Abstract
The normal microflora of the skin includes staphylococcal species that will induce inflammation when present below the dermis but are tolerated on the epidermal surface without initiating inflammation. Here we reveal a previously unknown mechanism by which a product of staphylococci inhibits skin inflammation. This inhibition is mediated by staphylococcal lipoteichoic acid (LTA), and acts selectively on keratinocytes triggered through Toll-like receptor (TLR) 3. The significance of this is seen by observations that TLR3 activation is required for normal inflammation after injury, and that keratinocytes require TLR3 to respond to RNA from damaged cells with the release of inflammatory cytokines. Staphylococcal LTA inhibits both inflammatory cytokine release from keratinocytes and inflammation triggered by injury through a TLR2-dependent mechanism. These findings show for the first time that the skin epithelium requires TLR3 for normal inflammation after wounding and that the microflora can modulate specific cutaneous inflammatory responses.
INTRODUCTION
All complex metazoans are colonized with a myriad of microbial organisms, a group that has been referred to as the “microbiome”. On the skin, bacterial colonization is abundant, diverse and constant, but inflammation is undesirable and an indication of disease. The two principal normal stimuli of inflammation are injury and infection. In infections, the detection of microbes is accomplished in part by Toll-like receptors (TLRs) that are best known as stimuli of inflammation. Although the role of TLRs in response to infection is well defined, the mechanisms involving TLRs that regulate inflammation in the skin injury are poorly understood.
Epithelial surfaces in the gut regulate the magnitude and duration of TLR signaling, thus shaping and maintaining normal mucosal immunity1-3 via the induction of proinflammatory and anti-inflammatory cytokine synthesis 4,5. Moreover, commensal bacteria Lactococcus lactis and Bacteroides vulgatus can prime the host to produce IL-10, trefoil factors and TGF-β suggested to modulate inflammation in the intestine 6-8. However, TLRs that have been generally thought to act as proinflammatory signals in response to microbial products also may recognize self-epitopes that are released from damaged cells or are present at the surface of apoptotic cells in autoimmune diseases9,10. These observations have raised the possibility that TLRs may play a role in initiating inflammation in response to injury11,12.
In skin injury, tissue damage results in necrosis and apoptosis, but it is not known if this event is involved in the initiation of the inflammatory response that is critical to normal wound repair. In the absence of infection, uncontrolled inflammation during wound repair is undesirable and may cause dysfunction during healing. Uncontrolled inflammation after minor trauma is also well known to exacerbate several human skin diseases such as psoriasis. Therefore, normal immune defense requires that a balance is maintained to minimize unnecessary inflammation yet rapidly respond to infection and injury. This balance is particularly difficult to maintain at epithelial surfaces that are in contact with the external environment and has frequent trauma and exposure to the products of the microbiome. The innate systems that enable the control of this inflammatory response are generally unknown.
Given the hypothesis that epithelial flora may serve to protect the host from unintended inflammatory diseases, and the importance of TLRs in the recognition of microbial products and potential role after tissue damage, we set out to study how these systems may be involved in homeostatic control of skin inflammation. Staphylococcal species are the most frequently cultured normal inhabitant of the healthy human skin 13 and have been hypothesized to serve a role in human health14. It has been entirely unknown how these bacteria could affect cutaneous homeostasis. In this study we describe a mechanism by which a product of staphylococci, LTA, suppresses skin inflammation during wound repair.
RESULTS
Staphylococcal products suppress inflammation
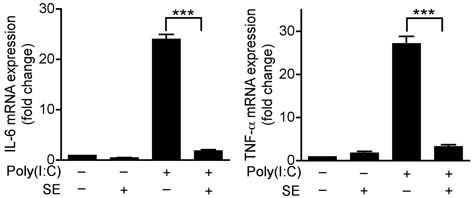
Recent observations in several systems have suggested that activation of TLRs on keratinocytes will trigger inappropriate production of proinflammatory cytokines in the epidermis as an element in the pathogenesis of several skin diseases 15-17. However, despite production of TLR ligands, several commensal bacterial species including Staphylococcus epidermidis (S. epidermidis) normally reside in contact with keratinocytes in the epidermis and do not initiate inflammation. We therefore hypothesized that S. epidermidis could influence the inflammatory response of keratinocytes through negative regulation of TLR signaling. Primary human keratinocytes treated with a panel of TLR ligands showed that polyriboinosinic polyribocytidylic acid [poly(I:C)], a stimulus for TLR318,19, was the most potent stimulus for expression of TNF-α by keratinocytes and that prior exposure to a <10 kDa product of S. epidermidis (SE) suppressed poly(I:C)-induced IL-6 and TNF-α expression (Fig. 1a and Supplementary Fig. 1a). This suppression persisted over time and affected both TNF-α mRNA and protein (Supplementary Fig. 1b).
Figure 1. S. epidermidis inhibits poly(I:C)-induced inflammatory cytokines produced by keratinocytes, and inflammation in mouse skin.
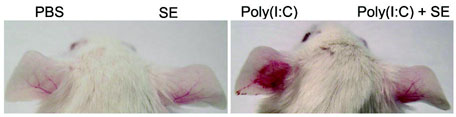
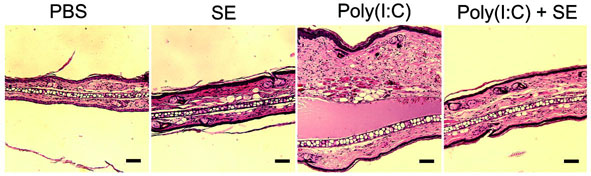
(a) Quantification of IL-6 and TNF-α mRNA expression of cultured normal human keratinocytes treated with 36 μg ml−1 of a sterile <10 kDa product of S. epidermidis conditioned culture media (SE) and 10 μg ml−1 of the TLR3 ligand [poly(I:C)] for 24 h. (b) Photographs of the ears of BALB/c mice 24 h after subcutaneous injection with PBS alone, SE alone, poly(I:C) alone or SE and poly(I:C). (c) H&E staining of ears treated as in (b), scale bars represent 100 μm. (d) Quantification of IL-6 and TNF-α expression in tissue from mouse ears treated as in (b). **P <0.01 and *** P <0.001. P-values were determined by Two-tailed t tests. All data are representative of three independent experiments with n = 3–6 per group, and are mean ± SEM.
