Abstract
Objectives
To characterize the relationship between changes in body mass index (BMI) and incident atrial fibrillation (AF) in a large cohort of women.
Background
Obesity and AF are increasing public health problems. The importance of dynamic obesity-associated AF risk is uncertain, and mediators are not well characterized.
Methods
Cases of AF were confirmed by medical record review in 34,309 participants in the Women’s Health Study. Baseline and updated measures of BMI were obtained from periodic questionnaires.
Results
Over 12.9 +/− 1.9 years of follow-up, 834 AF events were confirmed. BMI was linearly associated with AF risk, with a 4.7% (95% CI 3.4, 6.1, p<0.0001) increase in risk with each kg/m2. Adjustment for inflammatory markers minimally attenuated this risk. When updated measures of BMI were utilized to estimate dynamic risk, overweight (HR 1.22 95%CI 1.02, 1.45, p=0.03) and obesity (HR 1.65 95%CI 1.36, 2.00, p<0.0001) were associated with adjusted short term elevations in AF risk. Participants becoming obese during the first 60 months had a 41% adjusted increase in risk of developing AF (p=0.02) compared to those maintaining BMI <30 kg/m2. The prevalence of overweight and obesity increased over time. The adjusted proportion of incident AF attributable to short term elevations in BMI was substantial (18.3%).
Conclusions
In this population of apparently healthy women, BMI was associated with short and long term elevations in AF risk, accounting for a large proportion of incident AF independent of traditional risk factors. A strategy of weight control may reduce the increasing incidence of AF.
INTRODUCTION
Over the past three decades, there has been a rapid increase in the prevalence of atrial fibrillation (AF), which is not entirely explained by the aging of the population.1 At present, an estimated 2.3 million people are diagnosed with AF in the United States, and AF accounts for between 75,000 to 100,000 strokes per year.2 If this rapid growth continues, the number of individuals with AF is expected to rise to 12.1 million by 2050.3 Once AF develops, treatments aimed at eliminating AF are associated with limited long-term success and non-negligible risks.4, 5 Even when treatment is apparently successful, asymptomatic AF may persist and the risk of stroke may never be eliminated6. Therefore, the identification of modifiable risk factors for development of AF is of paramount importance.
As we have witnessed this rapid increase in AF, the prevalence of obesity and overweight has steadily increased as well, and recent estimates suggest that 32.2% of adults are obese (BMI >30 kg/m2) and 6.9% of women are extremely obese (BMI >40 kg/m2).7 Several prospective studies have reported significant associations between obesity and incident AF8–10. However, how weight change influences the risk of incident AF and what proportion of the rapid increase in AF prevalence is attributable to obesity are uncertain. In addition, the mechanism(s) by which obesity confers an elevated risk are not entirely clear. Prior studies have identified left atrial size9 and impaired left ventricular diastolic function11 as potential mediators of the relationship between obesity and AF. However, other potential mediators of obesity-associated AF have not been well characterized. Measures of abdominal adiposity have been associated with markers of inflammation12, and several lines of evidence support a link between markers of inflammation and initiation and maintenance of AF13. However, it is unclear whether these inflammatory markers are mediators of obesity-associated AF risk.
To address these gaps in our knowledge, we examined the relationship between baseline and updated measures of body mass index (BMI) and incident AF over 12 years of follow-up in a large prospective cohort of women free of cardiovascular disease (CVD) at baseline, the Women’s Health Study (WHS). We utilized updated measures to account for changes in BMI over time and to characterize the short term impact BMI has on AF risk. This cohort also provided us with the unique opportunity to investigate the role inflammatory mediators might play in obesity-associated AF.
METHODS
Study Sample
The design of the WHS has been published elsewhere.14 Briefly, the WHS was initially a randomized, double-blinded and placebo-controlled trial of low dose aspirin and vitamin E performed in 39,876 female health professionals without prior CVD. Randomized treatment ended in March 2004, and the cohort has been followed subsequently. Of the original cohort, 4324 opted out of the observational follow-up and 7 were excluded due to presence of CVD at baseline, leaving 35545 women potentially eligible for inclusion in this analysis. The investigation of AF was not pre-specified as part of the original WHS; however, the present and other analyses on AF were pre-specified in 2006 prior to confirmation of the endpoint.
For this analysis, we excluded 787 women with a history of AF at baseline, and 449 women with missing information on BMI at baseline. Thus, the study population for the present analysis consisted of 34309 women with a mean follow-up of 12.9 +/− 1.9 person-years. All participants gave written informed consent. The study was approved by the institutional review board of Brigham and Women’s Hospital in Boston, Massachusetts.
Assessment of BMI
BMI, self-reported weight in kilograms divided by the square of self-reported height in meters (kg/m2), was the primary measure of total body adiposity in this study. Participants reported their height on the baseline questionnaire, 72-and 108-month questionnaires, and at the beginning of the observational phase of the study. Participants were asked to report their weight on the 24-, 36-, 60-, 72-, and 108-month questionnaires during the randomized period and at the beginning of the observational phase and yearly thereafter. We analyzed BMI as a continuous variable, and divided into the WHO Categories for overweight and obesity (<25 kg/m2 Normal, 25–29.99 kg/m2 Overweight, ≥ 30 kg/m2 Obese).15
Study variables
Information on baseline variables was collected using questionnaires. Follow-up questionnaires asking participants about study outcomes and other information were sent every six months during the first year and every 12 months thereafter. Covariates of interest assessed at study entry and at varying time points during follow-up included diabetes, hypertension, hyperlipidemia, smoking, alcohol use, and physical activity16 Plasma levels of hsCRP17, ICAM-118, and fibrinogen19 were measured at baseline in 24,621 of the women included in this analysis. Because distributions of ICAM-1, fibrinogen, and hsCRP are skewed, log-transformed levels were used in regression analyses.
