Abstract
Membrane-associated mucins are altered on the ocular surface in non-Sjögren’s dry eye. This study sought to determine if inflammatory mediators, present in tears of dry eye patients, regulate membrane-associated mucins MUC1 and −16 at the level of gene expression, protein biosynthesis and/or ectodomain release. A human corneal limbal epithelial cell line (HCLE), which produces membrane-associated mucins, was used. Cells were treated with interleukin (IL)-6, -8, or -17, tumor necrosis factor-α (TNF-α), and Interferon-gamma (IFN-γ), or a combination of TNF-α and IFN-γ, or IFN-γ and IL-17, for 1, 6, 24, or 48 hours. Presence of receptors for these mediators was verified by RT-PCR. Effects of the cytokines on expression levels of MUC1 and −16 were determined by real-time PCR, and on mucin protein biosynthesis and ectodomain release in cell lysates and culture media, respectively, by immunoblot analysis. TNF-α and IFN-γ each significantly induced MUC1 expression, cellular protein content and ectodomain release over time. Combined treatment with the two cytokines was not additive. By comparison, one of the inflammatory mediators, IFN-γ, affected all three parameters—gene expression, cellular protein, and ectodomain release—for MUC16. Combined treatment with TNF-α and IFN-γ showed effects similar to IFN-γ alone, except that ectodomain release followed that of TNF-α, which induced MUC16 ectodomain release. In conclusion, inflammatory mediators present in tears of dry eye patients can affect MUC1 and −16 on corneal epithelial cells and may be responsible for alterations of surface mucins in dry eye.
Keywords: IFN-γ, TNF-α, MUC1, MUC16, membrane-associated mucins, ocular surface, dry eye
1. Introduction
The International Dry Eye Workshop defined Dry Eye in 2007 as a multifactorial disorder of the tear film and the ocular surface, associated with discomfort, impaired vision, and tear film instability (DEWS, 2007). Common clinical signs of patients with dry eye syndrome include inflammation, increased tear osmolarity, decreased tear breakup time, and ocular surface epithelial damage as evidenced by dye penetrance (DEWS, 2007; Lemp, M.A., 1995). An increased number of soluble inflammatory mediators, including interleukin (IL)-6 and -8, tumor necrosis factor-α (TNF-α) and Interferon-γ (IFN-γ) have been detected in tears of dry eye patients with and without meibomian gland disease (Lam, H. et al., 2009), in Sjögren’s dry eye (Pflugfelder, S.C. et al., 1999) and in a mouse model of dry eye (De Paiva, C.S. et al., 2007; Song, X.J. et al., 2003). The more recently discovered, CD4+ derived Th17 cell line and it’s hallmark cytokine IL-17 have been shown to be involved in human and mouse autoimmune disorders and/or chronic inflammation, e.g., EAU (Experimental Autoimmune Uveitis), Psoriasis, Rheumatoid Arthritis, MS (Multiple Sclerosis), or inflammatory bowel disease (Harrington, L.E. et al., 2006; Kikly, K. et al., 2006; Luger, D. et al., 2008), and recent data demonstrate increased Th17 lymphocytes in conjunctiva in ocular cicatricial pemphigoid (Lambiase et al., 2009; De Paiva et al., 2009).
Recent data suggest that membrane-associated mucins (MAMs) play a role in the pathogenesis of dry eye (Argueso, P. et al., 2003; Danjo, Y. et al., 1998). Three membrane-associated mucins, MUC1, −4 and −16, are expressed by the ocular surface epithelium and are present along the epithelium-tear film interface (Gipson, I.K. et al., 2004). Their extracellular domains—constitutively released from apical cell membranes—are present in tears along with the goblet cell mucin MUC5AC (Spurr-Michaud S et al., 2007).
In vitro data show lack of Rose Bengal penetrance into corneal epithelial cells that express MUC16 and increased penetrance of the dye into cells in which MUC16 expression has been reduced, using siRNA methods (Blalock, T. et al., 2007). These findings have led to the hypothesis that loss of membrane-associated mucins (or altered glycosylation of the MAMs) leads to the development of dry spots and the Rose Bengal staining observed in dry eye. Cytokines, found in inflammatory diseases like dry eye, may be responsible—through direct or indirect effects—for alterations in expression and/or release of membrane-tethered mucins.
Several studies have examined the effect of inflammation on expression of membrane-associated mucins in different wet-surfaced epithelia. Li et al. (Li, X. et al., 2003) found upregulation of MUC1 mRNA in response to IL-6, IFN-γ, and TNF-α in oral mucosal epithelia, and Hoebler et al. demonstrated changes in mucin gene expression on induction of inflammation in a mouse model of colitis (Hoebler, C. et al., 2006). Paulsen et al. showed, using a human corneal epithelial cell line, an increase of membrane-associated MUC16 release by inflammatory mediators TNF-α and IFN-γ, without affecting expression levels of MUC16 (Paulsen, F. et al., 2008), and a recent report demonstrated that MUC1 and −16 release is induced by TNF-α and neutrophil elastase (Blalock, T.D. et al., 2008). To extend these data and to obtain further insight into alteration of MAMs at the ocular surface after inflammatory cytokine exposure, we have assessed the effect of IL-6, -8, -17, IFN-γ, and TNF-α and a combination of IFN-γ with TNF-α or IL-17 on MUC1 and MUC16 mRNA expression, as well as on mucin cellular protein content and ectodomain release using an immortalized human corneal epithelial cell line optimized for membrane-associated mucin expression.