Cutaneous inflammation induced by poly(I:C) in mice were next examined to determine the in vivo relevance of the response observed to SE. Ears pretreated with SE prior to poly(I:C) showed less inflammation compared to those treated only with poly(I:C) (Fig. 1b,c). The decrease in inflammation corresponded with a decrease in the expression of IL-6 and TNF-α mRNA ( Fig. 1d). However, the inhibitory effects of SE in whole skin were not observed when inflammation was induced by lipopolysaccharide (LPS) (Supplementary Fig. 1c), or phorbol 12-myristate 13-acetate (PMA) (Supplementary Fig. 1d). Thus, these data show SE functions as a selective suppressor of poly(I:C)-mediated inflammation in the skin.
Analysis of similar soluble <10 kDa products of other staphylococcal strains demonstrated that the capacity to inhibit poly(I:C)-induced TNF-α expression was present in multiple staphylococcal strains, except one hospital-isolated strain of Staphylococcus aureus (S. aureus) Rosenbach (Supplementary Fig. 2). SE also suppressed the expression of IL-8, but was not able to suppress IFN-β or IL-1β (data not shown). Moreover, SE inhibited the capacity of mouse epidermal sheets to express TNF-α and IL-6 in response to poly(I:C) (Supplementary Fig. 3a). However, in contrast to keratinocytes and epidermal sheets, other cell types representative of those present in skin but below the epidermis were not inhibited by SE and had increased expression of IL-6 and TNF-α in response to poly(I:C) and SE (Supplementary Fig. 3 b–d). Taken together, these results show poly(I:C) is a potent stimulus to release proinflammatory cytokines in the skin, but a soluble low molecular weight product from some bacteria will inhibit this response selectively in keratinocytes.
TLR3 mediates a response to injury
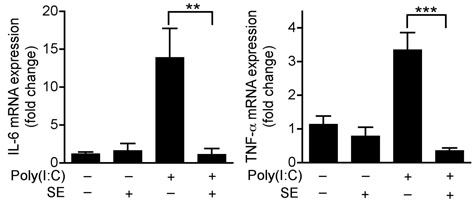
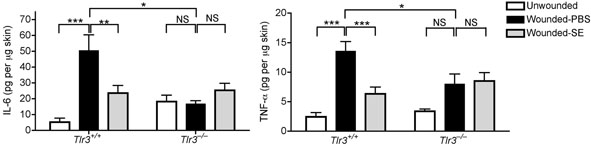
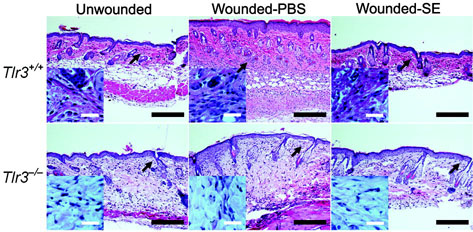
To understand the immunological relevance of suppression of TLR3-induced cytokine release by keratinocytes we next sought to investigate conditions in which TLR3 might be activated in skin. In gastrointestinal epithelium TLR3 has been implicated as a mechanism for detection of cell death 20. Skin injury results in the generation of necrotic and apoptotic cells 21,22. Therefore, we examined if TLR3 might be a mechanism for detection of skin injury and initiation of inflammation. To test this hypothesis, aseptic, full-thickness incisions were performed on the back of Tlr3-deficent mice and matched wild-type controls. Tlr3-deficent mice showed significantly less production of IL-6 and TNF-α at the wound edge compared to wild-type controls (Fig. 2a). This correlated with a decrease in inflammation and leukocyte recruitment (Fig. 2b and Supplementary Fig.4). Furthermore, consistent with the inhibitory effects of SE on poly(I:C)-induced skin inflammation, application of SE also inhibited wound-induced IL-6, TNF-α, and inflammation in wild-type mice, but had no significant effect on the injury response in Tlr3−/− mice (Fig. 2a,b and Supplementary Fig.4). Thus, these findings establish that Tlr3 is required for part of the normal inflammatory response following injury, and that SE acts on this pathway to decrease the magnitude of inflammation.
Figure 2. Inflammation in wounds is dependent on TLR3 and inhibited by S. epidermidis.
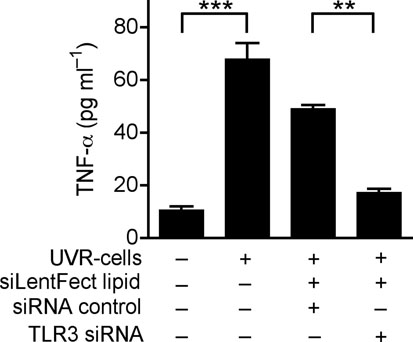
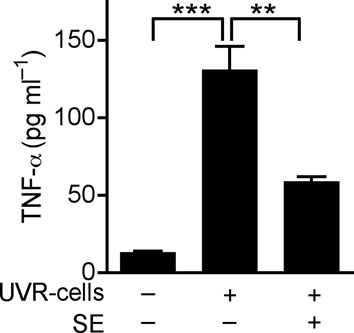
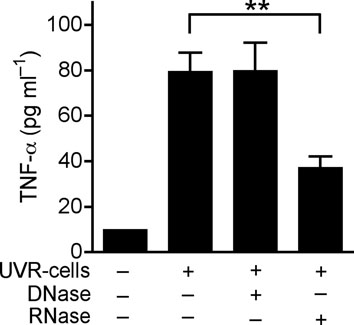
(a) Quantification of IL-6 and TNF-α production by ELISA in extracts of skin taken from 2 mm surrounding the wound edge 3 d after aseptic injury. (b) H&E staining of skin 2 mm adjacent to mouse wounds of wild-type (Tlr3+/+) and Tlr3-deficient (Tlr3−/−) mice treated as in (a). Black scale bars represent 200 μm and white scale represent 25 μm. Arrows designate region of 400X magnification shown in inset. (c) The induction of TNF-α by UVB-irradiated keratinocytes (UVR-cells) in untreated normal human keratinocytes and the blockage of UVR-cell-induced TNF-α by TLR3 siRNAs. (d) SE inhibited TNF-α production stimulated by UVR-cells. (e) RNase treatment decreased the capacity of UVR-cells to induce TNF-α in untreated normal human keratinocytes in culture, but not DNase treatment. * P <0.05, ** P <0.01 and *** P <0.001. n.s. no significance. P-values were analyzed by Two-way ANOVA in (a) or One-way ANOVA in (c)–(e). Data are the mean ± SEM. and are representative of two to four independent experiments with n = 4–7 per group.