Validation of incident AF
This female health professional cohort was asked to report diagnoses of incident AF at baseline, 48 months, and then annually. Beginning on September 19, 2006, those who reported an incident AF event on at least one yearly questionnaire were sent an additional questionnaire to confirm the episode and collect additional information. They were also asked for permission to review their medical records, particularly available electrocardiograms, rhythm strips, 24-hour electrocardiograms and information on cardiac structure and function. For deceased participants, we contacted family members to obtain consent and additional relevant information. A total of 1,425 self-reports of AF were made, 1,421 questionnaires were mailed out, and 1,324 questionnaires (93%) were received to identify patients for chart review. Of these, 834 cases of AF were confirmed by medical record review and 79 (9.5%), were asymptomatic at the time of diagnosis. An endpoint committee of physicians reviewed medical records for reported events according to predefined criteria. An incident AF event was confirmed if there was electrocardiographic evidence of AF or if a medical report indicated a personal history of AF. The earliest date in the medical records when documentation was believed to have occurred was set as the date of onset of AF. Only confirmed events were included in the analysis.
Statistical Analysis
For each woman, person-months of follow-up were calculated from the date of randomization to the date of AF, death or March 1, 2008, which ever came first. Age-adjusted Kaplan-Meier curves were used to plot survival free of atrial fibrillation for the three WHO categories of BMI, and differences between curves were tested with the log-rank test. We calculated age and multivariable-adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs) using Cox proportional hazards models. BMI was analyzed as a continuous variable and in WHO categories. All models were also adjusted for treatment arms of the randomized portion of the WHS (vitamin E, beta carotene, and aspirin use). The first model was age-adjusted (continuous). The second was a multivariable model that additionally adjusted for ethnicity, hypertension (defined as systolic blood pressure of ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, or report of diagnosis of hypertension by a physician), hypercholesterolemia (self-reported cholesterol of at least 240 mg/dl (6.22 mmol/l)), diabetes, alcohol consumption (rarely/never, <1 drink per week, 1 to 6 drinks per week, ≥1 drink per day), smoking (never, past, current), and physical activity ( < 1000 kilocalories per week, ≥1000 kilocalories per week).16To test for deviation from linearity, we included a quadratic term in the Cox- proportional hazard models containing continuous BMI, and non-parametrically using restricted cubic spline transformations20
To evaluate the degree to which the association between BMI and AF may be mediated by the development of intercurrent cardiovascular events, we refitted the Cox age-adjusted and multivariable proportional-hazards models after censoring all women with an intercurrent cardiovascular event at the date of the event. An intercurrent cardiovascular event was defined as confirmed myocardial infarction, stroke or coronary revascularization. To examine effect mediation by inflammation, we repeated the above age-adjusted and multivariable models among the 24,621 women in the biomarker cohort, and then performed an additional multivariable model that also included log-transformed fibrinogen, hsCRP, and ICAM-1 levels.
To investigate the relationship between the short term risk of BMI and subsequent risk of incident AF, we constructed time-varying Cox models where BMI categories were updated at each follow up and the most recent BMI measurement was used to estimate risk in the following time period. In multivariable models, other covariates were updated at various time points, and if data were missing at a given time point, the last observation was carried forward. Using these adjusted short term relative risks and the updated prevalence of obesity among the cases, we then estimated the population-attributable risk (PAR) proportion for overweight and obesity defined as pd*(RR-1/RR) where pd is the proportion of cases exposed to the risk factor and RR is the adjusted relative risk.21 We also evaluated effect modification through stratified analyses and multiplicative interaction terms. Multiplicative interaction terms between BMI and various baseline characteristics were evaluated in fully adjusted models using likelihood ratio tests. The proportional hazards assumption was examined for all models by including BMI by logarithm of time interaction into the model. No violation of this assumption was detected. All probability values were 2 tailed, and we considered P ≤ 0.05 as statistically significant. All analysis was performed with SAS version 9 (SAS institute Inc, Cary, NC).
RESULTS
Baseline BMI and AF
Baseline characteristics of the cohort by BMI WHO categories are shown in Table 1. At baseline, 6185 women (18% of the population) were categorized as obese (BMI > 30 kg/m2). As compared to women with a normal BMI (<25 kg/m2), obese women were more likely to have a history of diabetes, hypertension, hyperlipidemia, and were less physically active and less likely to consume alcoholic beverages.
Table 1.
