in chronic low back pain in postsurgical patients by controlled comparative local anesthetic blocks. Arch Phys Med Rehabil. 2007;88:449–55. doi: 10.1016/j.apmr.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Berg AO, Allan JD. Introducing the third U.S. Preventive Services Task Force. Am J Prev Med. 2001;20:S3–4. doi: 10.1016/s0749-3797(01)00271-9. [DOI] [PubMed] [Google Scholar]
- 20.Rubinstein SM, van Tulder M. A best-evidence review of diagnostic procedures for neck and low-back pain. Best Pract Res Clin Rheumatol. 2008;22:471–82. doi: 10.1016/j.berh.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Manchikanti L, Boswell MV, Datta S. et al. Comprehensive review of therapeutic interventions in managing chronic spinal pain. Pain Physician. 2009;12:E123–98. [PubMed] [Google Scholar]
- 22.Chou R, Atlas SJ, Stanos SP, Rosenquist RW. Nonsurgical interventional therapies for low back pain: A review of the evidence for an American Pain Society clinical practice guideline. Spine (Phila Pa 1976) 2009;34:1078–93. doi: 10.1097/BRS.0b013e3181a103b1. [DOI] [PubMed] [Google Scholar]
- 23.Staal JB, de Bie RA, de Vet HC. et al. Injection therapy for subacute and chronic low back pain: An updated Cochrane review. Spine (Phila Pa 1976) 2009;34:49–59. doi: 10.1097/BRS.0b013e3181909558. [DOI] [PubMed] [Google Scholar]
- 24.Carette S, Marcoux S, Truchon R. et al. A controlled trial of corticosteroid injections into facet joints for chronic low back pain. N Engl J Med. 1991;325:1002–7. doi: 10.1056/NEJM199110033251405. [DOI] [PubMed] [Google Scholar]
- 25.Schulte TL, Pietila TA, Heidenreich J. et al. Injection therapy of lumbar facet syndrome: A prospective study. Acta Neurochir (Wien) 2006;148:1165–72. doi: 10.1007/s00701-006-0897-z. [DOI] [PubMed] [Google Scholar]
- 26.Manchikanti L, Singh V, Falco FJE. et al. Lumbar facet joint nerve blocks in managing chronic facet joint pain: One-year follow-up of a randomized, double-blind controlled trial: Clinical Trial NCT00355914. Pain Physician. 2008;11:121–32. [PubMed] [Google Scholar]
- 27.Manchikanti L, Manchikanti KN, Manchukonda R. et al. Evaluation of lumbar facet joint nerve blocks in the management of chronic low back pain: A preliminary report of a randomized, double-blind controlled trial. Clinical Trial NCT000355914. Pain Physician. 2007;10:425–40. [PubMed] [Google Scholar]
- 28.Manchikanti L, Pampati V, Bakhit CE. et al. Effectiveness of lumbar facet joint nerve blocks in chronic low back pain: A randomized clinical trial. Pain Physician. 2001;4:101–17. [PubMed] [Google Scholar]
- 29.Nath S, Nath CA, Pettersson K. Percutaneous lumbar zygapophysial (facet) joint neurotomy using radiofrequency current, in the management of chronic low back pain. A randomized double-blind trial. Spine (Phila Pa 1976) 2008;33:1291–7. doi: 10.1097/BRS.0b013e31817329f0. [DOI] [PubMed] [Google Scholar]
- 30.Gofeld M, Jitendra J, Faclier G. Radiofrequency facet denervation of the lumbar zygapophysial joints: 10-year prospective clinical audit. Pain Physician. 2007;10:291–300. [PubMed] [Google Scholar]
- 31.Dreyfuss P, Halbrook B, Pauza K. et al. Efficacy and validity of radiofrequency neurotomy for chronic lumbar zygapophysial joint pain. Spine (Phila Pa 1976) 2000;25:1270–7. doi: 10.1097/00007632-200005150-00012. [DOI] [PubMed] [Google Scholar]