Wounding results in the rapid generation of abundant amounts of damaged cells, including necrotic and apoptotic keratinocytes. To test if a product of damaged keratinocytes can stimulate TLR3-dependent inflammation in adjacent normal cells, cultured keratinocytes were treated with UVB radiation to induce apoptosis and cell death. These cells (UVR-cells) were then collected and added to separate cultures of normal human keratinocytes. TNF-α release significantly increased when normal keratinocytes were exposed to UVR-cells but not when exposed to equal amounts of dead, non-irradiated cells (Fig. 2c and Supplementary Fig. 5a). This response was dependent on TLR3 since targeted knock down of TLR3 by siRNA abrogated the response to UVR cells (Fig. 2c and Supplementary Fig. 6). Consistent with response to poly(I;C) or wounding, SE also inhibited TNF-α release induced by UVR-cells (Fig. 2d). The product of UVR-cells that activated TLR3 in normal keratinocytes was RNA as RNase abrogated the capacity of UVR-cells to stimulate the production of TNF-α, but treatment with DNase did not (Fig. 2e). UVR-cells were also sorted into intact, Annexin V+ cells that excluded propidium iodide (PI), and dead cells whose membrane was permeable after UVB irradiation and thus stained with PI. Non-irradiated cells (normal cells) disrupted by sonication, or early apoptotic cells that were annexin V+ but excluded PI (annexin V+/ PI− cells), could not induce TNF-α release or TLR3 expression (Supplementary Fig.5a, b). However, UVR-cells staining with PI (PI+ cells) significantly increased TNF-α release and TLR3 expression (Supplementary Fig. 5a, b). Thus, these results show that RNA released from necrotic cells can induce TNF-α and TLR3 expression by keratinocytes.
LTA suppresses TLR3 inflammation
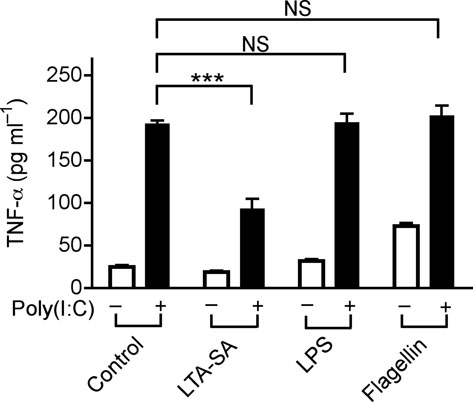
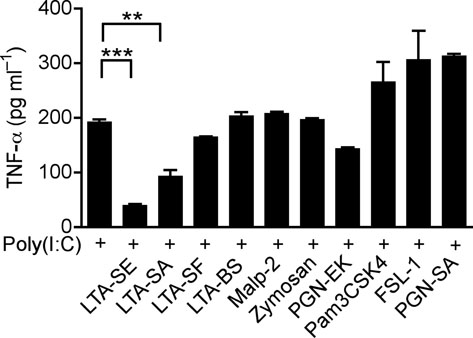
TLR-TLR cross-talk can suppress inflammatory responses 23,24, and LTA is a known molecular signal for recognition of staphylococci, thus we sought to determine whether this molecule could be responsible for the observed effects. LTA from S. epidermidis has two forms, cellular and exocellular LTA 25. Exocellular LTA can be recovered from liquid growth medium, and is the form present in SE, whereas commercial LTA is derived from the cell wall and membrane. Commercially available LTA from S. aureus (LTA-SA), but not LPS or Flagellin, suppressed the production of TNF-α induced by poly(I:C) (Fig.3a). LTA directly purified from SE (LTA-SE) or commercial LTAs from some other staphylococcal species suppressed poly(I:C)-induced TNF-α, whereas TLR2/1, TLR2/6 ligands (Pam3CSK4, FSL-1 and PGN-SA) either synergistically induced TNF-α, or failed to suppress TNF-α (Malp-2, PGN-EK and Zymosan) when combined with poly(I:C) (Fig. 3b). Interestingly, LTAs from Streptococcus faecalis and Bacillus subtilis (LTA-SF and LTA-BS) were not active (Fig. 3b).
Figure 3. Staphylococcal LTA inhibits poly(I:C)-induced TNF-α.