Baseline Characteristics According to BMI Categories
| Body Mass Index |
||||
|---|---|---|---|---|
| Total population N=34309 |
<25 kg/m2 N=17544 (51.1%) |
25.0–29.9 kg/m2 N=10580 (30.8%) |
≥30 kg/m2 N=6185 (18.0%) |
|
| Weight, kgs | 70.2 +/− 14.5 | 60.4 +/− 6.3 | 73.5 +/− 6.8 | 92.4 +/− 13.5 |
| Age, y | 54.6 +/− 7.0 | 54.4 +/−7.0 | 55.0+/−7.1 | 54.1+/−6.5 |
| Ethnicity, (%) white | 32389 (95.1) | 16663 (95.7) | 9953 (94.7) | 5773 (94.3) |
| Diabetes, (%) | 928 (2.7) | 172 (1.0) | 285 (2.7) | 471 (7.6) |
| Hypertension, (%) | 8667 (25.3) | 2777 (15.8) | 3027 (28.6) | 2863 (46.3) |
| Hyperlipidemia, (%) | 9980 (29.1) | 4338 (24.7) | 3508 (33.2) | 2134 (34.5) |
| Alcohol use, (%) | ||||
| Never | 15160 (44.2) | 6669 (38.0) | 4871 (46.0) | 3620 (58.5) |
| <1 drink per week | 4541 (13.2) | 2288 (13.1) | 1438 (13.6) | 815 (13.2) |
| 1–6 drinks per week | 11043 (32.2) | 6247 (35.6) | 3339 (31.6) | 1457 (23.6) |
| ≥1 drink per day | 3555 (10.4) | 2335 (13.3) | 929 (8.8) | 291 (4.7) |
| Smoking | ||||
| Never, (%) | 17689 (51.6) | 8960 (51.1) | 5463 (51.7) | 3266 (52.9) |
| Past, (%) | 12355 (36.0) | 6214 (35.4) | 3902 (36.9) | 2239 (36.2) |
| Current, (%) | 4239 (12.4) | 2358 (13.5) | 1207 (11.4) | 674 (10.9) |
| Physical Activity (%) | ||||
| ≥ 1000 kcal/week | 11728 (34.2) | 6499 (37.1) | 3582 (33.9) | 1647 (26.7) |
| <1000 kcal/week | 22580 (65.8) | 11044 (62.9) | 6998 (66.1) | 4538 (73.4) |
Hypertension is defined as self-reported systolic blood pressure of >140 mm Hg or diastolic blood pressure ≥90 mm Hg, or report of diagnosis of hypertension by a physician; history of hypercholesterolemia is self-reported cholesterol of at least 240 mg/dl (6.22 mmol/l)
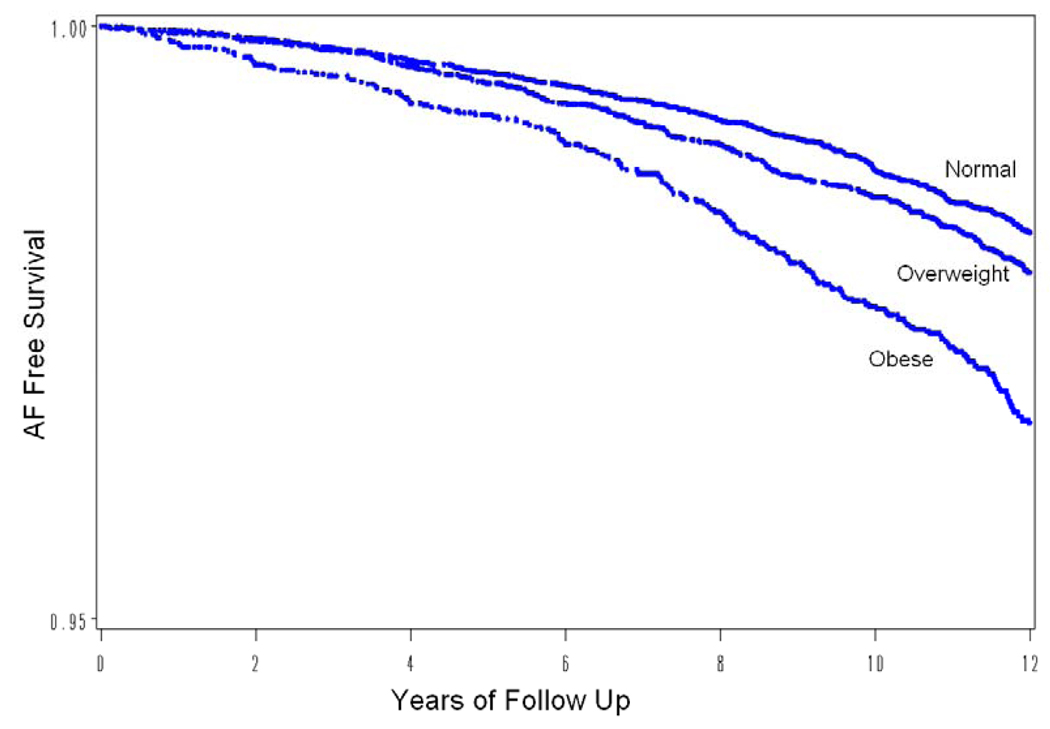
When examined as a continuous variable, BMI measured at baseline was associated with subsequent AF risk in age-adjusted and multivariable-adjusted Cox proportional hazards models (Table 2). Each 1-unit increase in BMI was associated with a 4.7% increase in risk of AF (95 % CI, 3.4–6.1%, p< .0001) even after controlling for obesity associated covariates age, ethnicity, diabetes, hypertension, hyperlipidemia, alcohol use, smoking, and physical activity, as well as aspirin, vitamin E and beta carotene randomization arm. When baseline BMI was divided into the WHO categories for obesity and overweight, a significant association with AF was observed for both the overweight and obese women in age adjusted models (Table 2), and the survival curves for freedom from AF continued to diverge over the course of the study (Figure 2, log-rank =P=0.001). However, the risk was not significantly elevated among overweight women (BMI 25–29.99 kg/m2) after multivariable adjustment. A quadratic term added to the model was not significant (p, quadratic term =0.58) suggesting lack of nonlinearity, and multivariable spline regression confirmed that the relationship between BMI and AF was linear (p for linear trend P<0.0001, p for deviation from linearity=0.58).
Table 2.