2. Materials and methods
2.1 Culture of corneal epithelial cells with inflammatory cytokines
An immortalized human corneal limbal epithelial cell line (HCLE), which was previously optimized for membrane-associated mucin production, was used in this study (Gipson, I.K. et al., 2003). Cells were plated on 6-well plastic plates in keratinocyte serum-free medium (K-sfm; Invitrogen Corp., Carlsbad, CA, USA), containing 25 μg/mL bovine pituitary extract, 0.2 ng/mL epidermal growth factor (EGF) (Invitrogen), and 0.4 mM CaCl2 (Sigma-Aldrich, Saint Louis, MO, USA). After growth to confluence at 37°C in 5% carbon dioxide, cells were switched to DMEM/F12 (Mediatech, Inc., Herndon, VA, USA) medium with high concentrations of calcium (1 mM CaCl2) supplemented with 10% calf serum and 10 ng/mL EGF for 7 days to promote stratification, differentiation, and mucin production. To determine the effect of IL-6, -8, -17, IFN-γ, and TNF-α on mucin gene message, protein expression, and release, HCLE cells were grown as described above, rinsed with serum-free DMEM/F12 and switched to unsupplemented DMEM/F12 medium for 24-hour serum starvation, and then cultured in DMEM/F12 containing 10 ng/ml IL-6 (Nishida, T. et al., 1992), 20 ng/ml IL-8 (Itoh, Y. et al., 2005), 50 ng/ml IL-17 (Chabaud, M. et al., 2001), 10 ng/ml IFN-γ (Silverman, M.D. et al., 2003) or 10 ng/ml TNF-α (Silverman, M.D. et al., 2003) (R&D Systems, Minneapolis, MN, USA) for 1, 6, or 24 hours. Concentrations of cytokines used for assays were chosen based on the literature. All cytokines were in PBS with 0.01% bovine serum albumin. In order to determine if the effects of the two cytokines TNF-α and IFN-γ or IL-17 and IFN-γ were additive (Kullberg, M.C. et al., 2006), cells were also cultured with 10 ng/ml TNF-α plus 10 ng/ml IFN-γ or 10 ng/ml IFN-γ plus 50 ng/ml IL-17. Due to a highly increased amount of cellular MUC1 protein from cell lysates induced by IFN-γ after 24-hour culture, we added a 48-hour time point for this inflammatory cytokine, to investigate if the upregulation in cellular protein would cause a subsequent increase in release of the mucins’ ectodomain. Bovine serum albumin 0.01% (Sigma-Aldrich) was used as vehicle control. Cell cultures were checked for cell shape and morphology, and photographed using phase-contrast microscopy (Nikon Elipse TS100, Melville, NY, USA), before harvesting. All treatments were done in triplicate and repeated to obtain N=6.
2.2 Conventional and real-time PCR to assess cytokine receptor presence and mucin gene expression
Total RNA was isolated from the cell cultures with TRIzol reagent (Invitrogen) according to the manufacturer’s recommended protocol. The possibility of genomic contamination of total RNA was eliminated by digestion with Amplification Grade DNase I (Invitrogen) prior to reverse transcription using the iScript cDNA Synthesis Kit (BioRad, Hercules, CA, USA) according to the manufacturer’s protocol as previously described (Blalock, T. et al., 2007).
RNA isolated from untreated, serum-starved samples was assayed by RT-PCR for IL-6 receptor (IL-6R) and the IL-6R associated glycoprotein 130 (gp130) necessary for mediator-receptor interaction, IL-8 receptors (IL-8RA and IL-8RB), IL-17 receptor A (IL-17RA), tumor necrosis factor receptor I (TNFRI) and II (TNFRII), and the IFN-γ receptors (IFN-γRα and IFN-γRβ) using previously published primer sets and conditions for 35 cycles of amplification with modifications noted below (Eckert, J. and Niemann, H., 1998; Gerritsma, J.S. et al., 1997; Knofler, M. et al., 2000; Maertzdorf, J. et al., 2002; Path, G. et al., 1997). PCR amplification for receptors was performed as follows: IL-6R 251 bp: 3 minutes at 94°C; 35 cycles of 30 seconds at 94°C, 30 seconds at 60°C, 30 seconds at 72°C; GP130 367 bp: 5 minutes at 95°C, 2 minutes at 72°C; 35 cycles of 15 seconds at 95°C, 15 seconds at 57°C, 15 seconds at 72°C; final elongation: 5 minutes at 72°C, TNFRI 488 bp and TNFRII 374 bp: 5 minutes at 94°C; 35 cycles of 30 seconds at 94°C, 30 seconds at 60°C, 30 seconds at 72°C; final elongation: 5 minutes at 72°C. IL-17RA 456 bp: 5 minutes at 95°C; 40 cycles of 60 seconds at 95°C, 60 seconds at 50°C, 60 seconds at 72°C; final elongation: 7 minutes at 72°C. IFN-γRα 685 bp and IFN-γRβ 418 bp: 5 minutes at 95°C; 35 cycles of 1.5 minutes at 94°C, 2.5 minutes at 56°C, 1.5 minutes at 72°C; final elongation: 10 minutes at 73°C. Amplifications were performed on samples lacking cDNA as negative controls. Amplified products were run on 2% agarose gels and visualized with ethidium bromide.
Real-time PCR amplification and relative quantification of the mucin genes expressed by the cell cultures was performed with double-labeled fluorogenic probes and primers (TaqMan; Applied Biosystems, Foster City, CA, USA) as previously described and validated (Gipson, I.K. et al., 2003). To normalize for any differences in starting amount of mRNA, GAPDH was used as the reference gene after confirmation of its consistent expression over the course of treatments. Mucin gene expression was determined relative to the vehicle control at each time point, using the ΔΔCT method (Gipson, I.K. et al., 2003).
2.3 Western blot assays
Proteins were extracted from cell cultures with 2% SDS plus complete protease inhibitors (Roche, Indianapolis, IN, USA) and quantified using BCA Protein Assay (Pierce, Rockford, IL, USA). Cell culture medium supernatants from each well were individually collected after centrifugation at 17320 x g for 30 minutes at 4°C to remove any desquamated cells. Mucins present in supernatants were then concentrated using 10 K Omega NanoSep spin tubes (Pall, East Hill, NY, USA) or Microcon Ultracel YM-10 Centrifugal Filter devices (Milipore, Bedford, MA, USA) and collected with 15 μl 2X reducing Laemmli sample buffer to allow examination of all material released into the medium for each culture well, as previously described (Blalock, T.D. et al., 2008).