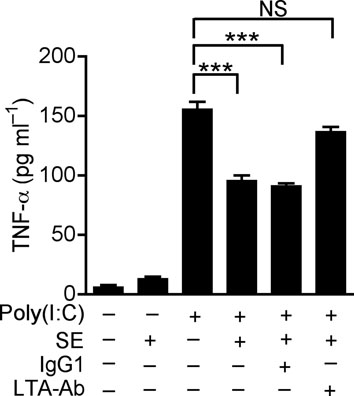
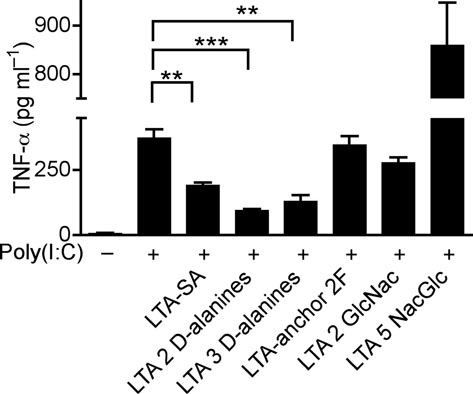
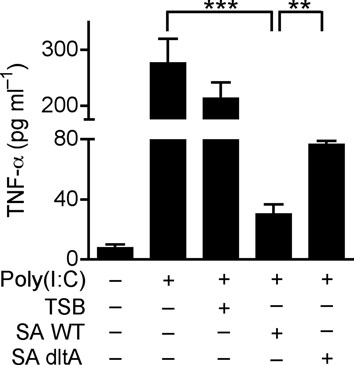
(a) Quantification of TNF-α in culture media of human keratinocytes after treatment with PBS or TLR-ligands alone (white bars), or in combination with 10 μg ml−1 of poly(I:C) (black bars); LTA-SA (10 μg ml−1), LPS (100 ng ml−1), Flagellin (50 ng ml−1). (b) Keratinocyte TNF-α stimulated with poly(I:C) (10 μg ml−1) combined with a panel of TLR2 ligands LTA-SE, LTA-SA, LTA-SF, LTA-BS, Zymosan, PGN-EK, PGN-SA (10 μg ml−1), Malp-2 (100 ng ml−1), Pam3CSK4, FSL-1 (1 μg ml−1). (c) Antibody to S. epidermidis LTA prevented SE from suppressing poly(I:C)-induced TNF-α in keratinocytes. (d) Quantification of TNF-α from keratinocytes treated with 10 μg ml−1 of poly(I:C) and 5 μg ml−1 of synthetic LTA containing alanines (LTA 2 D-alanines and LTA 3 D-alanines), or only the anchor (LTA-anchor 2F), or containing N-acetylglucosamine (LTA 2 GlcNac and LTA 5 NacGlc). (e) Quantification of TNF-α from keratinocytes stimulated by 10 μg ml−1 of poly(I:C) with a sterile <10 kDa product of S. aureus Sa113 parental strain conditioned culture media (SA wt; 36 μg ml−1), or a similar product of S. aureus Sa113 dltA mutant (SA dltA; 36 μg ml−1). ** P <0.01 and *** P <0.001. n.s. no significance. P-values were determined by One-way ANOVA. Data are the mean ± SEM of triplicates stimulations and are representative of two to four independent experiments.
The identity of LTA as the molecule in SE responsible for suppression of keratinocyte TNF-α production was confirmed by use of a specific LTA-neutralizing antibody25 (Fig. 3c). Furthermore, synthetic LTAs containing two or three D-alanine suppressed poly(I:C)-induced TNF-α, whereas synthetic LTAs only containing an anchor or containing two or five N-acetylglucosamine (NacGlc) failed to suppress TNF-α (Fig. 3d). The necessity of D-alanine modification was then confirmed by use of LTA from a S.aureus dltA mutant that lacks D-alanine modifications 26. Preparations from this mutant bacterial culture supernatant (SA dltA) partially lost the capacity to suppress poly(I:C)-induced TNF-α compared to its parental strain (SA wt) (Fig. 3e).
N-TRAF1 mediates LTA inhibition of TLR3
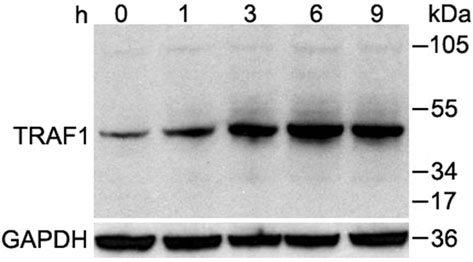
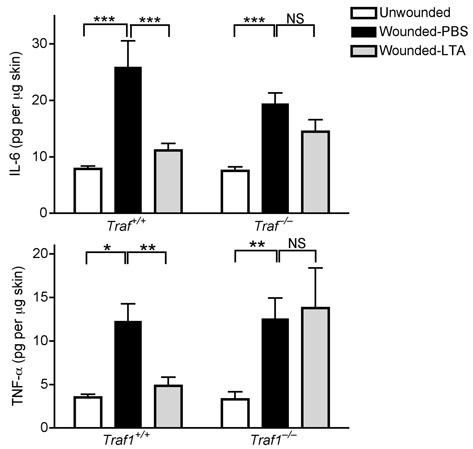
SE was observed to block nuclear translocation of NF-kB1/p50, but not the translocation of IRF3, both stimulated by poly(I:C) in keratinocytes (Supplementary Fig. 7a,b). Thus, we next investigated negative regulators involved in TLR-mediated NF-kB signaling including TNF receptor-associated factor 1 (TRAF1), TNF alpha-induced protein 3 (TNFAIP3, A20) and interleukin-1 receptor-associated kinase M (IRAK-M)27. LTA and SE significantly induced TRAF1 in keratinocytes within one hour and maximally between 6 and 9 hrs (Fig. 4a and Supplementary Fig. 8a). The expression of other negative regulatory genes (NLRX1, A20 or IRAK-M) involved in both the NF-kB and RIG-like helicase pathways, were not induced (Supplementary Fig. 8a). The role of TRAF1 as a mediator of the LTA inhibitory effect was confirmed by experiments in Traf1−/− mice where LTA was unable to suppress the production of IL-6 and TNF-α at wound edge compared to wild-type controls (Fig. 4b).
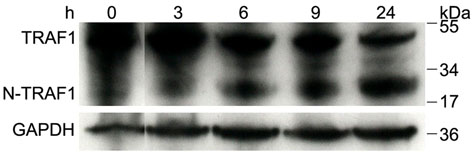
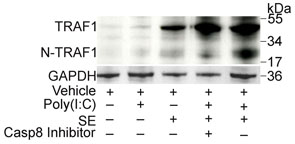
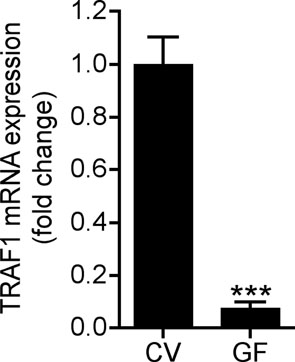
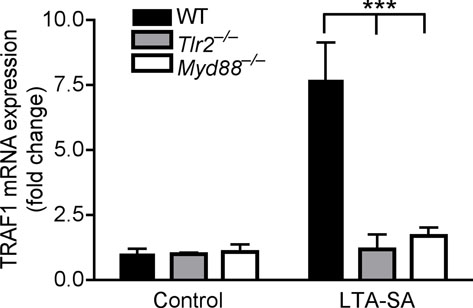
Figure 4. Staphylococcal LTA induces TRAF1 to inhibit TNF-α.