Hazard Ratios for Risk of Incident AF in Relation to Body Mass Index
| <25 kg/m2 | 25–30 kg/m2 | ≥ 30kg/m2 | Continuous BMI | |
|---|---|---|---|---|
| ALL ATRIAL FIBRILLATION EVENTS INCLUDED | ||||
| N participants (%) | 17544 (51.1) | 10580 (30.8) | 6185 (18.0) | 34309 |
| Number of events (%) | 344 (2.0) | 259 (2.5) | 231 (3.7) | 834 (2.4) |
| Age-adjusted incidence/1000 py | 1.55 | 1.85 | 2.98 | 1.90+ |
| Relative risks (Baseline examination) | ||||
| Age-adjusted (n=34309)* | Referent | 1.19 (1.01, 1.40) | 2.06 (1.74, 2.43) | 1.06 (1.05, 1.07) |
| Multivariable adjusted I (n=33990)** | Referent | 1.13 (0.96, 1.34) | 1.77 (1.47, 2.11) | 1.05 (1.03, 1.06) |
| Relative risks (Variables updated) | ||||
| Age-adjusted (n=34309)* | Referent | 1.28 (1.09, 1.52) | 1.98(1.67–2.36) | 1.06 (1.05, 1.07) |
| Multivariable adjusted I (n=34045)** | Referent | 1.22 (1.02, 1.45) | 1.65 (1.36, 2.00) | 1.04 (1.03, 1.06) |
| WOMEN CENSORED AT THE FIRST CARDIOVASCULAR EVENT | ||||
| Number of events (%) | 331 (1.9) | 233 (2.2) | 214(3.5) | 778 (2.3) |
| Age-adjusted incidence/1000 py | 1.49 | 1.67 | 2.76 | 1.77+ |
| Relative risks (Baseline) | ||||
| Age-adjusted (n=34309)* | Referent | 1.11(0.94–1.32) | 1.98 (1.66, 2.35) | 1.06 (1.05, 1.07) |
| Multivariable adjusted I (n=33990) ** | Referent | 1.07(0.90–1.27) | 1.75(1.45–2.11) | 1.05 (1.03, 1.06) |
| WOMEN IN THE BIOMARKER COHORT | ||||
| N participants (%) | 12774 (51.9) | 7528 (30.6) | 4319 (17.5) | 24621 |
| Number of events (%) | 268 (2.1) | 211 (2.8) | 167 (3.9) | 646 (2.6) |
| Age-adjusted incidence/1000 py | 1.66 | 2.13 | 3.05 | 2.05+ |
| Relative risks (Baseline) | ||||
| Age-adjusted (n=24621) * | Referent | 1.27 (1.06, 1.53) | 1.97 (1.62, 2.39) | 1.06 (1.04, 1.07) |
| Multivariable adjusted I (n=24394) ** | Referent | 1.20 (1.00, 1.45) | 1.68 (1.37, 2.07) | 1.04 (1.03, 1.06) |
| Multivariable adjusted II (n=24203) *** | Referent | 1.15 (0.95, 1.39) | 1.53 (1.22, 1.91) | 1.04 (1.02, 1.05) |
adjusted for age, vitamin E, beta carotene, and aspirin use
adjusted for age, race, vitamin E, beta carotene, aspirin use, diabetes, hypertension (defined as self-reported systolic blood pressure of ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, or report of diagnosis of hypertension by a physician), history of hypercholesterolemia (self-reported cholesterol of at least 240 mg/dl (6.22 mmol/l)), diabetes, alcohol consumption (rarely/never, <1 drink per week, 1 to 6 drinks per week, ≥1 drink per day), smoking (never, past, current), and physical activity ( < 1000 kilocalories per week, ≥1000 kilocalories per week)
adjusted for clinical variables above, and log-transformed hsCRP, ICAM and fibrinogen levels
this number reflects the overall age-adjusted incidence rate for the entire cohort
Figure 2.
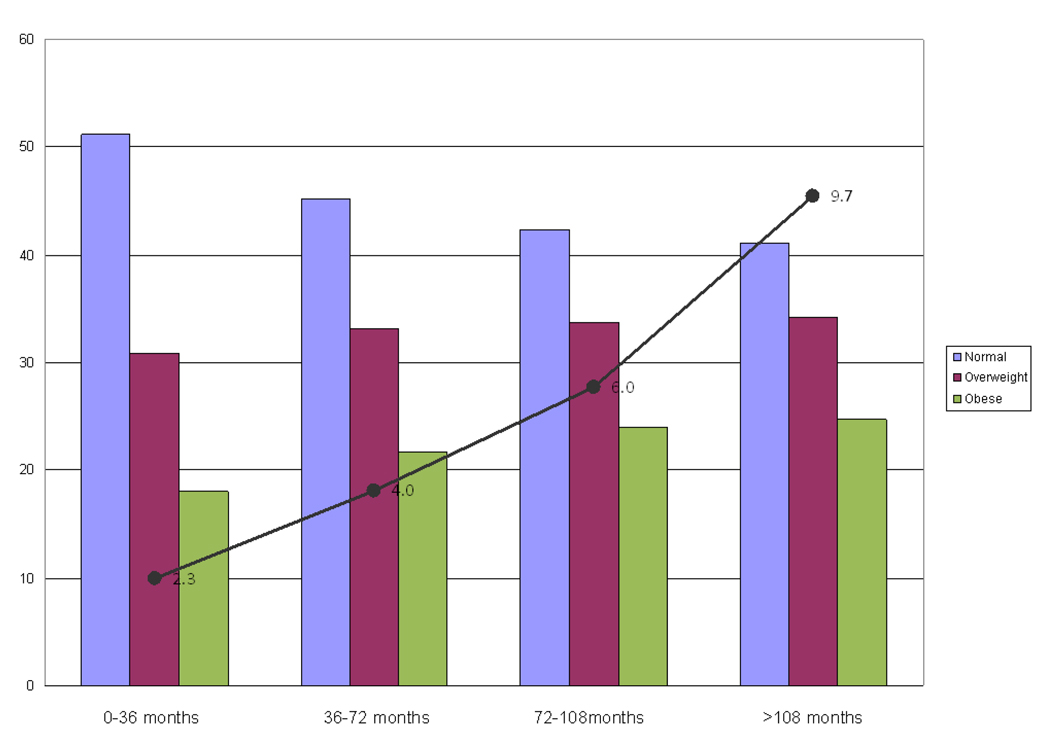
Incident AF and Percent of women in each WHO Category over 3 Year Time Periods. Shown is a bar graph of the percent of women in each WHO category at the beginning of four 36 month time periods over the course of the study. The incidence rate of AF (per 1000 participants per period) for each 36 month time period is superimposed on the graph for comparison.