SDS-agarose gel electrophoresis, a method commonly used for separation of large heavily glycosylated mucin proteins, was used to assay MUC1 and MUC16 protein levels (Thornton, D. et al., 2000). Ten to twenty-five μg of total protein was separated electrophoretically, at 4°C, under reducing conditions in a 1% (w/v), agarose gel, cast in a horizontal gel apparatus and run in 1X Tris/Glycine/SDS Buffer (Bio-Rad Laboratories, Hercules, CA, USA) for 1 hour 50 minutes at 50 mV as previously described (Spurr-Michaud S et al., 2007). For immunoblot detection of protein, proteins were transferred by vacuum blotting (model 785 vacuum blotter, Bio-Rad) to nitrocellulose and probed with anti-MUC1/episialin, clone 214D4 (Upstate, Lake Placid, NY) or MUC16 (CA125) Clone OC125 (Dako, Troy, MI, USA) as previously described (Spurr-Michaud S et al., 2007). To confirm equivalent protein loading of cell lysates, immunoblots were subsequently probed with an antibody recognizing the intracellular GAPDH enzyme (Rabbit polyclonal GAPDH, Abcam, Cambridge, UK). Blots were photographed using a digital camera (Kodak DC40, Rochester, NY, USA), and amount of mucin protein was determined by densitometry of bands on immunoblots using an image analysis program (Kodak 1D 2.0.2).
2.4 Statistical analysis
Results are expressed as the mean ± SEM. The statistical significance of the effect of the different cytokines compared to the vehicle control was determined with the Mann-Whitney U test, using InStat Statistical software (Graphpad Software, San Diego, CA, USA). Differences resulting in p values <0.05 were considered to be statistically significant. Statistical comparisons of data obtained from combined treatments with IFN-γ and TNF-α or IL-17 were done using the Kruskal-Wallis test.
3. Results
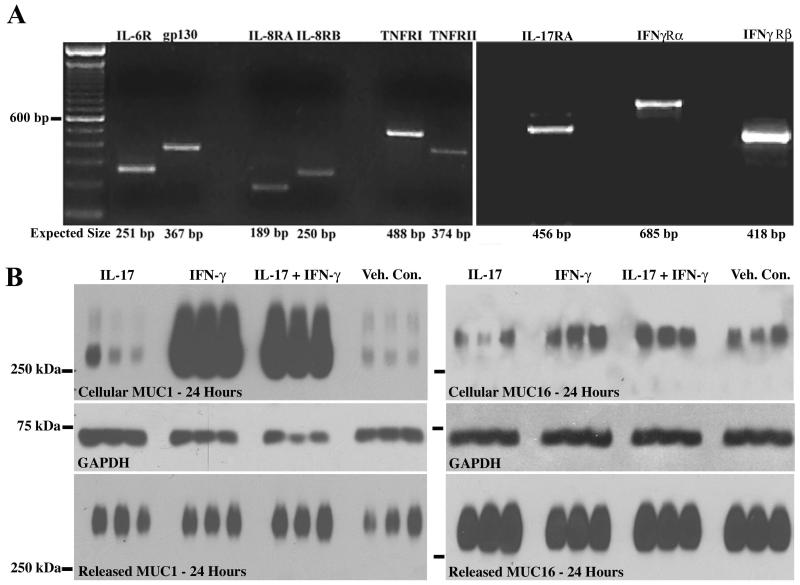
Inflammation of the ocular surface tissues occurs in several diseases, including dry eye. The surface epithelium affected by the disease is a stratified differentiated epithelium that expresses MUC1 and MUC16 apically. The human corneal epithelial cell line (HCLE) used in this study has been optimized to differentiate and stratify. The apical cells of the cultures produce the membrane mucins MUC1 and −16, which are present in the apical membrane glycocalyx as in native epithelia (Gipson, I.K. et al., 2003). Thus the direct effect of the inflammatory mediators demonstrated to be present in tears of dry eye patients with Sjögren’s disease—IL-6, IL-8, TNF-α and IFN-γ—and of IL-17 involved in autoimmune disorders and chronic inflammation of epithelia can be assessed in the HCLE cultures after serum deprivation. Analysis of mediator effects on cells in culture must be done on cells in which receptors of the mediators are present. Therefore, expression of receptors for the five inflammatory mediators in HCLE cells was analyzed and all were shown to be present by conventional PCR (Fig. 1A), IL-17 receptor A, and IL-6 and IL-8 receptors in human corneal cells have, to our knowledge, not been reported. Analysis of effects of the cytokines on cell shape or morphology showed no differences from vehicle control treated cells (data not shown).
Fig. 1.
A. HCLE cells express the receptors for the inflammatory mediators used in this study, indicating their ability to be responsive to the treatments. RT-PCR performed on RNA from untreated control cultures demonstrates the presence of message for the receptors for IL-6, -8, -17, TNF-α, and IFN-γ. The predicted base pair size for each of the receptors is indicated at the bottom of the figure. B. Representative immunoblots used to assay effects of cytokines on MUC1 and MUC16 cellular protein and ectodomain release. These blots demonstrate effect of 24 hour exposure to IL-17, IFN-γ, or IL-17 plus IFN-γ. GAPDH is the loading control for cellular protein quantitation.
3.1 Effects of inflammatory mediators on MUC1 expression, cell protein content, and ectodomain release
Representative data of effects of a cytokine (IFN-γ) on MUC1 expression as measured by qRT-PCR are shown in Table 1. Representative immunoblots showing the effect of cytokines (IL-17, IFN-γ, and IFN-γ plus IL-17) on MUC1 cellular protein amount and ectodomain release are shown in Fig. 1B.
Table 1. Ct Values from a Representative qRT-PCR Assay for Cytokine Effect on MUC1 and MUC 16 Expression.