(a) Western blot of TRAF1 in cultured human keratinocytes stimulated by LTA-SA for various times. The predicted molecular weight of TRAF1 is ca. 52 kDa. GAPDH was used as endogenous control. (b) Quantification of IL-6 and TNF-α of skin extracts from C57BL/6 Traf1-deficient mice and wild-type controls preinjected by PBS or LTA-SA 24 h and 2 h prior to wounding. 2 mm of skin around the wound edges was collected for ELISA. (c) Western blot with antibody raised against the N-terminus of human TRAF1 in keratinocyte extract treated with SE and poly(I:C) for the indicated time periods. The predicted molecular weight of N-TRAF1 is ca. 22 kDa. (d) Western blot showing that caspase 8 inhibitor prevented the cleavage of TRAF1 in cultured human keratinocytes. (e) Quantification of Traf1 mRNA expression in skin of germ-free mice and conventional mice. CV: conventional mice; GF: Germ-free mice. (f) Quantification of Traf1 mRNA expression induced by LTA-SA in skin of wild-type, Tlr2−/− and Myd88−/− mice. * P <0.05, ** P <0.01 and *** P <0.001. n.s. no significance. P-values were determined by using Two-way ANOVA in (b) and (f), and Two-tailed t tests in (e). Data are mean ± SEM, and are representative of two independent experiments with n = 3–7 per group.
It has been hypothesized that TRAF1 is processed by caspase 8 to an active N-terminal fragment (N-TRAF1) followed by binding to TRIF in order to serve as a negative regulator of TLR3. The cleavage of TRAF1 to N-TRAF1 was observed in the presence of SE and poly(I:C) (Fig. 4c) and occurred in a caspase 8-dependent manner since treatment with a caspase 8 inhibitor blocked the generation of N-TRAF1 (Fig. 4d). Association of TRAF1 with TRIF was observed by immunoprecipitation of SE-treated keratinocytes with TRAF1-specific antibody and detected with antibody to TRIF (Supplementary Fig. 8b). Furthermore, addition of a caspase 8 inhibitor increased TNF-α and completely abrogated the inhibitory effects of SE and LTA (Supplementary Fig. 8c,d).
TLR2 inhibits TLR3
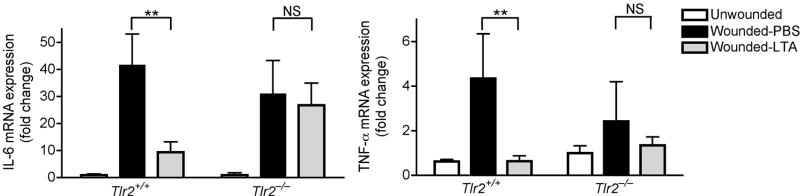
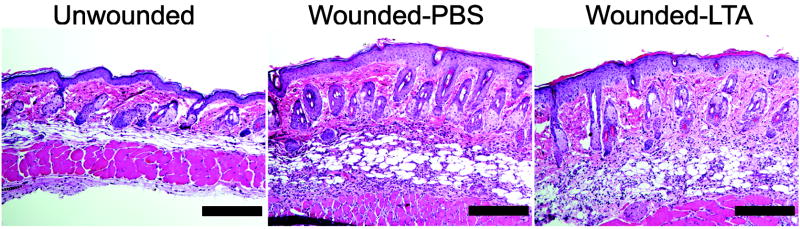
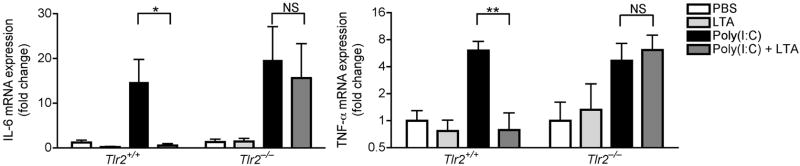
Having established the involvement of TRAF1 in suppression of keratinocyte cytokine release, and the role of the TLR2 ligand LTA in initiating this effect, we next sought to further confirm the mechanism and physiological relevance of these observations in vivo. Analysis of the skin from germ-free mice that were never exposed to bacterial products showed that the expression of Traf1 in their skin was dramatically decreased compared to skin of normal mice housed in a pathogen-free but non-sterile conditions (Fig. 4e). In addition to skin, the expression of Traf1 in small intestine and lung, but not heart, of germ-free mice was decreased compared to those tissues from conventional mice (Supplementary Fig. 8e). Furthermore, as predicted based on the known recognition system for LTA, diminished expression of Traf1 was also observed in Tlr2−/− or Myd88−/− mice intentionally exposed to LTA (Fig. 4f). Therefore, Tlr2−/− mice could be used as another model to test the consequences of diminished Traf1 to suppress Tlr3-dependent skin inflammation following injury. As predicted, LTA failed to suppress inflammation and the production of IL-6 and TNF-α at wound edge in Tlr2−/− mice (Fig. 5a, b), and a similar loss of responsiveness to LTA was seen in Tlr2−/− mice treated with poly(I:C) (Fig. 5c).
Figure 5. TLR2 is required for LTA to inhibit skin inflammation after injury or injection of poly(I:C).
(a) Quantification of IL-6 and TNF-α mRNA expression in skin from C57BL/6 Tlr2-deficient mice and wild-type controls preinjected by PBS or LTA-SA 24 h and 2 h prior to wounding. 2 mm of skin around the wound edges was collected for real-time RT-PCR. (b) H&E staining of skin in Tlr2-deficient mice treated as in (a). The scale bars represent 200 μm. (c) Quantification of IL-6 and TNF-α mRNA expression in skin from C57BL/6 Tlr2-deficient mice stimulated by subcutaneous injection of poly(I:C). Some ears were preinjected with LTA-SA, or PBS 2 h prior to injection of poly(I:C). * P <0.05 and ** P <0.01. n.s. no significance. P-values were evaluated by Two-way ANOVA. Data are the mean ± SEM of n = 5–7 and are representative of three independent experiments.