- 32.Merskey H, Bogduk N. Classification of Chronic Pain; Descriptions of Chronic Pain Syndromes and Definition of Pain Terms, 2nd ed; Task Force on Taxonomy of the International Association for the Study of Pain. Seattle: IASP Press; 1994. Lumbar zygapophysial joint pain; pp. 181–2. [Google Scholar]
- 33.Altman DG, Schulz KF, Moher D, et al; CONSORT GROUP (Consolidated Standards of Reporting Trials). The revised CONSORT statement for reporting randomized trials: Explanation and elaboration. Ann Intern Med. 2001;134:663–94. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 34.Moher D, Hopewell S, Schulz KF. et al. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fairbank JCT, Pynsent PB. The Oswestry disability index. Spine (Phila Pa 1976) 2000;25:2940–53. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 36.Manchikanti L, Singh V, Falco FJE. et al. Cervical medial branch blocks for chronic cervical facet joint pain: A randomized double-blind, controlled trial with one-year follow-up. Spine (Phila PA 1976) 2008;33:1813–20. doi: 10.1097/BRS.0b013e31817b8f88. [DOI] [PubMed] [Google Scholar]
- 37.Manchikanti L, Hirsch JA, Smith HS. Evidence-based medicine, systematic reviews, and guidelines in interventional pain management: Part 2: Randomized controlled trials. Pain Physician. 2008;11:717–73. [PubMed] [Google Scholar]
- 38.Manchikanti L, Singh V, Falco FJE. et al. Effectiveness of thoracic medial branch blocks in managing chronic pain: A preliminary report of a randomized, double-blind controlled trial; Clinical trial NCT00355706. Pain Physician. 2008;11:491–504. [PubMed] [Google Scholar]
- 39.Manchikanti L, Cash KA, McManus CD. et al. Preliminary results of randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: Part 1. Discogenic pain without disc herniation or radiculitis. Pain Physician. 2008;11:785–800. [PubMed] [Google Scholar]
- 40.Manchikanti L, Singh V, Cash KA. et al. Preliminary results of randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: Part 2. Disc herniation and radiculitis. Pain Physician. 2008;11:801–15. [PubMed] [Google Scholar]
- 41.Manchikanti L, Singh V, Cash KA. et al. Preliminary results of randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: Part 3. Post surgery syndrome. Pain Physician. 2008;11:817–31. [PubMed] [Google Scholar]
- 42.Manchikanti L, Cash KA, McManus CD. et al. Preliminary results of randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: Part 4. Spinal stenosis. Pain Physician. 2008;11:833–48. [PubMed] [Google Scholar]
- 43.Pereira J, Lawlor P, Vigano A. et al. Equianalgesic dose ratios for opioids. A critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22:672–87. doi: 10.1016/s0885-3924(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 44.van Kleef M, Barendse GA, Kessels A. et al. Randomized trial of radiofrequency lumbar facet denervation for chronic low back pain. Spine (Phila Pa 1976) 1999;24:1937–42. doi: 10.1097/00007632-199909150-00013. [DOI] [PubMed] [Google Scholar]
- 45.Lord SM, Barnsley L, Wallis BJ. et al. Percutaneous radiofrequency neurotomy for chronic cervical zygapophysial joint pain. N Engl J Med. 1996;335:1721–6. doi: 10.1056/NEJM199612053352302. [DOI] [PubMed] [Google Scholar]
- 46.Koes BW, Scholten RJ, Mens JM. et al. Efficacy of epidural steroid injections for low-back pain and sciatica: A systematic review of randomized clinical trials. Pain. 1995;63:279–88. doi: 10.1016/0304-3959(95)00124-7. [DOI] [PubMed] [Google Scholar]
