Abstract
Endothelium-dependent dilation (EDD) is impaired with aging, but there is significant variability among healthy middle-aged and older adults. We tested the hypothesis that EDD is related to white blood cell (WBC) count in healthy men and women aged 55–75 years (n=48) who have a WBC count within the clinically normal range. The peak forearm blood flow (FBF) response to intra-brachial artery infusion of acetylcholine was inversely related to WBC count (r=−0.38, P=0.004) and was 34% smaller in subjects with higher vs. lower WBC count (>vs.< median of 5.0×109 cells/L, P=0.001). Vascular smooth muscle responsiveness to nitric oxide (NO) (peak FBF response to sodium nitroprusside) was inversely related to WBC count (r=−0.30, P=0.02), but did not fully explain the associations with EDD. Inhibition of NO with NG-monomethyl-L-arginine reduced EDD in subjects with lower (−56%, P=0.01), but not higher WBC count. Tetrahydrobiopterin selectively improved EDD in subjects with higher WBC count (+35%, P=0.01) by increasing NO bioavailability. EDD was related (P<0.05) to neutrophil, eosinophil and monocyte, but not lymphocyte or basophil counts. Myeloperoxidase, which is secreted by neutrophils and monocytes, consumes NO and produces molecules that oxidize tetrahydrobiopterin, was inversely related to EDD (r=−0.35, P=0.02) and was 42% higher in subjects higher WBC count (P=0.02). No other factors contributed to the relation between EDD and WBC count. Among healthy middle-aged and older adults, impaired EDD is related to higher neutrophil, eosinophil and monocyte-based WBC count mediated by reduced responsiveness to NO and increased myeloperoxidase-associated reductions in tetrahydrobiopterin and NO bioavailability.
Keywords: endothelium-dependent dilation, aging, nitric oxide, tetrahydrobiopterin
Because age is the major risk factor for cardiovascular diseases (CVD), middle-aged and older adults are at elevated risk for CVD in the absence of other conventional risk factors.1 Much of this increased risk is related to vascular endothelial dysfunction, a key feature of which is an impaired ability of peripheral arteries to dilate in response to a pharmacological or flow-induced stimulus.1, 2 Endothelial dysfunction, characterized by impaired endothelium-dependent dilation (EDD), is a predictor of future CVD-related events in older adults without clinical disease at baseline.3, 4
EDD varies widely even among healthy middle-aged and older adults.5, 6 However, the factors that explain this inter-individual variability are not well understood. One such factor may be white blood cell (WBC) count. Within the normal clinical range, higher WBC count is associated with increased risk of future CV events.7, 8 Although only limited data are available, WBC count is inversely related to EDD among patients with clinical diseases such as type 2 diabetes and hypertension, and in smokers.9–11 It is unknown if EDD is related to WBC count among non-smoking, unmedicated middle-aged and older adults without chronic disease.
Little is known about the mechanisms that may link WBC count to EDD. In patients with type 2 diabetes, a higher WBC count is associated with a reduced dilation in response to the nitric oxide (NO) donor glyceryl trinitrate.11 This suggests that vascular smooth muscle responsiveness to NO, the major dilating molecule produced by the endothelium, may be reduced in patients with a higher WBC count. Aging generally is associated with reduced vascular NO bioavailability,6 in part as a result of reduced bioactivity of tetrahydrobiopterin,12 an essential co-factor for NO production by endothelial NO synthase.13 It is possible that middle-aged and older adults with higher WBC count may have greater impairments in EDD because of reduced tetrahydrobiopterin-mediated NO production and bioavailability.
Finally, the types of WBCs responsible for an association between total WBC count and EDD is important to establish and may have implications regarding the mechanisms involved. For example, myeloperoxidase is a peroxidase synthesized by neutrophils and monocytes that directly consumes NO and produces reactive oxygen species that oxidize tetrahydrobiopterin, collectively resulting in reduced NO bioavailability.14, 15 Higher circulating concentrations of myeloperoxidase are associated with impaired EDD in patients with rheumatoid arthritis16 and cardiovascular disorders,17 but its relation to WBC count and EDD in middle-aged and older adults without chronic disease is unknown.
In the present study, we tested the hypothesis that EDD is inversely related to WBC count among non-smoking, unmedicated middle-aged and older adults free of chronic disease. To do so, we first examined the relation between WBC count and EDD within an overall sample of healthy adults aged 55–75 years. We then determined if EDD differed in groups of middle-aged and older adults with lower vs. higher WBC count compared to a reference group of young controls. We also determined which types of WBCs were related to EDD and gained insight into the potential mechanisms by which higher WBC count may be associated with impaired EDD.