| I Hour | MUC1 | MUC16 | GAPDH |
|---|---|---|---|
| IFN-γ 1 | 33.21 | 29.47 | 23.50 |
| IFN-γ 2 | 31.92 | 30.25 | 22.65 |
| IFN-γ 3 | 33.34 | 29.91 | 24.34 |
| VC 1 | 32.18 | 28.62 | 22.55 |
| VC 2 | 29.62 | 26.43 | 20.00 |
| VC 3 | 29.32 | 25.97 | 20.63 |
| 6 Hours | MUC1 | MUC16 | GAPDH |
|---|---|---|---|
| IFN-γ 1 | 31.54 | 29.67 | 26.28 |
| IFN-γ 2 | 30.94 | 28.84 | 25.84 |
| IFN-γ 3 | 31.86 | 29.16 | 25.55 |
| VC 1 | 31.61 | 28.60 | 23.92 |
| VC 2 | 32.30 | 29.18 | 24.43 |
| VC 3 | 32.47 | 28.95 | 24.49 |
| 24 Hours | MUC1 | MUC16 | GAPDH |
|---|---|---|---|
| IFN-γ 1 | 26.38 | 26.12 | 21.97 |
| IFN-γ 2 | 27.35 | 26.42 | 21.93 |
| IFN-γ 3 | 26.20 | 25.61 | 21.42 |
| VC 1 | 28.79 | 27.88 | 22.84 |
| VC 2 | 31.03 | 27.66 | 22.12 |
| VC 3 | 34.12 | 30.49 | 26.49 |
| 48 Hours | MUC1 | MUC16 | GAPDH |
|---|---|---|---|
| IFN-γ 1 | 28.60 | 27.00 | 25.83 |
| IFN-γ 2 | 26.41 | 25.11 | 23.62 |
| IFN-γ 3 | 37.22 | 33.33 | 34.09 |
| VC 1 | 31.29 | 26.97 | 24.10 |
| VC 2 | 31.86 | 27.88 | 24.53 |
| VC 3 | 33.38 | 29.92 | 25.36 |
IFN-γ: Interferon-γ Treatment
VC: Vehicle Control
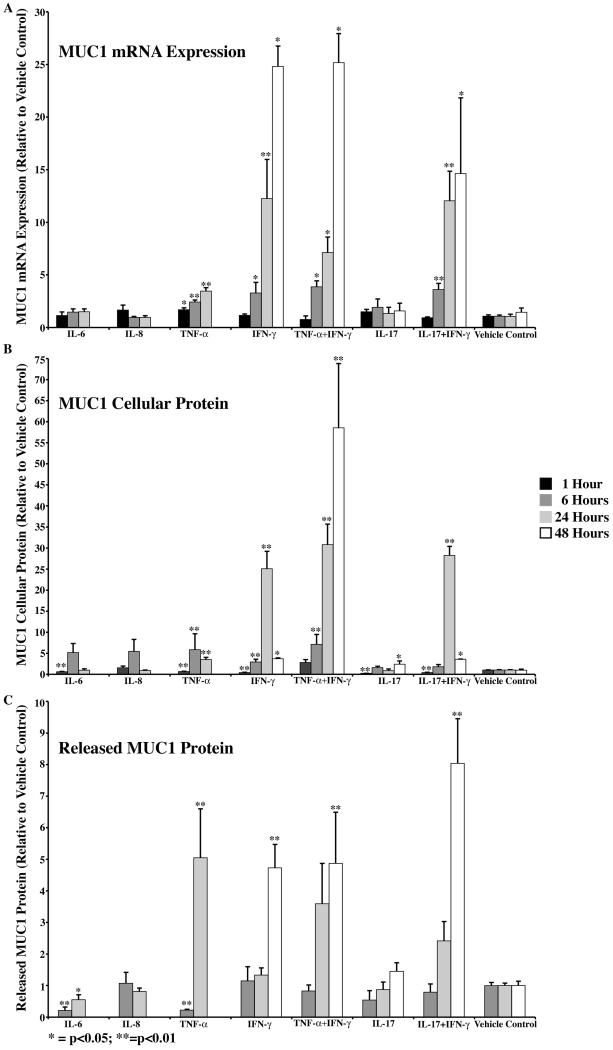
Three of the five inflammatory mediators tested, IL-6, TNF-α, and IFN-γ had significant effects on MUC1 expression, cellular protein amount and/or ectodomain release (Table 2, Fig. 2). IL-8 and IL-17 had essentially no significant effects on the mucin, except that, at 1 hour and 48 hours, IL-17 showed decreased or increased cellular MUC1, respectively (Fig. 2). IL-6 downregulated MUC1 cellular protein at 1 hour, followed by a decrease in MUC1 ectodomain release at 6 and 24 hours of culture with the cytokine (Fig. 2). TNF-α and IFN-γ had similar effects on the mucin; both significantly upregulated MUC1 expression with a concomitant increase in both cellular MUC1 and ectodomain release. The only differences between the effects of the two cytokines were temporal, in that upregulation of expression of MUC1 was earlier at the 1-hour time point with TNF-α, and the increased ectodomain release induced by IFN-γ occurred later, at 48 hours of culture. In both instances, the cytokines induced an increase in MUC1 mRNA over time, such that by 24 hours there was a 4- and 12-fold increase in message by TNF-α and IFN-γ, respectively, with a continued increase (25 fold) in mRNA after 48-hour treatment with IFN-γ. As a result of the mRNA increase, protein biosynthesis significantly increased by 5 fold after 6 hours with TNF-α and 25 fold after 24 hours with IFN-γ. The effect on biosynthesis of MUC1 by TNF-α peaked at 6 hours and was followed by a 5-fold increase in ectodomain release at 24 hours. By comparison, the effect of IFN-γ on the amount of MUC1 protein in cells peaked at 24 hours, and was 25-fold over control. Ectodomain release as a response to increased amount of MUC1 cell protein, was delayed by comparison to TNF-α, and reached 5 fold over vehicle control by 48 hours.
Table 2.
Significant differences in MUC1 mRNA expression, cellular and released protein induced by cytokines relative to vehicle control
| Inflammatory | Mediator | 1 Hour | 6Hours | 24 Hours | |
|---|---|---|---|---|---|
| IL-6 | Expression | † | |||
|
|
|||||
| Cellular |
|
||||
|
|
|||||
| Released | 0 |
|
 *
*
|
||
|
|
|||||
| IL-8 | Expression | ||||
|
|
|||||
| Cellular | |||||
|
|
|||||
| Released | 0 | ||||
|
|
|||||
| TNF-α | Expression |
 *
*
|
|
|
|
|
|
|||||
| Cellular |
|
|
|
||
|
| |||||
| Released | 0 |
|
|
48 Hours | |
|
| |||||
| IFN-γ | Expression |
 *
*
|
|
 *
*
|
|
|
| |||||
| Cellular |
|
|
|
 *
*
|
|
|
| |||||
| Released | 0 |
|
|||
|
| |||||
| TNF + IFN-γ |
Expression |
 *
*
|
 *
*
|
 *
*
|
|
|
| |||||
| Cellular |
|
|
|
||
|
| |||||
| Released | 0 |
|
|||
|
| |||||
| IL-17 | Expression | ||||
|
| |||||
| Cellular |
|
 *
*
|
|||
|
| |||||
| Released | 0 | ||||
|
| |||||
| IL-17 + IFN-γ |
Expression |
|
|
 *
*
|
|
|
| |||||
| Cellular |
|
|
 *
*
|
||
|
| |||||
| Released | 0 |
|
|||
 = Significant increase
= Significant increase
 = Significant decrease; p<0.01
= Significant decrease; p<0.01
p<0.05
0 = None detected in treated or controls
Blank entries indicate data not significantly different than vehicle control.