DISCUSSION
Commensal microorganisms have been proposed to influence host immune responses and host-pathogen interactions in the gut28. In the current study we hypothesized that specific elements of the resident microbiota of normal human skin can modulate cutaneous immune responses triggered by TLR ligands. Our results confirmed this hypothesis while also revealing for the first time that TLR3 is a critical element in the induction of inflammation after skin injury. Inhibition of this inflammatory event is accomplished by specific staphylococcal LTAs, and mediated by TLR2 on keratinocytes. The mechanism for LTA-TLR2 mediated suppression of TLR3 signaling is by induction of the negative regulatory factor TRAF1, an event we show is important to limit the extent of cutaneous inflammation. Thus, we show for the first time that a sensitive balance exists between stimulating and inhibitory mediators during wound healing, and that epithelial cells uniquely detect these signals to achieve tissue homeostasis following injury.
It was previously unclear why keratinocytes are highly sensitive to TLR3 ligands since the classical ligand for TLR3, viral dsRNA, is not a frequent initiator of major inflammatory responses in the skin. The epidermis can be exposed to dsRNA from viral infections, but this is a relatively uncommon event compared to other skin pathogens. We show here that the significance of the high sensitivity to TLR3 ligands is likely because the epidermis uses TLR3 for recognition of injury to self. Our data show RNA from necrotic cells trigger TLR3 on undamaged keratinocytes, leading to local release of proinflammatory cytokines. This is probably a frequent mechanism for detection of injury and maintenance of homeostasis as necrotic cells are abundant at the wound edge. Therefore, it is reasonable to speculate that TLR3 in the normal epidermis is an important sensor of injury, and that systems must exist to modulate this response to prevent excessive or unwanted inflammation. Furthermore, since SE or LTA not only inhibited proinflammatory cytokine production by isolated keratinocytes in culture, but also inhibited inflammation in vivo, this argues that the production of proinflammatory cytokines by keratinocytes is a major contributor to some forms of skin inflammation.
Our findings demonstrate that LTA produced by staphylococcal species have a uniqe anti-inflammatory action on keratinocytes. In contrast, LTA initiates the opposite response when exposed to other immune cells. It is logical that LTA would have distinct effects on cytokine release depending on the cell type exposed. LTA acts as a proinflammatory factor for cells that normally exist in a sterile environment, such as macrophages (Supplementary Fig.3b), monocytes and mast cells29,30. These cells are not normally exposed to the surface microbiome and appropriately recognize LTA as foreign. However, keratinocytes are unique in that they are frequently exposed to LTA. Furthermore, the structure of LTA appears to be important for the nature of the keratinocyte response. Addition of D-alanine to the LTA core is an important factor to dictate activity but further analysis is necessary to understand these LTA structure-function relationships. Moreover, other TLR2 ligands that depend on formation of a heterodimer with TLR1 or TLR6 do not inhibit TLR3 in keratinocytes but rather have a proinflammatory effect. Thus, the specificity of the response is dictated by cell type and specific structure of the TLR2 ligand produced by the microbe. In particular, S. epidermidis, a normal inhabitant of the skin, may have a uniquely structured TLR2 ligand that maximizes anti-inflammatory action yet minimizes the capacity to initiate inflammation.
To limit TLR-induced inflammation several negative regulatory systems exist including sequestration of signaling molecules, blockade of their recruitment, degradation of target proteins, or inhibition of transcription27,31. These negative regulators can be part of microbial virulence. Examples of this include decoy receptors in some bacterial infections that prevent a direct interaction between TLRs and their microbial ligands, and vaccinia virus production of several proteins that interfere with viral recognition through both TLR3 and helicases32-34. Therefore, there is potential for pathogenic staphylococci such as S. aureus to exploit suppression of keratinocyte activation as a mechanism of virulence, while S. epidermidis may benefit the host by dampening unwanted inflammation. This hypothesis requires further testing but is supported by data obtained from germ-free mice that shows these animals lack normal expression of Traf1 in the skin. These observations support additional findings that the ability of TLR2 to recognize commensal bacteria is not irrelevant under normal conditions. Rather, the activation of TLR2 in keratinocytes has its own beneficial effect in maintaining homeostasis.
Taken together, these findings are best appreciated when one recognizes that inflammation is an undesirable condition on skin but is fundamentally a necessary protective response after injury. Prolonged and dysregulated production of inflammatory cytokines leads to excessive neutrophil influx, resulting in sustained inflammatory responses and poor healing, subsequently causing extensive tissue damage35,36. On the other hand, without an appropriate inflammatory response wound healing is also delayed and the host is more susceptible to microbial invasion. Local modulation of the inflammatory response by products of bacterial commensals at the site of such an injury might be a beneficial therapeutic strategy for management of wound healing complicated by excessive inflammation, or control of other inflammatory skin disorders. The trick will be to evoke a reduction in the detrimental aspects of inflammation without increasing the risk of wound infection. Our findings emphasize the potential benefit of the resident bacteria on skin, and the potential negative consequences of complete depletion of microflora from skin by indiscriminate use of topical and systemic antibiotics.
METHODS
Mice
C57BL/6 wild-type, Tlr3-deficient, Tlr2-deficient and BABL/c wild-type mice were housed in VA San Diego Healthcare System Veterinary Medical Unit (VMU) and Traf1-dificient mice were purchased from the Jackson Laboratory while swiss webster conventional and germ-free mice were purchased from Taconic. All animal experiments were approved by VA San Diego Healthcare System Institutional Animal Care and Use Committee.
Bacterial extracts preparation and LTA Purification
Bacteria were grown in Tryptic soy broth (TSB) at 37 °C for 15–16 hours. For scale-up preparation, overnight cultures were diluted 1:100 into TSB and grown for another 15–16 hours. We then collected bacterial cultures and filtered them by 0.22 μM Stericup (Millipore). We used MacroSep 10K OMEGA column (VWR) to collect the less than 10 kDa fractions from bacterial supernatants and determined concentrations of bacterial extracts by BCA™ Protein Assay Kit (Pierce). We purified LTA from S. epidermidis conditioned culture media (SE) by using the protocol of Grundling and Schneewind(2007)37.