When women who developed interim CVD were censored from the analysis, the relationship between BMI and AF was minimally changed (Table 2). To examine effect mediation by inflammation, age-adjusted and multivariable adjusted models limited to women who donated blood samples at baseline were performed (Table 2), and relationships between BMI and AF were similar to those observed in the entire cohort. Further adjustment for fibrinogen, hsCRP and ICAM-1 levels resulted in an 8.9% attenuation of the HR for developing AF associated with obesity (HR 1.68, 95% CI 1.37, 2.07 reduced to 1.53, 95%CI 1.22, 1.91, Multivariable adjusted model II, Table 2).
Updated Measures of BMI and AF risk
We next examined the dynamic association between BMI and AF by updating measures of BMI, and BMI in the overweight range was now associated with a significant elevation in the risk of AF. Even after adjustment for updated measures of potential biologic intermediaries including diabetes, hypertension, and physical activity, overweight status remained significantly associated with short term elevations in AF risk (HR 1.22, 95%CI 1.02, 1.45, p=0.03). Again, the significant elevation in risk persisted for the obese even after adjustment for these updated biologic intermediaries (HR 1.65 95%CI 1.36, 2.00, p<0.0001).
To further investigate the relationship between change in BMI and AF, we examined whether a change in BMI over the first 60 months of the study influenced risk of AF after that time point (Table 3). The majority of women did not change categories, and the 5095 women who remained obese had a trend toward a higher risk of AF after 60 months, whereas the 2,411 women who developed new obesity had a significantly higher adjusted risk of incident AF after year 5 (HR1.41, 95%CI 1.05, 1.90, p=0.02) compared with those who maintained a BMI <30kg/m2 over the same period. In contrast, women who were obese at baseline, but then attained a BMI < 30 by year 5, no longer had a significantly elevated risk of subsequent AF in adjusted analyses (HR 1.01, 95%CI 0.58, 1.79, p=0.96) compared with those who maintained a BMI <30kg/m2 over the same period. However, these analyses were limited by small numbers of women (n= 599) who achieved this degree of weight loss and as a result CIs are wide.
Table 3.
Weight Change over 60 months and Risk of Incident Atrial Fibrillation
| Body Mass Index (kg/m2) over 60 Months of Follow Up |
||||
|---|---|---|---|---|
| Stable <30 N=24204, 422 AF Events |
Reduced <30 N=599, 14 AF events |
Stable ≥30 N=5095, 157 AF events |
Increased ≥30 N=2411, 57 AF events |
|
|
Age-adjusted* (N=32309, 650 AF events) |
Referent | 1.00(0.57, 1.76) p= 0.99 |
1.34(0.97, 1.86) p= 0.08 |
1.39(1.03, 1.87) p= 0.03 |
|
Multivariable-adjusted** (N=32016, 645 AF events) |
Referent | 1.01(0.58, 1.79) p= 0.96 |
1.32(0.95, 1.84) p= 0.096 |
1.41(1.05, 1.90) p=0.02 |
adjusted for age, BMI, vitamin E, beta carotene, and aspirin use
adjusted for age, BMI, race, vitamin E, beta carotene, aspirin use, diabetes, hypertension, hyperlipidemia, alcohol use, smoking, and degree of physical activity
Population Attributable Risk Proportion
The prevalence of overweight and obesity increased over the course of the study. At baseline, the prevalence of overweight and obesity was 30.8% and 18.0%, respectively, which rose to 34.2% and 24.2% respectively by the last time period, and over the same time-period the incidence of new AF increased as well (Figure 2). Using a weighted average over time periods, the average prevalence of overweight and obesity over the course of the study was 34.1% and 23.2%. Utilizing updated measures of BMI, the age-adjusted population attributable risk proportion associated with obesity and overweight were estimated to be 0.153 and .074, respectively. Even after accounting for other potential confounders, some of which might be in the causal pathway (hypertension and diabetes), the estimated population attributable risk proportion remained substantial at 0.122 and 0.061 for obesity and overweight, respectively, with a total of 18.3% of AF cases attributable to short term elevations in BMI > 25 kg/m2.
Sub Group Analyses: (Figure 3)
Figure 3.