Fig. 2.
MUC1 expression, cellular protein amount and/or ectodomain release was affected by four of the five inflammatory mediators tested. TNF-α and IFN-γ significantly increased expression and cellular protein by 6 and 24 hours, respectively, followed by ectodomain release at 24 (TNF-α) or 48 hours (IFN-γ). IL-6 decreased cellular MUC1 amount at 1 hour, followed by a decrease in ectodomain release at 6 and 24 hours. IL-17 also decreased the cellular amount of MUC1 at 1 hour, but by 48 hours, there was a significant increase. No significant effects were seen after treatment with IL-8. Combined treatment with TNF-α and IFN-γ or with IL-17 and IFN-γ had no effect over treatment with the cytokines alone.
Since both TNF-α and IFN-γ had strong effects on MUC1 expression and ectodomain release, we cultured HCLE cells in the presence of both cytokines to determine if their effects were additive. The data indicate that effects of combined treatment essentially follow that of IFN-γ alone and were not additive (Fig. 2, Table 2).
IL-17 and IFN-γ have been demonstrated to act synergistically to induce intestinal inflammation (Kullberg, M.C. et al., 2006), thus, we cultured HCLE cells with both IL-17 and IFN-γ. There was no significant difference between treating with IFN-γ alone or in combination with IL-17 (Fig. 2).
3.2 Effects of the inflammatory mediators on MUC16 expression, cell protein content and ectodomain release
Representative data of effects of a cytokine (IFN-γ) on MUC16 expression as measured by qRT-PCR are shown in Table 1. An example of an immunoblot used to measure effects of cytokines (IL-17, IFN-γ, and IFN-γ plus IL-17) on MUC16 cell protein and ectodomain release is shown in Fig. 1B.
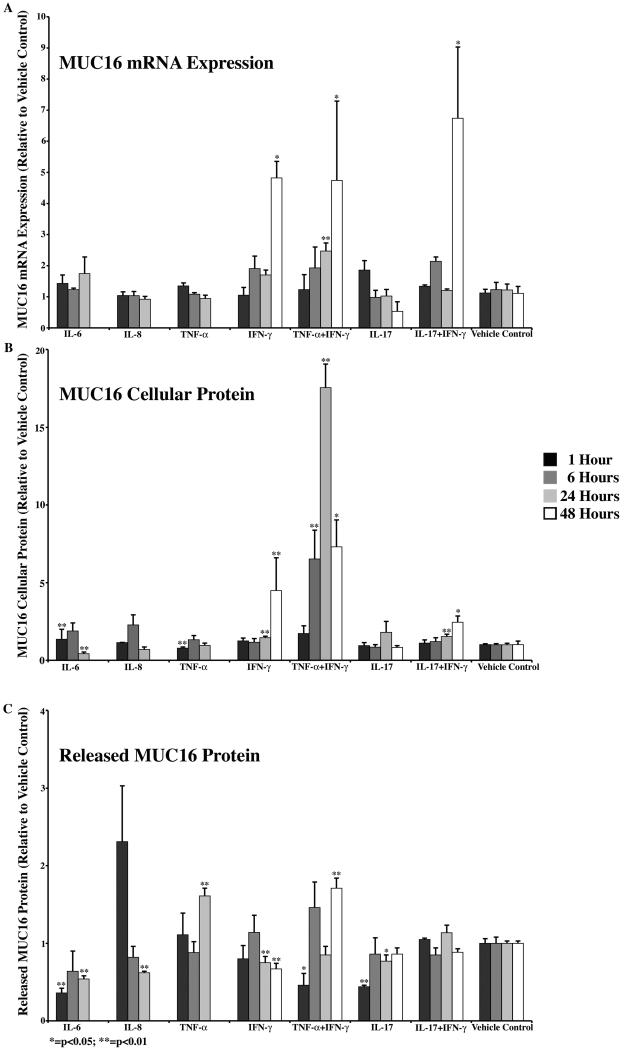
Four of the five inflammatory mediators had effects on MUC16 cellular protein or ectodomain release without significant changes in expression (Table 3, Fig. 3). IL-6 induced an initial increase in MUC16 cellular protein at 1 hour, followed by a significant decrease by 24 hours. In addition, release of the ectodomain of the mucin was decreased at both 1 and 24 hours. IL-8 had only one observed effect on MUC16, that of significantly decreasing its ectodomain release at the 24-hour time point. TNF-α induced a decrease in MUC16 cellular protein at 1 hour and an increase in ectodomain release by 24 hours. IL-17 did not significantly affect cell protein content, but ectodomain release was significantly downregulated at 1 hour and 24 hours. By comparison, IFN-γ or combinations of the cytokine with either TNF-α or IL-17 induced MUC16 expression at 24 and/or 48 hours. IFN-γ alone induced a significant increase in cell protein by 24 hours, with significant downregulation of ectodomain release at 24 and 48 hours. As with MUC1, treatment of HCLE cells with IFN-γ in combination with TNF-α or IL-17 produced no significant effects on MUC16 parameters over treatment with individual cytokines alone (p<0.01) (Fig. 3).
Table 3.