UVB-induced apoptosis and necrosis
We irradiated cultured human keratinocytes by UVB at 15 mJ/cm2 for 1 min. After 24 h, we collected the UVB-irradiated cells and sorted them into Annexin V+/ PI− cells and PI+ cells. To stimulate TNF-α we added these sorted UVR-cells to untreated normal human keratinocytes in culture, respectively. We used sonicated-non-irradiated cells (normal cells) as control. We measured TNF-α in culture media 24 h after treatment with these cells.
LTA neutralization
We used 300 μg ml−1 of S. epidermidis LTA monoclonal antibody (abcam) or monoclonal mouse IgG1 isotype control (EXBIO) to incubate with 30 μg of SE for 20 h at 4 °C before we added SE to stimulate cells. After 24 h stimulation, we collected cell supernatants for TNF-α ELISA assay.
RNA interference
We seeded neonatal human epidermal keratinocytes at first or second passage in 24-well plate and transfected cells with 10 nM four pairs of siRNA oligonucleotides targeted to TLR3 (Dharmacon; SMART Pool) and non-targeted control siRNA (Dharmacon) by using silentFect (BioRad) as we described before 38. We tested the efficiency of TLR3 siRNA blockage by western blot. After 24 h transfection, we added UVR-cells to stimulate cells for 24 h. We evaluated the production of TNF-α by ELISA.
Caspase 8 inactivation
We applied 100 nM caspase 8 inhibitor (Z-IETD; R&D) 10 min before we added10 μg ml−1 of poly(I:C) with or without 36 μg ml−1 of SE or 10 μg ml−1 of LTA in cultured human keratinocytes and waited for 6 h or 48 h. We analyzed the inhibition of TRAF1 processing by western blot and the production of TNF-α by ELISA.
Immunoprecipitation and immunoblot
We stimulated cultured human keratinocytes at indicated time points. We lysed cells by using RIPA buffer (pH 7.4) containing protease inhibitor cocktail (Roche) and then sonicated them on ice-cold water. We measured protein concentrations of the extracts by BCA™ Protein Assay Kit (Pierce) and used15 μg of total protein for immunoprecipitation and 3 μg of total protein for western blot. SDS-PAGE was used to separate bands and TRAF1 or the association of TRAF1 and TRIF was detected by immunoblot with TRAF1-specific antibody (Santa Cruz) or TRIF-specific antibody (Cell signaling).
Cutaneous inflammation in vivo
We shaved the backs of age-matched adult littermates and removed hair by using chemical depilation (Nair; Church & Dwight) as previously described39. We intradermally injected 100 μL of PBS or 100 μL of SE (24 μg) or 100 μL of LTA-SA (50 μg) 24 h and 2 h prior to wounding back skin of wild-type or Tlr3-deficient mice or Traf1-deficient mice by biopsy punches. 3 d later, we collected 2 mm of skin around wound edges either for ELISA assay or stored it in formalin (Protocol) for H&E staining.
For cutaneous inflammation in ears, we injected12 μg of SE or 10 μg of LTA-SA into the ear lobes of C57BL/6 wild-type (or BABL/c wild-type mice) and Tlr2-deficient mice 2 h before we injected 50 μg of poly(I:C) or 20 μg of LPS. After 24 h we cut mouse ears to either store them in formalin for H&E staining or homogenize them for RNA isolation. We analyzed the expression of cytokines by real-time RT-PCR.
For phorbol 12-myristate 13 –acetate (PMA)- induced skin inflammation, we injected 12 μg of SE in BABL/c wild-type mice ear lobes 2 h before we topically treated mouse ears with 20 μL of 1mg ml−1PMA (Sigma). After 6 h, we took ears for cytokines analysis.
Statistical analysis
All data are present as mean ± SEM. We used two-tailed t tests to determine significances between two groups. We did analyses of multiple groups by One-way or Two-way ANOVA with Bonferroni post test of GraphPad Prism Version 4. For all statistical tests, we considered P values <0.05 to be statistically significant.
Supplementary Material
Acknowledgments
We thank A. Peschel for advice with blocking LTA activity by the antibody; E. Raz from University of California, San Diego for providing C57BL/6 Tlr3-deficient mouse breeding pair; V. Nizet and E. Tistiskov for helpful discussion; G. Cheng, B. Beutler, R. Modlin and E. Raz for critical reading and helpful advice; D. Bird for histological sections. This work was supported by US National Institutes of Health grants R56AI083358, R01AR052728, R01 AI052453, and a US Veterans Administration Merit Award to R.L.G and US National Institutes of Health grants DC00129 and DC006279 to A.F.R.
Footnotes
AUTHOR CONTRIBUTIONS Y. L. and R.L.G. designed the experiments; Y. L. performed most of the experiments and analyzed data; A.D.N. helped in culture of bone-marrow-derived dendritic cells and preparation of UVB-irradiated apoptotic/necrotic cells; T.N. and A.L. helped with mouse experiments; Y.Y. and Z.-R. W. designed the LPS-induced inflammation model; A.L.C. helped to perform initial experiments; L.V.H. provided germ-free mouse ear and intestine samples; S.V.A. provided synthetic LTAs; K.A.R. showed how to make mouse wound model; C.-M. H. helped to modify the protocol for animal study; A.F.R. provided Tlr2-deficient mice; Y. L. and R.L.G. wrote and prepared the manuscript.
The authors declare no conflict of interest.