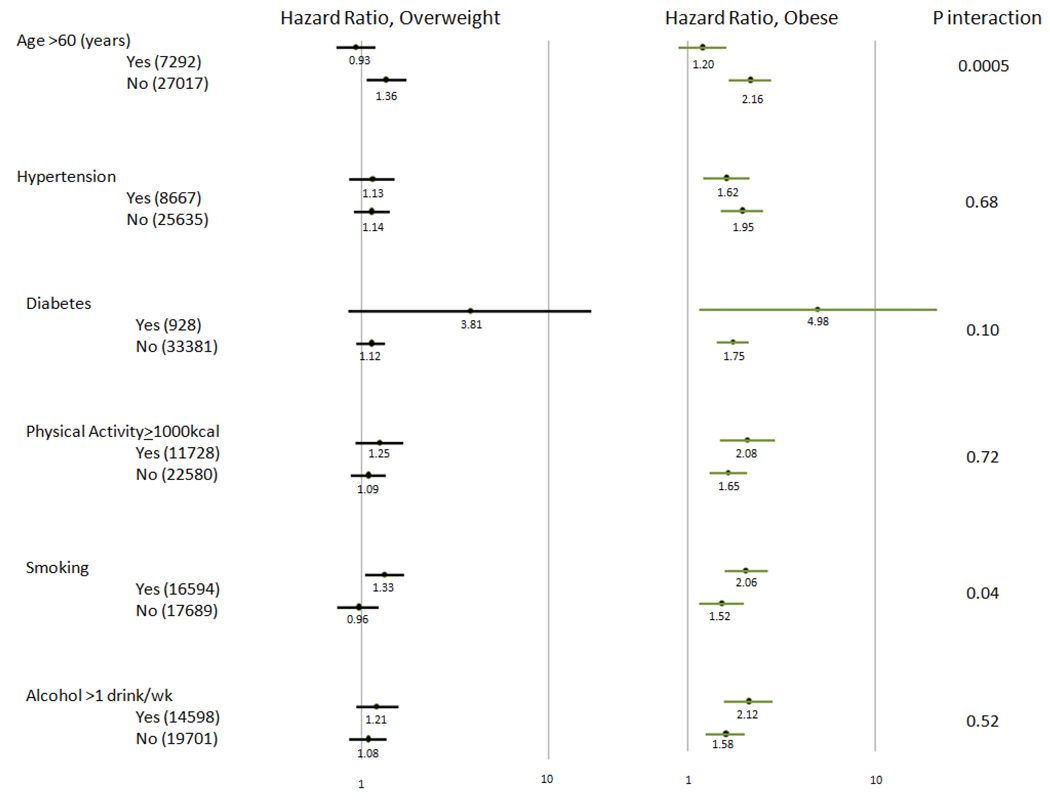
Overweight and Obesity and risk of incident atrial fibrillation, stratified by various baseline characteristics
Shown are hazard ratios for incident AF stratified by various baseline characteristics. The number of patients in each category is indicated in the first column. Hazard ratios and 95% confidence intervals are shown on a logarithmic scale with overweight in black and obese in green. All hazard ratios are adjusted for adjusted for age, race, vitamin E, beta carotene, aspirin use, diabetes, hypertension (defined as self-reported systolic blood pressure of ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, or report of diagnosis of hypertension by a physician), history of hypercholesterolemia (self-reported cholesterol of at least 240 mg/dl (6.22 mmol/l)), diabetes, alcohol consumption (rarely/never, <1 drink per week, 1 to 6 drinks per week, ≥1 drink per day), smoking (never, past, current), and physical activity (< 1000 kilocalories per week, ≥ 1000 kilocalories per week)
In pre-specified stratified analyses, the association between BMI- and incident AF was stronger among women 60 years of age or younger as compared to those over age 60 at baseline. (P, interaction = 0.0005). In the younger women, the HR for incident AF was 1.36 (1.08, 1.72, p=0.01) and 2.16 (1.69, 2.76, p<0.0001) among the overweight and obese respectively as compared to the non-obese. Alternatively, overweight and obesity were not significantly associated with incident AF in multivariable models among the smaller subgroup of women who were over age 60 at baseline. In addition to age, there was also evidence for a more marginal interaction according to smoking status, with higher obesity associated HR among current and past smokers (p for interaction =0.04). Our findings were otherwise consistent across all subgroups.
DISCUSSION
In this cohort of female health care professionals without prior evidence of CVD, BMI is strongly associated with subsequent development of AF even after accounting for the interim development of CVD and other important AF risk factors such as diabetes and hypertension. The relationship is linear, with a 4.7% increase in risk of incident AF for each kg/m2 increase in BMI. In our full multivariable models, 1.65–1.77-fold elevations in AF risk were observed among the obese (BMI >30 kg/m2), with greater than two-fold elevations in risk among obese women who were 60 years of age or younger at study entry. Adjustment for inflammatory markers measured at baseline in the biomarker cohort only minimally attenuated the obesity-associated risk of AF, suggesting that inflammation is not a major mediator of the AF risk associated with obesity.
When BMI was updated over the course of the study, significant short-term elevations in risk were associated with elevated BMI in both the overweight and obese ranges even after adjustment for updated biologic intermediaries. Study participants who became obese during the first 60 months of follow up had a 41% adjusted increase in risk of developing AF (p=0.02) compared to those who maintained BMI <30 kg/m2. These results on short term risk suggest that the AF risk associated with obesity may be modifiable by weight change.
To our knowledge, this is one of the first studies examining the short-term influence of BMI changes over time and subsequent risk of AF. Our results utilizing updated BMI measures are consistent with data from other population based cohort studies utilizing a single measure of BMI8, 9, 22–24, although the linear relationship observed here was not apparent in all of the individual studies. In a meta-analysis of 5 population based cohorts 10, baseline BMI was associated with a graded risk of AF, with estimated risk elevations of 39% and 87% in the overweight and obese respectively as compared to those of normal weight. A recent study of 6903 Swedish men demonstrated that long-term weight gain from age 20 to midlife was associated with an increased risk of AF consistent with our findings on short-term risk.25
Prior population-based studies did not report AF risk according to subgroups, and the reason for the interaction between age and obesity-associated AF risk in our data is not clear. We observed a similar age-interaction for the AF risk associated with habitual vigorous exercise among men in the Physicians Health Study,26 and it is possible that the influence of other AF risk factors may differ according to age. Women with a genetic or physiologic predisposition to developing obesity-associated AF may do so at a younger age, and therefore, these women would be excluded from analyses of older populations. This finding warrants further investigation since it raises potential alternative mechanisms for the rapid increase in the prevalence of new onset AF not reliant on the aging of the U.S. population.