- 47.Birkenmaier C, Veihelmann A, Trouillier HH. et al. Medial branch blocks versus pericapsular blocks in selecting patients for percutaneous cryodenervation of lumbar facet joints. Reg Anesth Pain Med. 2007;32:27–33. doi: 10.1016/j.rapm.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 48.Guideline for the Evaluation and Management of Low Back Pain: Evidence Review. Chou R, Huffman L; [American Pain Society, Glenview, IL, 2009]. www.ampainsoc.org/pub/pdf/LBPEvidRev.pdf. [Google Scholar]
- 49.Tsui BC, Wagner A, Finucane B. Electrophysiologic effect of injectates on peripheral nerve stimulation. Reg Anesth Pain Med. 2004;29:189–93. doi: 10.1016/j.rapm.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Pham Dang C, Lelong A, Guilley J. et al. Effect on neurostimulation of injectates used for perineural space expansion before placement of a stimulation catheter: Normal saline versus dextrose 5% in water. Reg Anesth Pain Med. 2009;34:398–403. doi: 10.1097/AAP.0b013e3181b48648. [DOI] [PubMed] [Google Scholar]
- 51.Johnson CR, Barr RC, Klein SM. A computer model of electrical stimulation of peripheral nerves in regional anesthesia. Anesthesiology. 2007;106:323–30. doi: 10.1097/00000542-200702000-00021. [DOI] [PubMed] [Google Scholar]
- 52.Eippert F, Finsterbusch J, Bingel U. et al. Direct evidence for spinal cord involvement in placebo analgesia. Science. 2009;326:404. doi: 10.1126/science.1180142. [DOI] [PubMed] [Google Scholar]
- 53.Indahl A, Kaigle AM, Reikeräs O. et al. Interaction between the porcine lumbar intervertebral disc, zygapophysial joints, and paraspinal muscles. Spine (Phila Pa 1976) 1997;22:2834–40. doi: 10.1097/00007632-199712150-00006. [DOI] [PubMed] [Google Scholar]
- 54.Indahl A, Kaigle A, Reikeräs O. et al. Electromyographic response of the porcine multifidus musculature after nerve stimulation. Spine (Phila Pa 1976) 1995;20:2652–8. doi: 10.1097/00007632-199512150-00006. [DOI] [PubMed] [Google Scholar]
- 55.Tsui BC, Kropelin B, Ganapathy S. et al. Dextrose 5% in water: Fluid medium maintaining electrical stimulation of peripheral nerve during stimulating catheter placement. Acta Anaesthesiol Scand. 2005;49:1562–5. doi: 10.1111/j.1399-6576.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- 56.Levin JH. Prospective, double-blind, randomized placebo-controlled trials in interventional spine: What the highest quality literature tells us. Spine J. 2009;9:690–703. doi: 10.1016/j.spinee.2008.06.447. [DOI] [PubMed] [Google Scholar]
- 57.Buchbinder R, Osborne RH, Ebeling PR. et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361:557–68. doi: 10.1056/NEJMoa0900429. [DOI] [PubMed] [Google Scholar]
- 58.Kallmes DF, Comstock BA, Heagerty PJ. et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361:569–79. doi: 10.1056/NEJMoa0900563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weinstein JN. Balancing science and informed choice in decisions about vertebroplasty. N Engl J Med. 2009;361:619–21. doi: 10.1056/NEJMe0905889. [DOI] [PubMed] [Google Scholar]
- 60.Clark W, Lyon S, Burnes J. Trials of vertebroplasty for vertebral factures. N Engl J Med. 2009;361:2097–8. doi: 10.1056/NEJMc096289. [DOI] [PubMed] [Google Scholar]
- 61.Baerlocher MO, Munk PL, Liu DM. Trials of vertebroplasty for fractures. N Engl J Med. 2009;361:2098. [PubMed] [Google Scholar]