References
- 1.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Xu J, et al. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. 2003;299:2074–6. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 3.Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–29. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Netea MG, Van der Meer JW, Kullberg BJ. Toll-like receptors as an escape mechanism from the host defense. Trends Microbiol. 2004;12:484–8. doi: 10.1016/j.tim.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Kelly D, Conway S, Aminov R. Commensal gut bacteria: mechanisms of immune modulation. Trends Immunol. 2005;26:326–33. doi: 10.1016/j.it.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Steidler L, et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–5. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 7.Vandenbroucke K, et al. Active delivery of trefoil factors by genetically modified Lactococcus lactis prevents and heals acute colitis in mice. Gastroenterology. 2004;127:502–13. doi: 10.1053/j.gastro.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 8.Haller D, et al. Transforming growth factor-beta 1 inhibits non-pathogenic Gram negative bacteria-induced NF-kappa B recruitment to the interleukin-6 gene promoter in intestinal epithelial cells through modulation of histone acetylation. J Biol Chem. 2003;278:23851–60. doi: 10.1074/jbc.M300075200. [DOI] [PubMed] [Google Scholar]
- 9.Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annu Rev Immunol. 2007;25:419–41. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- 10.Leadbetter EA, et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–7. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 11.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 12.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–35. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziebuhr W, et al. Nosocomial infections by Staphylococcus epidermidis: how a commensal bacterium turns into a pathogen. Int J Antimicrob Agents. 2006;28(Suppl 1):S14–20. doi: 10.1016/j.ijantimicag.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Bibel DJ, et al. Competitive adherence as a mechanism of bacterial interference. Can J Microbiol. 1983;29:700–3. doi: 10.1139/m83-114. [DOI] [PubMed] [Google Scholar]
- 15.Lebre MC, et al. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J Invest Dermatol. 2007;127:331–41. doi: 10.1038/sj.jid.5700530. [DOI] [PubMed] [Google Scholar]
- 16.Pivarcsi A, et al. Expression and function of Toll-like receptors 2 and 4 in human keratinocytes. Int Immunol. 2003;15:721–30. doi: 10.1093/intimm/dxg068. [DOI] [PubMed] [Google Scholar]
- 17.Baker BS, Ovigne JM, Powles AV, Corcoran S, Fry L. Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol. 2003;148:670–9. doi: 10.1046/j.1365-2133.2003.05287.x. [DOI] [PubMed] [Google Scholar]
- 18.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto C, Nakagawa Y, Ohara K, Takahashi H. Polyriboinosinic polyribocytidylic acid [poly(I:C)]/TLR3 signaling allows class I processing of exogenous protein and induction of HIV-specific CD8+ cytotoxic T lymphocytes. Int Immunol. 2004;16:55–63. doi: 10.1093/intimm/dxh025. [DOI] [PubMed] [Google Scholar]
- 20.Cavassani KA, et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205:2609–21. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown DL, Kao WW, Greenhalgh DG. Apoptosis down-regulates inflammation under the advancing epithelial wound edge: delayed patterns in diabetes and improvement with topical growth factors. Surgery. 1997;121:372–80. doi: 10.1016/s0039-6060(97)90306-8. [DOI] [PubMed] [Google Scholar]
- 22.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–89. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanhoutte F, et al. Toll-like receptor (TLR)2 and TLR3 synergy and cross-inhibition in murine myeloid dendritic cells. Immunol Lett. 2008;116:86–94. doi: 10.1016/j.imlet.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh TK, et al. TLR-TLR cross talk in human PBMC resulting in synergistic and antagonistic regulation of type-1 and 2 interferons, IL-12 and TNF-alpha. Int Immunopharmacol. 2007;7:1111–21. doi: 10.1016/j.intimp.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Lambert PA, Worthington T, Tebbs SE, Elliott TS. Lipid S, a novel Staphylococcus epidermidis exocellular antigen with potential for the serodiagnosis of infections. FEMS Immunol Med Microbiol. 2000;29:195–202. doi: 10.1111/j.1574-695X.2000.tb01523.x. [DOI] [PubMed] [Google Scholar]
- 26.Weidenmaier C, et al. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat Med. 2004;10:243–5. doi: 10.1038/nm991. [DOI] [PubMed] [Google Scholar]
- 27.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Hooper LV. Do symbiotic bacteria subvert host immunity? Nat Rev Microbiol. 2009;7:367–74. doi: 10.1038/nrmicro2114. [DOI] [PubMed] [Google Scholar]
- 29.Timmerman CP, et al. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect Immun. 1993;61:4167–72. doi: 10.1128/iai.61.10.4167-4172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshioka M, et al. Lipoteichoic acid downregulates FcepsilonRI expression on human mast cells through Toll-like receptor 2. J Allergy Clin Immunol. 2007;120:452–61. doi: 10.1016/j.jaci.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 31.Han J, Ulevitch RJ. Limiting inflammatory responses during activation of innate immunity. Nat Immunol. 2005;6:1198–205. doi: 10.1038/ni1274. [DOI] [PubMed] [Google Scholar]
- 32.Stack J, et al. Vaccinia virus protein A46R targets multiple Toll-like-interleukin-1 receptor adaptors and contributes to virulence. J Exp Med. 2005;201:1007–18. doi: 10.1084/jem.20041442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harte MT, et al. The poxvirus protein A52R targets Toll-like receptor signaling complexes to suppress host defense. J Exp Med. 2003;197:343–51. doi: 10.1084/jem.20021652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson AJ, Locarnini SA. Toll-like receptors, RIG-I-like RNA helicases and the antiviral innate immune response. Immunol Cell Biol. 2007;85:435–45. doi: 10.1038/sj.icb.7100100. [DOI] [PubMed] [Google Scholar]
- 35.Szpaderska AM, DiPietro LA. Inflammation in surgical wound healing: friend or foe? Surgery. 2005;137:571–3. doi: 10.1016/j.surg.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Wetzler C, Kampfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol. 2000;115:245–53. doi: 10.1046/j.1523-1747.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- 37.Grundling A, Schneewind O. Synthesis of glycerol phosphate lipoteichoic acid in Staphylococcus aureus. Proc Natl Acad Sci U S A. 2007;104:8478–83. doi: 10.1073/pnas.0701821104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schauber J, et al. Histone Acetylation in Keratinocytes Enables Control of the Expression of Cathelicidin and CD14 by 1,25-Dihydroxyvitamin D(3) J Invest Dermatol. 2007 doi: 10.1038/sj.jid.5701102. [DOI] [PubMed] [Google Scholar]
- 39.Nizet V, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–7. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.