Methods
Subjects
For the primary sample, data were obtained from 48 men and women aged 55–75 years. The subjects were divided into two equal groups based on the median WBC count (5.0 × 109 cells/L). Reference data for EDD and NO responsiveness were included on a group of healthy young adult controls (18–35 years; n=17, 13M). Subjects were free of clinical CVD, diabetes and other chronic diseases as assessed by medical history, physical examination, blood chemistries, ECG and blood pressure responses to incremental treadmill exercise performed to volitional exhaustion. Subjects were nonsmokers, not regularly exercising, not taking medications and refrained from dietary supplements for 4 weeks prior to the study. No subjects had an abnormally high WBC count (>10.0 ×109 cells/L) that would indicate an acute inflammatory response. All procedures were approved by the Human Research Committee of the University of Colorado at Boulder. The nature, benefits, and risks of the study were explained to the volunteers and their written informed consent was obtained prior to participation.
Procedures
All measurements were performed at the University of Coloradoat Boulder Clinical and Translational Research Center after a 12-hfast and a 24-h abstention from alcohol and physical activity.
Subject characteristics
Arterial blood pressure was measured over the brachial artery during seated rest using a semiautomated device (Dinamap Pro 100, GE Health Care, Waukesha, WI). Fasting plasma metabolic factors were determined by the Clinical and Translational Research Center core laboratory using standard assays. White blood cell count was measured by standard Coulter counter technique (Beckman Coulter Ac·T 5diff CP, Fullerton, CA). ELISA was used to measure serum concentrations of myeloperoxidase (Prognostix, Cleveland, OH), oxidized low density lipoprotein (LDL)(ALPCO, Salem, NH), tumor necrosis factor (TNF) α and interleukin (IL)-6 (R&D Systems, Minneapolis, MN). C-reactive protein was measured using a high-sensitivity Chemistry Immuno Analyzer (AU400e, Olympus America, Center Valley, PA).
Vasodilatory responses
Forearm blood flow (FBF) responses to incremental intra-brachial artery infusion of acetylcholine (1.0, 2.0, 4.0, and 8.0 μg·100 ml/forearm volume/min) (i.e., EDD) and sodium nitroprusside(0.5, 1.0, and 2.0 μg·100 ml/forearm volume/min) were measured in the experimental (nondominant)and the control (dominant) forearm of all subjects using strain-gauge venous occlusion plethysmography (Hokanson, Bellevue, WA) as described previously.18–20 The contribution of NO to the FBF responses to acetylcholine was determined in a subset of subjects (lower WBC count: n=4, 2m/2f; higher WBC count: n=4, 3m/1f) by co-infusing NG-monomethyl-L-arginine (L-NMMA, Clinalfa AG, Bubendorf, Switzerland, 5 mg/min, 10-min loading dose) into the brachial artery during the incremental infusion of acetylcholine. The role of tetrahydrobiopterin bioactivity in the FBF responses to acetylcholine and its NO component was determined in a subset of subjects (lower WBC count: n=11, 4m/7f; higher WBC count: n=12, 5m/7f) by co-infusing tetrahydrobiopterin (Clinalfa AG, 500 μg/min, 10-min loading dose) into the brachial artery during the incremental infusion of acetylcholine in the absence and presence of L-NMMA.
Data analysis
Statistical analyses were performed with SPSS (version 17.0.2; Chicago, IL). Pearson correlation analysis was used to assess bivariate relations of interest and multivariate analysis was used to determine the effect of additional factors on those relations. Differences in subject characteristics were determined by t-test for independent sample comparisons. The FBF responses to incremental doses of acetylcholine and sodium nitroprusside were analyzed by repeated-measures ANOVA. ANCOVA was used to determine the effects of an outside factor on group differences in primary outcome variables. Statistical significance for all analyses was set at P < 0.05. Values are mean ± SE.
Results
Subject Characteristics
Characteristics for the lower and higher WBC count subject groups are presented in Table 1. WBC count was 50% greater in the group with higher WBC count (P<0.001). Values for risk factors were within the normal clinical ranges for both groups. There were no group differences in age, blood pressure, or plasma lipids. The group with higher WBC count had higher fasting blood glucose (P=0.004). Circulating concentrations of C-reactive protein tended to be greater (P=0.06) in the subjects with higher WBC count, whereas IL-6, TNFα and oxidized LDL were not different in the two groups.
Table 1.
Group Subject Characteristics
| Variable | Lower WBC Count | Higher WBC Count |
|---|---|---|
| N (m/f) | 24 (13/11) | 24 (13/11) |
| White blood cell count, 109 cells/L | 4.1 ± 0.1 | 6.0 ± 0.2 * |
| Age, yr | 63 ± 1 | 63 ± 1 |
| Body mass index, kg/m2 | 26 ± 1 | 28 ± 1 |
| Waist:Hip Ratio | 0.85 ± 0.02 | 0.86 ± 0.02 |
| Systolic blood pressure, mm Hg | 123 ± 3 | 121 ± 2 |
| Diastolic blood pressure, mm Hg | 75 ± 2 | 75 ± 2 |
| Total cholesterol, mg/dl | 210 ± 5 | 198 ± 6 |
| LDL-Cholesterol, mg/dl | 117 ± 5 | 115 ± 5 |
| HDL-Cholesterol, mg/dl | 60 ± 3 | 53 ± 3 |
| Triglycerides, mg/dl | 110 ± 10 | 121 ± 8 |
| Fasting blood glucose, mg/dl | 88 ± 1 | 95 ± 2 * |
| C-reactive protein, mg/L | 1.0 ± 0.3 | 1.7 ± 0.3 |
| Interleukin-6, pg/ml | 1.2 ± 0.2 | 1.4 ± 0.2 |
| Tumor necrosis factor α, pg/ml | 1.5 ± 0.2 | 1.7 ± 0.2 |
| Myeloperoxidase, pmol/L | 327 ± 30 | 465 ± 52 * |
| Oxidized LDL, U/L | 60 ± 3 | 59 ± 3 |
Data are mean±SE.