Significant differences in MUC16 mRNA expression, cellular and released protein induced by cytokines relative to vehicle control
| Inflammatory | Mediator | 1 Hour | 6Hours | 24 Hours | |
|---|---|---|---|---|---|
| IL-6 | Expression | † | |||
|
|
|||||
| Cellular |
|
|
|||
|
|
|||||
| Released |
|
|
|||
|
|
|||||
| IL-8 | Expression | ||||
|
|
|||||
| Cellular | |||||
|
|
|||||
| Released |
|
||||
|
|
|||||
| TNF-α | Expression | ||||
|
|
|||||
| Cellular |
|
||||
|
| |||||
| Released |
|
48 Hours | |||
|
| |||||
| IFN-γ | Expression |
 *
*
|
|||
|
| |||||
| Cellular |
|
|
|||
|
| |||||
| Released |
|
|
|||
|
| |||||
| TNF + IFN-γ |
Expression |
|
 *
*
|
||
|
| |||||
| Cellular |
|
|
 *
*
|
||
|
| |||||
| Released |
 *
*
|
|
|||
|
| |||||
| IL-17 | Expression | ||||
|
| |||||
| Cellular | |||||
|
| |||||
| Released |
|
 *
*
|
|||
|
| |||||
| IL-17 + IFN-γ |
Expression |
 *
*
|
|||
|
| |||||
| Cellular |
|
 *
*
|
|||
|
| |||||
| Released | |||||
 = Significant increase
= Significant increase
 = Significant decrease; p<0.01
= Significant decrease; p<0.01
p<0.05
Blank entries indicate data was not significantly different from vehicle control.
Fig. 3.
MUC16 cellular protein amount and/or ectodomain release was significantly affected by all five of the inflammatory mediators tested. There was a decrease in ectodomain release after 1 hour of treatment with IL-17. IL-6 increased cellular protein and decreased ectodomain release at 1 hour and decreased both at 24 hours. IL-8 decreased ectodomain release at 24 hours. TNF-α decreased cellular protein at 1 hour and increased ectodomain release at 24 hours. IFN-γ induced the most significant effects, increasing cellular protein at 24 and 48 hours, decreasing ectodomain release at 24 and 48 hours without changes in expression level at 24 hours. By 48 hours, expression levels were significantly upregulated. Combined treatment with TNF-α and IFN-γ or IL-17 and IFN-γ had no effect over treatment with the cytokines alone.
4.0 Discussion
Data from this in vitro study suggest that inflammation with its accompanied release of inflammatory cytokines can influence membrane mucin expression and ectodomain release at the ocular surface. Human corneal epithelial cells, since they express the appropriate cytokine receptors, can respond to IL-6, IL-8, IL-17, TNF-α and IFN-γ. The data indicate that the cytokines regulate MUC1 and MUC16 in a mucin and cytokine-specific pattern.
Several patterns of regulation of the mucins emerge from the data (Tables 2 and 3). The first pattern is one in which expression, protein biosynthesis, as well as ectodomain release are all upregulated in a sequential way, indicating that the cytokine affects the mucins’ production and subsequent turnover at the transcription level. This pattern was most evident with MUC1, and was induced by two cytokines, TNF-α and IFN-γ. The effect on MUC1 mRNA and protein appeared to be more dramatic with IFN-γ since increases in message and protein were 14 and 25 fold, respectively (Fig. 2). Perhaps the transcriptional upregulation of MUC1 is a compensatory response on the part of the epithelium in response to inflammation, since the mucin is now known to be a multifunctional molecule with signaling capabilities (Hollingsworth, M.A. and Swanson, B.J., 2004), It may have unique functions at the ocular surface—sensing and signaling—in this case, in response to inflammation. Regulation of all three parameters, expression, protein biosynthesis, and ectodomain release, was also observed with MUC16, but not in as sequential a pattern, since it did not appear until 48 hours in culture. IFN-γ alone or in combination with TNF-α increased expression at 48 and 24 hours, respectively, and this correlated with increased cellular protein and changes in ectodomain release.
The second pattern of effect of cytokines on the mucins was one in which the amount of mucin in cells and the amount of ectodomain released occurred without changes in mucin mRNA expression. This pattern may reflect independent regulation of activity of ectodomain release, leading to a concomitant change in amount of total cellular protein. Although the pattern was seen for both mucins, it was predominant for MUC16. The data demonstrated downregulation of ectodomain release, less than 1 fold of vehicle control by IL-6 for both MUC1 and MUC16, and of MUC16 by IL-8, IL-17, and IFN-γ. On the other hand, TNF-α increased MUC16 ectodomain release by almost 2 fold. An increase in ectodomain release of MUC16 of this magnitude may result in sufficient loss of surface mucin to allow penetrance of rose bengal dye as seen in dry eye patients. Recent work demonstrated increased penetrance of the dye into HCLE cells in which MUC16 expression has been abrogated by siRNA methods, as well as after release of MUC16 from the cell surface in response to neutrophil elastase (Blalock, T. et al., 2007; Blalock, T.D. et al., 2008). Loss of MUC16 from the corneal surface could potentially cause loss of tear film stability and rapid tear breakup.
Regulation of ectodomain release of MUC1 and −16 by cytokines, particularly by TNF-α and IFN-γ may occur through their regulation of ectodomain sheddases such as metalloproteinases. It has recently been shown that MMP7 and −9 induce MUC16 ectodomain release in HCLE cells (Blalock, T.D. et al., 2008). TNF-α has also been shown to induce MUC1 ectodomain release in uterine epithelia through the TNF-α converting enzyme (TACE) induction of ADAM17, a membrane metalloproteinase (Thathiah, A. et al., 2003). MMP9 has been reported to be present in higher amounts in tears of dry eye patients (Afonso, A.A. et al., 1999; Solomon, A. et al., 2001). IL-17 and IFN-γ are known to induce expression of MMP-9 in mouse airways and tumor tissue, respectively (Prause, O. et al., 2004; Xiong, T. et al., 2008). Perhaps metalloproteinases in tears are acting as local sheddases, inducing regional areas of dye penetrance, leading to tear film instability. In a human uterine epithelial cell line, MUC1 release was induced by MT1-MMP in a TACE/ADAM17 independent way (Thathiah, A. and Carson, D.D., 2004). As was recently published, IFN-γ stimulates MT1-MMP expression and protein synthesis in human monocytic cells (Galboiz, Y. et al., 2002). That could explain the dramatic release of MUC1 by IFN-γ.
Data obtained in this study confirm and extend several studies demonstrating regulation of MUC1 by TNF-α and IFN-γ. Carson et al. demonstrated that TNF-α induced an increase in MUC1 gene expression and ectodomain release in a human uterine epithelial cell line after 24-hour treatment (Thathiah, A. et al., 2004), and Li et al. demonstrated upregulation of MUC1 mRNA with a concomitant increase in MUC1 protein in human oral epidermoid carcinoma cells by TNF-α and IFN-γ (Li, X. et al., 2003).Our data extend their findings to corneal epithelium and suggest that these cytokines have similar effects on MUC1 across epithelial types.