There are many reasons why dynamic changes in BMI might be expected to modify AF risk independent of obesity associated co-morbidities. Obesity is associated with increased left atrial size and decreased left ventricular diastolic function which itself leads to increased left atrial pressure.9, 27 Weight reduction has also been linked to regression of left atrial enlargement.28 Dynamic changes in left atrial size and pressure likely affect both the atrial substrate and triggers for AF. Increased left atrial pressure may acutely lead to increases in atrial ectopy that triggers AF.11 Further, more prolonged BMI-mediated left atrial stretch may lead to development of fibrosis and atrial enlargement on a structural basis.29 Some of these obesity-associated atrial changes could be reversible or modifiable with weight loss, while other changes may be irreversible.
If the observed dynamic associations between BMI and AF are causal, the public health impact of the current obesity epidemic on the growing AF burden could be quite substantial with respect to clinical outcomes, quality of life and health care costs associated with AF. In our study, the prevalence of obesity increased over the course of the study from 18.0% to 24.2%, and 12.2% of incident AF cases were estimated to be attributable to obesity independent of other measured risk factors. When one also takes into account the modestly but significantly elevated risks observed in over a third of the women who were overweight, the percentage of AF cases attributable to an elevated BMI increases to 18.3%. Given the even higher prevalence of obesity and overweight in most Western populations, with current estimates for obesity approaching one third of the population,7 the attributable risk proportion associated with obesity and overweight in the general population is likely even higher.
The present study has several strengths and limitations that warrant consideration. Strengths of the present study include its prospective design, large sample size, updated measures of BMI, and long-term follow-up with a large number of confirmed events. Several limitations should also be considered. First, cases of AF were identified by self report and electrocardiographic screening was not performed in this cohort. Therefore, asymptomatic cases of AF would have been missed if not detected through the participant’s usual medical care. Although the percentage of AF cases that were asymptomatic in this health professional cohort with access to healthcare was similar to that in cohorts employing screening electrocardiograms30, 31, it is likely that a more rigorous electrocardiographic screening method such as ambulatory ECG monitoring may have detected more asymptomatic episodes32. Additionally, due to the sometimes subtle nature of symptoms, AF onset can be difficult to ascertain exactly.
Second, body weight and height, as well as data on all potential confounders, were self-reported, potentially leading to some misclassification; which if non-differential would bias our results towards the null. However high correlations have been demonstrated between self-reported and directly measured weight (r=0.96) in a comparable cohort of female health professionals.33 BMI as a measure of adiposity in general may also misclassify those with high muscle mass, though BMI is highly correlated with absolute fat mass in women.34 Third, the selective nature of the cohort, initially healthy, middle-aged women health professionals primarily of Caucasian origin may limit the generalizability of the findings specifically to men or other non-Caucasian female populations where risk factors for AF may differ. Lastly, we were not able to include echocardiographic measures in our multivariable analysis, as these were not measured systematically in the entire cohort.
Conclusions
BMI is linearly associated with risk of incident AF in this large cohort of women, and the association is stronger among younger women. Only a portion of the obesity-associated AF risk is mediated by inflammation and traditional risk factors. With updated measured of BMI, significant short-term elevations in AF risk persisted in both the overweight and obese ranges after controlling for these potential mediators. An estimated 18.3 percent of the incident AF in this cohort was attributable directly to short term elevations in BMI above the normal range. Taken together, these data suggest that weight control may be a reasonable strategy for reducing the increasing population burden of AF.
Figure 1.
Survival free of atrial fibrillation by BMI category. Shown is an age-adjusted Kaplan Meier survival curve plotting survival free of atrial fibrillation divided into categories of BMI (Normal < 25 kg/m2, Overweight 25–30 kg/m2, and Obese ≥ 30 kg/m2). The log rank test shows statistical significance with a p value of < 0.0001.
ACKNOWLEDGEMENTS
The authors thank Dr. M.V. Moorthy, PhD for his helpful contributions to the statistical analysis. The study was supported by grants from the Harris Family Foundation to Dr. Tedrow and HL-093613 from the NHLBI to Dr. Albert. The Women's Health Study was supported by grants HL-043851 and HL-080467 from the NHLBI and CA-047988 from the NCI (PI, Buring). The Donald W. Reynolds Foundation, Las Vegas, NV funded the biomarker measurements (Dr. Ridker). The funding organizations had no role in the design and conduct of the study or the preparation, review or approval of the manuscript.