p<0.05 vs. lower WBC count.
Vasodilatory Responses
EDD
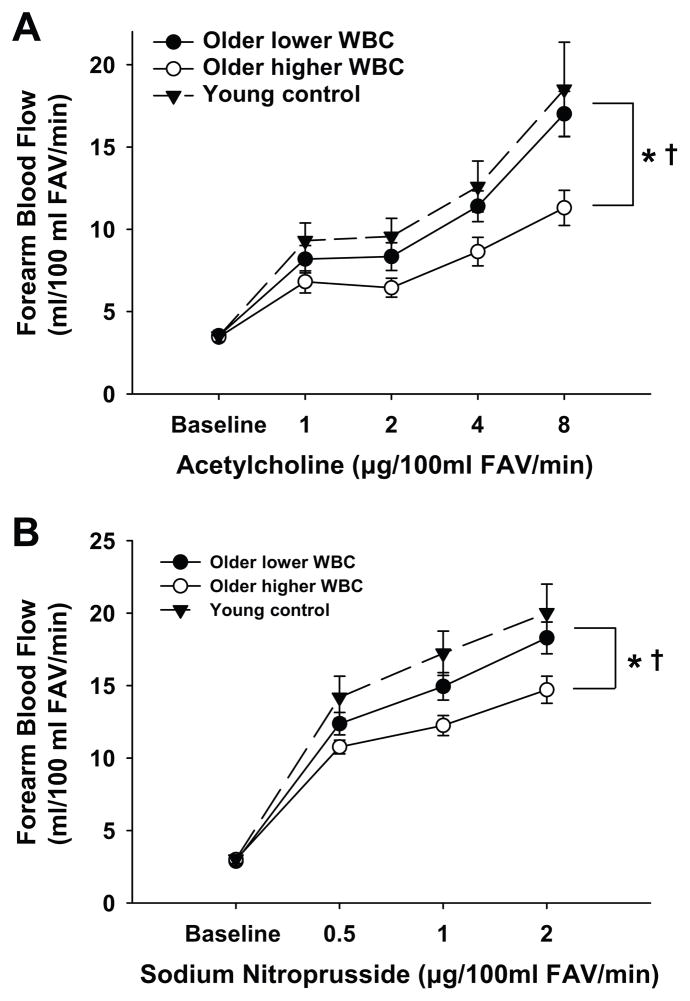
Among all middle-aged and older subjects, the peak FBF response to acetylcholine was inversely related to WBC count (r=−0.38, P=0.004). When subjects were divided into groups based on WBC count, the FBF responses to acetylcholine were smaller in subjects with a higher WBC count, with a peak vasodilatory response 34% less than the group with a lower WBC count (P=0.02, Figure 1A). The FBF responses to acetylcholine in the groups with higher and lower WBC count were smaller (P=0.006) and not different (P=0.55), respectively, compared with young adult controls (age=25±1 yr, WBC count=5.4 × 109 cells/L). Thus, EDD is impaired in middle-aged and older adults with a higher WBC count compared with their peers with a lower WBC count and young adults.
Figure 1. White blood cell (WBC) count, endothelium-dependent dilation (EDD) and nitric oxide (NO) responsiveness.
Middle-aged and older adults (ma/o) with a higher WBC count had (A) impaired EDD, assessed by the peak forearm blood flow (FBF) response to acetylcholine (ACh), and (B) impaired NO responsiveness, assessed by the peak forearm blood flow (FBF) response to sodium nitroprusside (SNP), when compared with ma/o with a lower WBC count and young controls. *P<0.05 vs. lower WBC count group. †P<0.05 vs. young controls. FAV indicates forearm volume.
Sensitivity to NO
Among all subjects, the peak FBF response to sodium nitroprusside was inversely related to WBC count (r=−0.30, P=0.02). Consistent with this relation, subjects with a higher WBC count had smaller FBF responses to sodium nitroprusside, with a peak response 18% less than the group with a lower WBC count (P=0.04, Figure 1B). The FBF responses to sodium nitroprusside in the groups with higher and lower WBC count were smaller (P=0.007) and not different (P=0.36), respectively, compared with the young adult controls. These observations suggest that vascular smooth muscle relaxation and vasodilation in response to NO is reduced in middle-aged and older adults with a higher WBC count.