- 62.Lotz JC. Trials of vertebroplasty for vertebral fractures. N Engl J Med. 2009;361:2098. [PubMed] [Google Scholar]
- 63.Grey A, Bolland M. Trials of vertebroplasty for vertebral fractures. N Engl J Med. 2009;361:2098–9. [PubMed] [Google Scholar]
- 64.Manchikanti L, Pampati V, Fellows B. et al. The diagnostic validity and therapeutic value of lumbar facet joint nerve blocks with or without adjuvant agents. Curr Rev Pain. 2000;4:337–44. doi: 10.1007/s11916-000-0016-4. [DOI] [PubMed] [Google Scholar]
- 65.Manchikanti KN, Pampati V, Damron KS. et al. A double-blind, controlled evaluation of the value of Sarapin in neural blockade. Pain Physician. 2004;7:59–62. [PubMed] [Google Scholar]
- 66.Hayashi N, Weinstein JN, Meller ST. et al. The effect of epidural injection of betamethasone or bupivacaine in a rat model of lumbar radiculopathy. Spine (Phila Pa 1976) 1998;23:877–85. doi: 10.1097/00007632-199804150-00008. [DOI] [PubMed] [Google Scholar]
- 67.Lee HM, Weinstein JN, Meller ST. et al. The role of steroids and their effects on phospholipase A2. An animal model of radiculopathy. Spine (Phila Pa 1976) 1998;23:1191–6. doi: 10.1097/00007632-199806010-00001. [DOI] [PubMed] [Google Scholar]
- 68.Johansson A, Hao J, Sjolund B. Local corticosteroid application blocks transmission in normal nociceptive C-fibres. Acta Anaesthesiol Scand. 1990;34:335–8. doi: 10.1111/j.1399-6576.1990.tb03097.x. [DOI] [PubMed] [Google Scholar]
- 69.Pasqualucci A, Varrassi G, Braschi A. et al. Epidural local anesthetic plus corticosteroid for the treatment of cervical brachial radicular pain: Single injection verus continuous infusion. Clin J Pain. 2007;23:551–7. doi: 10.1097/AJP.0b013e318074c95c. [DOI] [PubMed] [Google Scholar]
- 70.Arner S, Lindblom U, Meyerson BA. et al. Prolonged relief of neuralgia after regional anesthetic block. A call for further experimental and systematic clinical studies. Pain. 1990;43:287–97. doi: 10.1016/0304-3959(90)90026-A. [DOI] [PubMed] [Google Scholar]
- 71.Wertheim HM, Rovenstine EA. Suprascapular nerve block. Anesthesiology. 1941;2:541–5. [Google Scholar]
- 72.Bisby MA. Inhibition of axonal transport in nerves chronically treated with local anesthetics. Exp Neurol. 1975;47:481–89. doi: 10.1016/0014-4886(75)90080-1. [DOI] [PubMed] [Google Scholar]
- 73.Lavoie PA, Khazen T, Filion PR. Mechanisms of the inhibition of fast axonal transport by local anesthetics. Neuropharmacology. 1989;28:175–81. doi: 10.1016/0028-3908(89)90054-3. [DOI] [PubMed] [Google Scholar]
- 74.Katz WA, Rothenberg R. The nature of pain: Pathophysiology. J Clin Rheumatol. 2005;11:S11–5. doi: 10.1097/01.rhu.0000158686.43637.af. [DOI] [PubMed] [Google Scholar]
- 75.Melzack R, Coderre TJ, Katz J. et al. Central neuroplasticity and pathological pain. Ann N Y Acad Sci. 2001;933:157–74. doi: 10.1111/j.1749-6632.2001.tb05822.x. [DOI] [PubMed] [Google Scholar]
- 76.Cassuto J, Sinclair R, Bonderovic M. Anti-inflammatory properties of local anesthetics and their present and potential clinical implications. Acta Anaesthesiol Scand. 2006;50:265–82. doi: 10.1111/j.1399-6576.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- 77.Riew KD, Park JB, Cho YS. et al. Nerve root blocks in the treatment of lumbar radicular pain. A minimum five-year follow-up. J Bone Joint Surg Am. 2006;88:1722–5. doi: 10.2106/JBJS.E.00278. [DOI] [PubMed] [Google Scholar]