The data on regulation of MUC16 by TNF-α and IFN-γ also confirm the work of Paulsen in that both studies show no significant effect on MUC16 expression but significant effects on the mucins’ ectodomain release in human corneal epithelial cell lines. Our data show, however, opposite effects of IFN-γ on MUC16 ectodomain release (Paulsen, F. et al., 2008). Data reported here extend the work of Paulsen in that we used epithelial cultures, which have stratified and differentiated to express mucins apically rather than cells at confluence, which are undifferentiated. Also, this study assessed effects of cytokines in a temporal manner, including assay of total cellular mucin protein in addition to mRNA levels and ectodomain release.
The data obtained from the in vitro assays reported herein are indicative of potential regulation of membrane mucins on the ocular surface. Taken together, these data strongly suggest that IFN-γ and TNF-α have effects on both MUC1 and MUC16 at the transcriptional level as well as at the level of ectodomain release. Since cytokines may be present in combinations on an inflamed ocular surface, net effects of these combinations could, depending on ratios, either increase translation and ectodomain release or prevent ectodomain release. Measurement of mucin ectodomain levels, along with cytokine levels, in tears of patients with inflamed ocular surface will provide answers to overall questions regarding effect of inflammation on glycocalyx integrity.
Obviously, there are concerns that the concentrations of mediator used in this study may not be physiologic, although amounts of inflammatory mediators that are locally released by immune cells cannot be quantitated. Another concern is that epithelia grown in isolation from stromal interactions may respond differently. Nevertheless, the data do open potential mechanisms for ocular surface damage as seen in drying and cicatrizing eye disease.
In summary, data reported here indicate that TNF-α and IFN-γ have dramatic effects on both MUC1 and MUC16, the two major mucins found on the corneal surface. Regulation of MUC1 by the cytokines appears to be at the transcriptional level early after exposure, whereas regulation of MUC16 may be affecting the mechanisms of ectodomain release at later time points.
Acknowledgements
This work was supported by a sponsored research agreement from Alcon, Inc., Fort Worth, TX, and by NEI Grant RO1-EY03306 to Ilene K. Gipson
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afonso AA, Monroy D, Stern ME, Feuer WJ, Tseng SC, Pflugfelder SC. Correlation of tear fluorescein clearance and Schirmer test scores with ocular irritation symptoms. Ophthalmology. 1999;106:803–810. doi: 10.1016/S0161-6420(99)90170-7. [DOI] [PubMed] [Google Scholar]
- Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest. Ophthalmol. Vis. Sci. 2003;44:2487–2495. doi: 10.1167/iovs.02-0862. [DOI] [PubMed] [Google Scholar]
- Blalock T, Spurr-Michaud S, Tisdale A, Heimer S, Gilmore M, Ramesh V, Gipson I. Functions of MUC16 in corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 2007;48:4509–4518. doi: 10.1167/iovs.07-0430. [DOI] [PubMed] [Google Scholar]
- Blalock TD, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Release of membrane-associated mucins from ocular surface epithelia. Invest. Ophthalmol. Vis. Sci. 2008;49:1864–1871. doi: 10.1167/iovs.07-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabaud M, Lubberts E, Joosten L, van Den Berg W, Miossec P. IL-17 derived from juxta-articular bone and synovium contributes to joint degradation in rheumatoid arthritis. Arthritis research. 2001;3:168–177. doi: 10.1186/ar294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danjo Y, Watanabe H, Tisdale AS, George M, Tsumura T, Abelson MB, Gipson IK. Alteration of mucin in human conjunctival epithelia in dry eye. Invest. Ophthalmol. Vis. Sci. 1998;39:2602–2609. [PubMed] [Google Scholar]
- De Paiva CS, Villarreal AL, Corrales RM, Rahman HT, Chang VY, Farley WJ, Stern ME, Niederkorn JY, Li DQ, Pflugfelder SC. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest. Ophthalmol. Vis. Sci. 2007;48:2553–2560. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- De Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JD, III, Fang B, Zheng X, Ma P, Farley WJ, Siemasko KF, Niederkorn JY, Stern ME, Li D-Q, Pflugfelder SC. IL-17 Disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2:243–253. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEWS Report of the International Dry Eye WorkShop (DEWS) The ocular surface. 2007;5:67–202. doi: 10.1016/s1542-0124(12)70086-1. [DOI] [PubMed] [Google Scholar]
- Eckert J, Niemann H. mRNA expression of leukaemia inhibitory factor (LIF) and its receptor subunits glycoprotein 130 and LIF-receptor-beta in bovine embryos derived in vitro or in vivo. Mol. Hum. Reprod. 1998;4:957–965. doi: 10.1093/molehr/4.10.957. [DOI] [PubMed] [Google Scholar]
- Galboiz Y, Shapiro S, Lahat N, Miller A. Modulation of monocytes matrix metalloproteinase-2, MT1-MMP and TIMP-2 by interferon-gamma and -beta: implications to multiple sclerosis. J. Neuroimmunol. 2002;131:191–200. doi: 10.1016/s0165-5728(02)00266-7. [DOI] [PubMed] [Google Scholar]
- Gerritsma JS, Gerritsen AF, De Ley M, van Es LA, Daha MR. Interferon-gamma induces biosynthesis of complement components C2, C4 and factor H by human proximal tubular epithelial cells. Cytokine. 1997;9:276–283. doi: 10.1006/cyto.1996.0164. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Hori Y, Argueso P. Character of ocular surface mucins and their alteration in dry eye disease. The ocular surface. 2004;2:131–148. doi: 10.1016/s1542-0124(12)70149-0. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Argueso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest. Ophthalmol. Vis. Sci. 2003;44:2496–2506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr. Opin. Immunol. 2006;18:349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Hoebler C, Gaudier E, De Coppet P, Rival M, Cherbut C. MUC genes are differently expressed during onset and maintenance of inflammation in dextran sodium sulfate-treated mice. Dig. Dis. Sci. 2006;51:381–389. doi: 10.1007/s10620-006-3142-y. [DOI] [PubMed] [Google Scholar]