To determine if differences in vasodilatory responsiveness to NO explained the relation between EDD and WBC count, we performed a multivariate analysis in the overall group. The peak FBF response to sodium nitroprusside contributed to the relation between WBC count and peak FBF response to acetylcholine (P<0.001). However, the WBC count-peak FBF response to acetylcholine association remained significant after adjustment for the peak FBF response to sodium nitroprusside (partial correlation coefficient: r=−0.27, P=0.04). Similar results were obtained by ANCOVA with the group comparisons. These results indicate that reduced sensitivity to NO contributes to, but does not completely explain, the greater impairments in EDD in the subjects with a higher compared with lower WBC count.
Role of NO Bioavailability
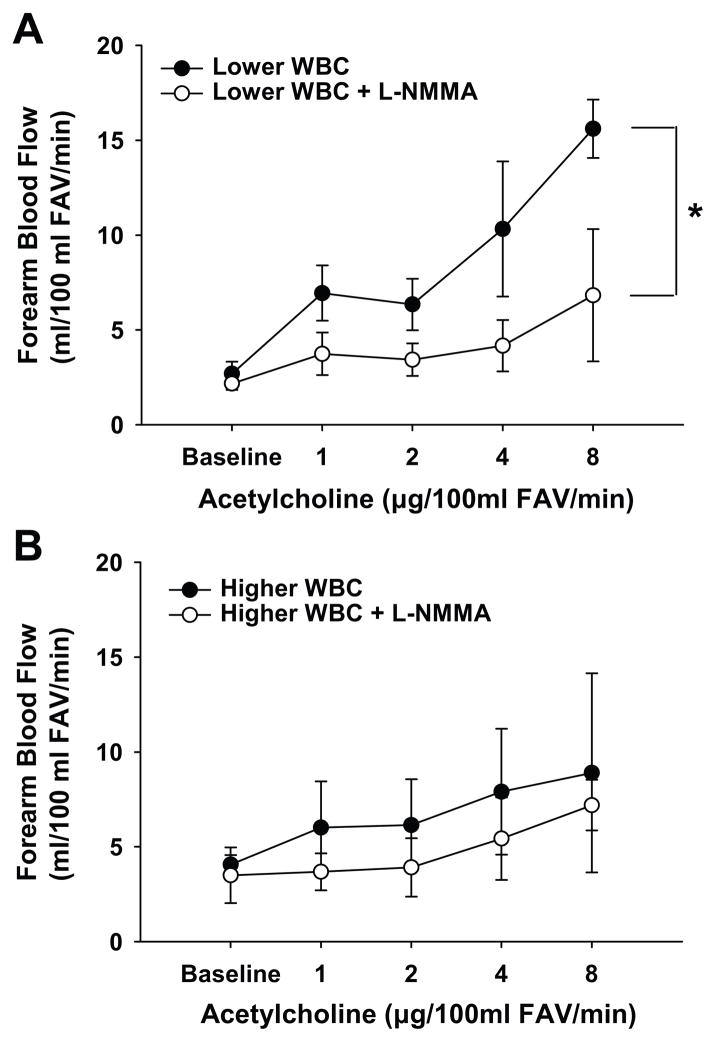
Inhibition of NO production with L-NMMA reduced the FBF response to acetylcholine in subjects with a lower WBC count (P=0.01), but did not significantly affect the response in those with a higher WBC count (P=0.30) (Figure 2). As a result, there were no differences in the FBF responses to acetylcholine between the groups in the absence of NO production (P=0.48). This indicates that the greater impairment in baseline EDD in the subjects with a higher WBC count is mediated by reduced NO bioavailability.
Figure 2. Role of nitric oxide (NO) bioavailability in white blood cell (WBC) count-endothelium-dependent dilation association.
The forearm blood flow response to acetylcholine was reduced with co-infusion of the NO inhibitor NG-monomethyl-L-arginine (L-NMMA) in subjects with a lower WBC count (A), but did not change in subjects with a higher WBC count (B) such that there were no group differences in the absence of NO production. *P<0.05 vs. acetylcholine alone. FAV indicates forearm volume.
Role of Tetrahydrobiopterin
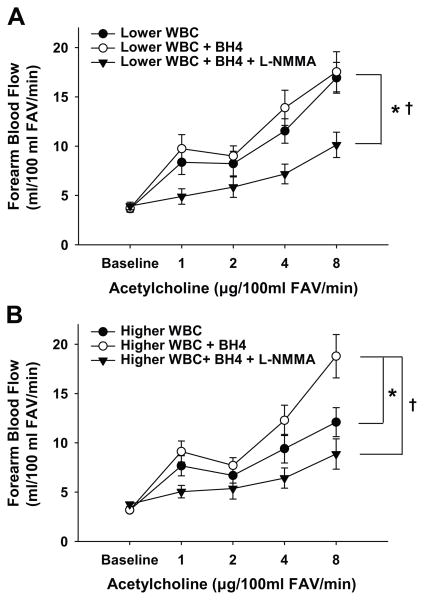
Infusion of tetrahydrobiopterin improved the FBF responses to acetylcholine in subjects with a higher WBC count (P=0.01), but had no effect in the subjects with a lower WBC count (P=0.41, Figure 3). This suggests that the impaired FBF responses to acetylcholine in middle-aged and older adults with a higher WBC count are mediated, at least in part, by reduced vascular bioactivity of tetrahydrobiopterin.