ABBREVIATIONS
- AF
atrial fibbrillation
- BMI
body mass index
- WHO
World Health Organization
- CVD
cardiovascular disease
- HR
hazard ratio
- CI
Confidence Interval
- WHS
Women’s Health Study
- hsCRP
high-sensitivity C-reactive protein
- ICAM-1
intercellular adhesion molecule 1
REFERENCES
- 1.Tsang TS, Petty GW, Barnes ME, et al. The prevalence of atrial fibrillation in incident stroke cases and matched population controls in Rochester, Minnesota: changes over three decades. J Am Coll Cardiol. 2003;42(1):93–100. doi: 10.1016/s0735-1097(03)00500-x. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 3.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 4.Waldo AL. A perspective on antiarrhythmic drug therapy to treat atrial fibrillation: there remains an unmet need. Am Heart J. 2006;151(4):771–778. doi: 10.1016/j.ahj.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Cappato R, Calkins H, Chen SA, et al. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2009;53(19):1798–1803. doi: 10.1016/j.jacc.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Sherman DG, Kim SG, Boop BS, et al. Occurrence and characteristics of stroke events in the Atrial Fibrillation Follow-up Investigation of Sinus Rhythm Management (AFFIRM) study. Arch Intern Med. 2005;165(10):1185–1191. doi: 10.1001/archinte.165.10.1185. [DOI] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. Jama. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 8.Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: The Danish Diet, Cancer, and Health Study. The American Journal of Medicine. 2005;118:489–495. doi: 10.1016/j.amjmed.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 9.Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. Jama. 2004;292(20):2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 10.Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity--results of a meta-analysis. Am Heart J. 2008;155(2):310–315. doi: 10.1016/j.ahj.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Gersh BJ, Tsang TS, Barnes M, Seward JB. The changing epidemiology and natural history of nonvalvular atrial fibrillation: the role of novel risk factors. European Heart Journal. 2005;7:C5–C11. [Google Scholar]
- 12.Rexrode KM, Pradhan A, Manson JE, Buring JE, Ridker PM. Relationship of total and abdominal adiposity with CRP and IL-6 in women. Ann Epidemiol. 2003;13(10):674–682. doi: 10.1016/s1047-2797(03)00053-x. [DOI] [PubMed] [Google Scholar]
- 13.Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a Risk Factor for Atrial Fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 14.Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women's Health Study. J Womens Health Gend Based Med. 2000;9(1):19–27. doi: 10.1089/152460900318911. [DOI] [PubMed] [Google Scholar]
- 15.WHO. Obesity: preventing and managing the global epidemic. Geneva, Switzerland: Technical Report Series Number 894; Report on a WHO Consultation on Obesity. 1997 [PubMed]
- 16.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116(19):2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 18.Albert MA, Glynn RJ, Buring JE, Ridker PM. Relation Between Soluble Intercellular Adhesion Molecule- 1, Homocysteine, and Fibrinogen Levels and Race/Ethnicity in Women Without Cardiovascular Disease. Am J Cardiol. 2007;99:1246–1251. doi: 10.1016/j.amjcard.2006.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mora S, Rifai N, Buring JE, Ridker PM. Additive value of immunoassay-measured fibrinogen and high-sensitivity C-reactive protein levels for predicting incident cardiovascular events. Circulation. 2006;114(5):381–387. doi: 10.1161/CIRCULATIONAHA.106.634089. [DOI] [PubMed] [Google Scholar]
- 20.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6(4):356–365. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88(1):15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy NF, MacIntyre K, Stewart S, Hart CL, Hole D, McMurray JJ. Long-term cardiovascular consequences of obesity: 20-year follow-up of more than 15 000 middle-aged men and women (the Renfrew-Paisley study) Eur Heart J. 2006;27(1):96–106. doi: 10.1093/eurheartj/ehi506. [DOI] [PubMed] [Google Scholar]
- 23.Wilhelmsen L, Rosengren A, Lappas G. Hospitalizations for atrial fibrillation in the general male population: morbidity and risk factors. J Intern Med. 2001;250(5):382–389. doi: 10.1046/j.1365-2796.2001.00902.x. [DOI] [PubMed] [Google Scholar]
- 24.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98(5):476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 25.Rosengren A, Hauptman PJ, Lappas G, Olsson L, Wilhelmsen L, Swedberg K. Big men and atrial fibrillation: effects of body size and weight gain on risk of atrial fibrillation in men. Eur Heart J. 2009;30(9):1113–1120. doi: 10.1093/eurheartj/ehp076. [DOI] [PubMed] [Google Scholar]
- 26.Aizer A, Gaziano JM, Cook NR, Manson JE, Buring JE, Albert CM. Relation of vigorous exercise to risk of atrial fibrillation. Am J Cardiol. 2009;103(11):1572–1577. doi: 10.1016/j.amjcard.2009.01.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Risks for atrial fibrillation and congestive heart failure in patients >/=65 years of age with abnormal left ventricular diastolic relaxation. Am J Cardiol. 2004;93(1):54–58. doi: 10.1016/j.amjcard.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Alaud-din A, Meterissian S, Lisbona R, MacLean LD, Forse RA. Assessment of cardiac function in patients who were morbidly obese. Surgery. 1990;108(4):809–818. discussion 18–20. [PubMed] [Google Scholar]
- 29.Tsang TS, Barnes ME, Miyasaka Y, et al. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Heart J. 2008;29(18):2227–2233. doi: 10.1093/eurheartj/ehn324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukamal KJ, Tolstrup JS, Friberg J, Jensen G, Gronbaek M. Alcohol consumption and risk of atrial fibrillation in men and women: the Copenhagen City Heart Study. Circulation. 2005;112(12):1736–1742. doi: 10.1161/CIRCULATIONAHA.105.547844. [DOI] [PubMed] [Google Scholar]
- 31.Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96(7):2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 32.Israel CW, Gronefeld G, Ehrlich JR, Li YG, Hohnloser SH. Long-term risk of recurrent atrial fibrillation as documented by an implantable monitoring device: implications for optimal patient care. J Am Coll Cardiol. 2004;43(1):47–52. doi: 10.1016/j.jacc.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 33.Willett W, Stampfer MJ, Bain C, et al. Cigarette smoking, relative weight, and menopause. Am J Epidemiol. 1983;117(6):651–658. doi: 10.1093/oxfordjournals.aje.a113598. [DOI] [PubMed] [Google Scholar]
- 34.Spiegelman D, Israel RG, Bouchard C, Willett WC. Absolute fat mass, percent body fat, and body-fat distribution: which is the real determinant of blood pressure and serum glucose? Am J Clin Nutr. 1992;55(6):1033–1044. doi: 10.1093/ajcn/55.6.1033. [DOI] [PubMed] [Google Scholar]