- Hollingsworth MA, Swanson BJ. Mucins in cancer: Protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Joh T, Tanida S, Sasaki M, Kataoka H, Itoh K, Oshima T, Ogasawara N, Togawa S, Wada T, Kubota H, Mori Y, Ohara H, Nomura T, Higashiyama S, Itoh M. IL-8 promotes cell proliferation and migration through metalloproteinase-cleavage proHB-EGF in human colon carcinoma cells. Cytokine. 2005;29:275–282. doi: 10.1016/j.cyto.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr. Opin. Immunol. 2006;18:670–675. doi: 10.1016/j.coi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Knofler M, Mosl B, Bauer S, Griesinger G, Husslein P. TNF-alpha/TNFRI in primary and immortalized first trimester cytotrophoblasts. Placenta. 2000;21:525–535. doi: 10.1053/plac.1999.0501. [DOI] [PubMed] [Google Scholar]
- Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, Cua DJ, Powrie F, Cheever AW, Maloy KJ, Sher A. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J. Exp. Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. Am. J. Ophthalmol. 2009;147:198–205 e191. doi: 10.1016/j.ajo.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambiase A, Micera A, Mantelli F, Moretti C, Di Zazzo A, Perrella E, Bonini S, Bonini S. T-helper 17 lymphocytes in ocular cicatricial pemphigoid. Mol. Vis. 2009;15:1449–1455. [PMC free article] [PubMed] [Google Scholar]
- Lemp MA. Report of the National Eye Institute/Industry Workshop on clinical trials in dry eyes. CLAO J. 1995;21:221–232. [PubMed] [Google Scholar]
- Li X, Wang L, Nunes DP, Troxler RF, Offner GD. Pro-inflammatory cytokines up-regulate MUC1 gene expression in oral epithelial cells. J. Dent. Res. 2003;82:883–887. doi: 10.1177/154405910308201107. [DOI] [PubMed] [Google Scholar]
- Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J. Exp. Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertzdorf J, Osterhaus AD, Verjans GM. IL-17 expression in human herpetic stromal keratitis: modulatory effects on chemokine production by corneal fibroblasts. J. Immunol. 2002;169:5897–5903. doi: 10.4049/jimmunol.169.10.5897. [DOI] [PubMed] [Google Scholar]
- Nishida T, Nakamura M, Mishima H, Otori T. Interleukin 6 promotes epithelial migration by a fibronectin-dependent mechanism. J. Cell. Physiol. 1992;153:1–5. doi: 10.1002/jcp.1041530102. [DOI] [PubMed] [Google Scholar]
- Path G, Bornstein SR, Ehrhart-Bornstein M, Scherbaum WA. Interleukin-6 and the interleukin-6 receptor in the human adrenal gland: expression and effects on steroidogenesis. J. Clin. Endocrinol. Metab. 1997;82:2343–2349. doi: 10.1210/jcem.82.7.4072. [DOI] [PubMed] [Google Scholar]
- Paulsen F, Jager K, Worlitzsch D, Brauer L, Schulze U, Schafer G, Sel S. Regulation of MUC16 by inflammatory mediators in ocular surface epithelial cell lines. Ann Anat. 2008;190:59–70. doi: 10.1016/j.aanat.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren’s syndrome keratoconjunctivitis sicca. Curr. Eye Res. 1999;19:201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- Prause O, Bozinovski S, Anderson GP, Linden A. Increased matrix metalloproteinase-9 concentration and activity after stimulation with interleukin-17 in mouse airways. Thorax. 2004;59:313–317. doi: 10.1136/thx.2003.008854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MD, Zamora DO, Pan Y, Texeira PV, Baek SH, Planck SR, Rosenbaum JT. Constitutive and inflammatory mediator-regulated fractalkine expression in human ocular tissues and cultured cells. Invest. Ophthalmol. Vis. Sci. 2003;44:1608–1615. doi: 10.1167/iovs.02-0233. [DOI] [PubMed] [Google Scholar]
- Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest. Ophthalmol. Vis. Sci. 2001;42:2283–2292. [PubMed] [Google Scholar]
- Song XJ, Li DQ, Farley W, Luo LH, Heuckeroth RO, Milbrandt J, Pflugfelder SC. Neurturin-deficient mice develop dry eye and keratoconjunctivitis sicca. Invest. Ophthalmol. Vis. Sci. 2003;44:4223–4229. doi: 10.1167/iovs.02-1319. [DOI] [PubMed] [Google Scholar]
- Spurr-Michaud S, Argüeso P, Gipson I. Assay of mucins in human tear fluid. Exp. Eye Res. 2007;84:939–950. doi: 10.1016/j.exer.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thathiah A, Blobel CP, Carson DD. Tumor necrosis factor-alpha converting enzyme/ADAM 17 mediates MUC1 shedding. J. Biol. Chem. 2003;278:3386–3394. doi: 10.1074/jbc.M208326200. [DOI] [PubMed] [Google Scholar]
- Thathiah A, Brayman M, Dharmaraj N, Julian JJ, Lagow EL, Carson DD. Tumor necrosis factor alpha stimulates MUC1 synthesis and ectodomain release in a human uterine epithelial cell line. Endocrinology. 2004;145:4192–4203. doi: 10.1210/en.2004-0399. [DOI] [PubMed] [Google Scholar]
- Thathiah A, Carson DD. MT1-MMP mediates MUC1 shedding independent of TACE/ADAM17. Biochem. J. 2004;382:363–373. doi: 10.1042/BJ20040513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton D, Khan N, Sheehan D. Separation and identification of mucins and their glycoforms. In: Corfield AP, editor. Glycoprotein Methods and Protocols: The Mucins. Humana Press; Totowa: 2000. pp. 77–85. [DOI] [PubMed] [Google Scholar]
- Xiong T, Peng H, Chen G, Yuan Y. The expression and activity of MMPs are increased in residual tumor tissues after the termination of immunotherapy. Journal of Huazhong University of Science and Technology. 2008;28:375–378. doi: 10.1007/s11596-008-0401-5. Medical sciences = Hua zhong ke ji da xue xue bao. Yi xue Ying De wen ban = Huazhong keji daxue xuebao. [DOI] [PubMed] [Google Scholar]