Figure 3. Role of tetrahydrobiopterin (BH4) modulation of nitric oxide (NO) bioavailability in white blood cell (WBC) count-endothelium dependent dilation association.
The forearm blood flow response (FBF) to acetylcholine was enhanced during co-infusion of BH4 in subjects with higher WBC count (B), but did not change for subjects with lower WBC count (A) such that there were no group differences when BH4 was supplemented. Co-infusion with the NO inhibitor NG-monomethyl-L-arginine (L-NMMA) during acetylcholine and BH4 infusion resulted in a decline in FBF response in both groups (A and B) such that there were no group differences in effects of BH4 in the absence of NO production. *P<0.05 vs. acetylcholine alone. †P<0.05 vs. acetylcholine with BH4. FAV indicates forearm volume.
Inhibition of NO production using L-NMMA reduced the FBF responses to co-infusion of acetylcholine and tetrahydrobiopterin in both groups (both P≤0.008, Figure 3), abolishing the vasodilatory-enhancing effects of tetrahydrobiopterin in the subjects with a higher WBC count. These findings provide evidence that increased NO bioavailability was the mechanism for the tetrahydrobiopterin-mediated improvements in EDD in middle-aged and older adults with a higher WBC count.
WBC Subpopulations
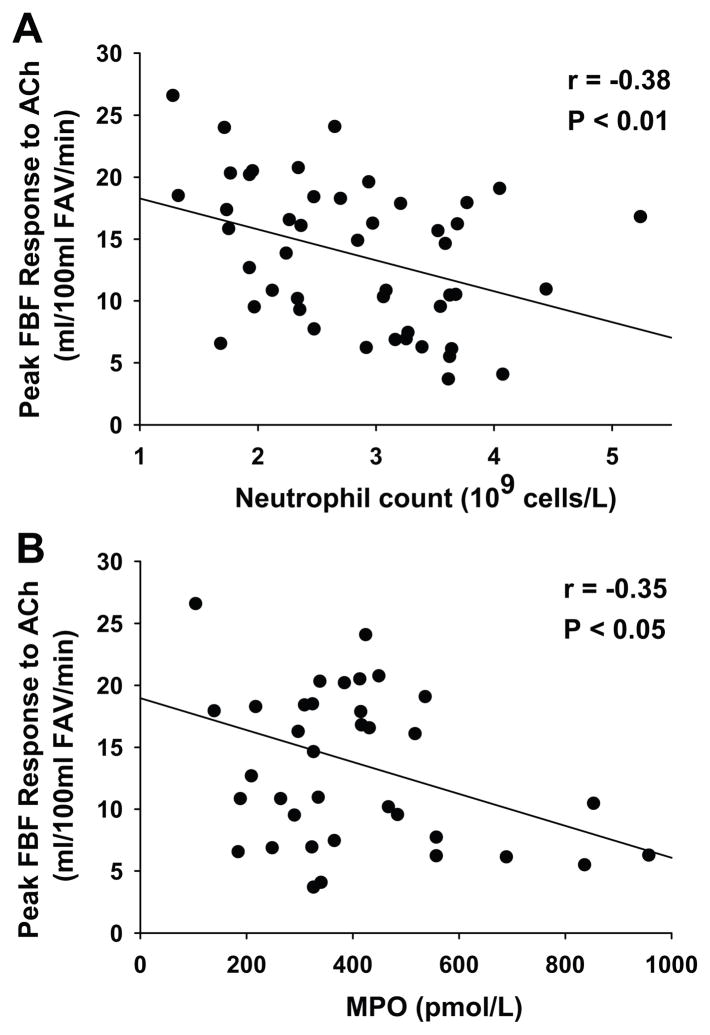
Among all subjects, neutrophil count demonstrated the strongest relation to the peak FBF response to acetylcholine (r=−0.38, P=0.005, Figure 4A). Eosinophil and monocyte count also were related to the peak FBF response to acetylcholine (Table 2). Basophil and lymphocyte count were not related to the peak FBF response to acetylcholine (Table 2). These findings indicate that neutrophils and, to a lesser extent, eosinophils and monocytes, were the subpopulations of WBCs that contributed most to the relation between EDD and WBC count.
Figure 4. Neutrophil count, myeloperoxidase and endothelium-dependent dilation (EDD).
EDD assessed by the peak forearm blood flow (FBF) response to acetylcholine (ACh) was inversely related to (A) neutrophil count and (B) serum myeloperoxidase concentrations. FAV indicates forearm volume.
Table 2.
Associations Between Types of WBCs and EDD
| White blood cell Differential Count (109 cells/L) | Peak FBF Response to ACh (ml/100ml FAV/min) | |
|---|---|---|
| r | P | |
| Neutrophil | −0.38 | <0.01 |
| Eosinophil | −0.30 | 0.02 |
| Monocyte | −0.27 | 0.03 |
| Basophil | −0.20 | 0.09 |
| Lymphocyte | −0.15 | 0.15 |
FBF, forearm blood flow; ACh, acetylcholine; FAV, forearm volume.
Role of Myeloperoxidase
Serum myeloperoxidase was 42% greater in the subjects with a higher WBC count (P=0.01) and was inversely related to the peak FBF response to acetylcholine in the overall group (r=−0.35, P=0.02, Figure 4B). Multivariate analysis indicated that myeloperoxidase contributed to the relation between WBC count and peak FBF response to acetylcholine (P=0.05), but WBC count remained a significant predictor of the peak acetylcholine response after adjustment for myeloperoxidase (partial correlation coefficient: r=−0.33, P=0.02). Similar results were obtained with ANCOVA in the group comparisons. These results suggest that myeloperoxidase contributes to, but does not completely explain, the relation between WBC count and EDD.
Relations to Other Factors
The peak FBF response to acetylcholine was inversely related to plasma C-reactive protein (r=−0.34, P=0.02). However, accounting for C-reactive protein with multivariate analysis did not influence the relation between the peak FBF response to acetylcholine and WBC count (partial correlation coefficient: r=0.39, P=0.005). These findings indicate that the greater impairment in EDD in the subjects with higher WBC count was not related to their greater C-reactive protein concentrations.
Peak FBF responses to acetylcholine were not related to measures of body fatness, blood pressure, plasma lipids, fasting blood glucose levels or serum concentrations of IL-6, TNFα or oxidized LDL. Collectively, these results indicate that WBC count was the best predictor of EDD among all subject characteristics and circulating factors.
Other Relations to WBC Count
WBC count was related to myeloperoxidase (r=0.33, P=0.02), C-reactive protein (r=0.32, P=0.02) and fasting blood glucose (r=0.45, P=0.001).
Discussion
Our results provide evidence that acetylcholine-induced EDD, a common expression of vascular endothelial function and predictor of future CVD risk, is inversely related to WBC count among non-smoking, unmedicated middle-aged and older men and women without clinical disease. Within the clinically normal range of WBC count, a group with higher WBC concentrations had impaired EDD compared with their peers with a lower WBC count and young adults. WBC count was a stronger predictor of EDD than other subject characteristics and circulating factors, including other markers of inflammation such as serum C-reactive protein. The inverse relation between WBC count and EDD observed among middle-aged and older adults is consistent with and extends previous observations in patients with diabetes and hypertension and in smokers.9–11 We also determined that this overall relation with WBC count was due to significant associations between EDD and neutrophil (strongest), eosinophil and monocyte counts. Finally, our findings indicate that the mechanisms contributing to the WBC count-EDD relation include impaired vascular smooth muscle responsiveness to NO and tetrahydrobiopterin-linked reductions in NO bioavailability associated with increases in circulating myeloperoxidase.
Responsiveness to NO
We found that vasodilation to the NO donor sodium nitroprusside was impaired in subjects with a higher WBC count, consistent with previous findings in patients with diabetes.11 A reduced vasodilatory response to sodium nitroprusside reflects a decrease in NO signaling in vascular smooth muscle cells, most likely as a result of impaired cyclic GMP signaling.21 However, in the present study vasodilatory responsiveness to sodium nitroprusside did not fully explain the relation between EDD and WBC count among individuals or between groups, suggesting that other mechanisms are involved.
NO and Tetrahydrobiopterin Bioavailability
In the present study, the greater impairment in EDD in subjects with higher WBC count was mediated by reduced NO bioavailability because inhibition of NO production using L-NMMA abolished baseline group differences in EDD.
One factor contributing to NO production is tetrahydrobiopterin, an essential cofactor for NO synthase. Infusion of tetrahydrobiopterin improves EDD on average in middle-aged and older adults,12 suggesting that reduced bioactivity of tetrahydrobiopterin contributes to age-associated reductions in EDD, at least in some individuals. In the present study, infusion of tetrahydrobiopterin increased EDD in the group with a higher WBC count, but had no effect in the group with a lower WBC count. This indicates that among healthy non-smoking middle-aged and older adults, a higher WBC count is associated with reduced vascular tetrahydrobiopterin bioactivity.
Reduced tetrahydrobiopterin bioactivity should limit production of NO by the vascular endothelium via “uncoupling” of endothelial NO synthase,22 with a consequent reduction in NO bioavailability. Consistent with this idea, we found that co-infusion of L-NMMA abolished the selective tetrahydrobiopterin-associated improvement in EDD in the subjects with a higher WBC count, resulting in similar responses in the two groups. These data support the concept that the greater impairment in EDD in middle-aged and older adults with a higher WBC count is mediated by reduced tetrahydrobiopterin bioactivity-dependent decreases in NO bioavailability.
Because tetrahydrobiopterin restored EDD in the subjects with a higher WBC count, and reduced NO responsiveness was found to contribute to impaired EDD in these subjects, it is possible that tetrahydrobiopterin restored EDD in part by increasing responsiveness to NO. We did not determine the effects of tetrahydrobiopterin on the FBF responses to sodium nitrioprusside in the present study and, therefore, are unable to provide direct insight into this possibility.
Types of WBCs Involved
Our results indicate that the inverse relation between EDD and total WBC count was due to significant inverse relations between EDD and neutrophils, eosinophils and monocytes. Among these cell types, the strongest relation to EDD was with neutrophils, a cell population that is a strong predictor of future CV events.23–25 In contrast, EDD was not related to basophils or lymphocytes. The weakest relation to EDD was with lymphocytes, which, among WBC types, are the weakest predictors of CVD risk.23, 24
Myeloperoxidase
Because neutrophils produce the majority of circulating myeloperoxidase26 and serum myeloperoxidase is a predictor of EDD in patients with clinical disease,16, 17 myeloperoxidase concentrations and their relation to EDD were assessed in the present study. We found that serum myeloperoxidase was inversely related to EDD in our overall sample and contributed to the relation between EDD and WBC count. Consistent with this, serum myeloperoxidase concentrations were significantly greater in the subjects with higher WBC count. Myeloperoxidase reduces NO bioavailability by a number of mechanisms including direct consumption of NO and production of reactive oxygen species that oxidize tetrahydrobiopterin to its inactive form, which, in turn, uncouples endothelial NO synthase.14, 15 As such, it is possible that the greater serum myeloperoxidase in subjects with a higher WBC count contributed to their impaired EDD by reducing NO bioavailability via increased consumption of NO and decreased tetrahydrobiopterin bioactivity and NO production.
Circulating Inflammatory Proteins
We found that C-reactive protein, an acute phase protein and most commonly used clinical marker of systemic inflammation, was greater in subjects with a higher WBC count, with concentrations corresponding to a moderately increased risk of CVD.27 In contrast, serum concentrations of the cytokines IL-6 and TNFα did not differ between groups. However, controlling for C-reactive protein concentration did not alter the relation between EDD and WBC count among individuals. Thus, markers of systemic inflammation do not obviously explain the relation between EDD and WBC count in present study.
Local Interactions
The influence of WBC count on EDD may be mediated in part by local interactions with the vascular wall. WBCs are immune cells that constantly interact with the endothelial cell layer via rolling, adhesion and infiltration into the vascular wall.28, 29 Upon interacting with the vascular wall, WBCs can produce and release reactive oxygen species and cytokines, which could, in turn, influence gene and protein expression, intra-cellular signaling and vasodilatory responsiveness.30 Thus, the modulatory influence of WBC count on EDD in middle-aged and older adults may be, in part, the result of physical or chemical interactions with the vascular endothelium.
Perspectives
Our results demonstrate that EDD is inversely related to WBC count among non-smoking middle-aged and older adults without clinical disease. Thus, WBC count appears to be a key factor that influences EDD and contributes to its variability in this group. Importantly, our findings show that the mechanisms linking WBC count to EDD in these subjects involve decreased vascular smooth muscle sensitivity to NO and tetrahydrobiopterin-associated reductions in NO bioavailability. The relation between EDD and WBC count is due to inverse relations between EDD and selective populations of WBCs, with neutrophils having the strongest association. Increased myeloperoxidase produced by neutrophils could be an important mechanism for reduced tetrahydrobiopterin bioactivity and NO bioavailability. Indeed, WBC count and serum myeloperoxidase were more strongly related to EDD than any other subject characteristic or circulating factor in the present study. Overall, our findings may have important clinical implications for identifying and treating middle-aged and older adults who are at greater risk for vascular endothelial dysfunction and CV events.
Acknowledgments
We would like to thank Kristen Jablonski, Thomas LaRocca, and Brooke Lawson for technical assistance.
Sources of Funding
This work was supported by National Institutes of Health awards AG031617, AG006537, AG013038, AG015897, AG022241, AG000279, and RR00051 and American Heart Association 0715735Z.
Footnotes
Conflicts of Interest: None
References
- 1.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 2.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol. 2008;51:997–1002. doi: 10.1016/j.jacc.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 4.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 5.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 6.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 7.Margolis KL, Manson JE, Greenland P, Rodabough RJ, Bray PF, Safford M, Grimm RH, Jr, Howard BV, Assaf AR, Prentice R. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: the Women’s Health Initiative Observational Study. Arch Intern Med. 2005;165:500–508. doi: 10.1001/archinte.165.5.500. [DOI] [PubMed] [Google Scholar]
- 8.Grimm RH, Jr, Neaton JD, Ludwig W. Prognostic importance of the white blood cell count for coronary, cancer, and all-cause mortality. JAMA. 1985;254:1932–1937. [PubMed] [Google Scholar]
- 9.Elkind MS, Sciacca RR, Boden-Albala B, Tondella ML, Feikin DR, Fields BS, Sacco RL, Di Tullio MR, Homma S. Leukocyte count is associated with reduced endothelial reactivity. Atherosclerosis. 2005;181:329–338. doi: 10.1016/j.atherosclerosis.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Lavi S, Prasad A, Yang EH, Mathew V, Simari RD, Rihal CS, Lerman LO, Lerman A. Smoking is associated with epicardial coronary endothelial dysfunction and elevated white blood cell count in patients with chest pain and early coronary artery disease. Circulation. 2007;115:2621–2627. doi: 10.1161/CIRCULATIONAHA.106.641654. [DOI] [PubMed] [Google Scholar]
- 11.Woodman RJ, Watts GF, Puddey IB, Burke V, Mori TA, Hodgson JM, Beilin LJ. Leukocyte count and vascular function in Type 2 diabetic subjects with treated hypertension. Atherosclerosis. 2002;163:175–181. doi: 10.1016/s0021-9150(01)00770-5. [DOI] [PubMed] [Google Scholar]
- 12.Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Hara K, Jitsuiki D, Goto C, Oshima T, Chayama K, Yoshizumi M. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis. 2006;186:390–395. doi: 10.1016/j.atherosclerosis.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt K, Werner ER, Mayer B, Wachter H, Kukovetz WR. Tetrahydrobiopterin-dependent formation of endothelium-derived relaxing factor (nitric oxide) in aortic endothelial cells. Biochem J. 1992;281 ( Pt 2):297–300. doi: 10.1042/bj2810297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abu-Soud HM, Hazen SL. Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem. 2000;275:37524–37532. doi: 10.1074/jbc.275.48.37524. [DOI] [PubMed] [Google Scholar]
- 15.Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A, Castro L, Lusis AJ, Nauseef WM, White CR, Freeman BA. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–2394. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- 16.Maki-Petaja KM, Cheriyan J, Booth AD, Hall FC, Brown J, Wallace SM, Ashby MJ, McEniery CM, Wilkinson IB. Inducible nitric oxide synthase activity is increased in patients with rheumatoid arthritis and contributes to endothelial dysfunction. Int J Cardiol. 2008;129:399–405. doi: 10.1016/j.ijcard.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Vita JA, Brennan ML, Gokce N, Mann SA, Goormastic M, Shishehbor MH, Penn MS, Keaney JF, Jr, Hazen SL. Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation. 2004;110:1134–1139. doi: 10.1161/01.CIR.0000140262.20831.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donato AJ, Eskurza I, Jablonski KL, Gano LB, Pierce GL, Seals DR. Cytochrome P-450 2C9 signaling does not contribute to age-associated vascular endothelial dysfunction in humans. J Appl Physiol. 2008;105:1359–1363. doi: 10.1152/japplphysiol.90629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 20.Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension. 2008;52:72–79. doi: 10.1161/HYPERTENSIONAHA.108.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munzel T, Daiber A, Ullrich V, Mulsch A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler Thromb Vasc Biol. 2005;25:1551–1557. doi: 10.1161/01.ATV.0000168896.64927.bb. [DOI] [PubMed] [Google Scholar]
- 22.Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun. 1999;263:681–684. doi: 10.1006/bbrc.1999.1422. [DOI] [PubMed] [Google Scholar]
- 23.Prentice RL, Szatrowski TP, Fujikura T, Kato H, Mason MW, Hamilton HH. Leukocyte counts and coronary heart disease in a Japanese cohort. Am J Epidemiol. 1982;116:496–509. doi: 10.1093/oxfordjournals.aje.a113434. [DOI] [PubMed] [Google Scholar]
- 24.Sweetnam PM, Thomas HF, Yarnell JW, Baker IA, Elwood PC. Total and differential leukocyte counts as predictors of ischemic heart disease: the Caerphilly and Speedwell studies. Am J Epidemiol. 1997;145:416–421. doi: 10.1093/oxfordjournals.aje.a009123. [DOI] [PubMed] [Google Scholar]
- 25.Wheeler JG, Mussolino ME, Gillum RF, Danesh J. Associations between differential leucocyte count and incident coronary heart disease: 1764 incident cases from seven prospective studies of 30,374 individuals. Eur Heart J. 2004;25:1287–1292. doi: 10.1016/j.ehj.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Schultz J, Kaminker K. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch Biochem Biophys. 1962;96:465–467. doi: 10.1016/0003-9861(62)90321-1. [DOI] [PubMed] [Google Scholar]
- 27.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 28.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 29.Petri B, Phillipson M, Kubes P. The physiology of leukocyte recruitment: an in vivo perspective. J Immunol. 2008;180:6439–6446. doi: 10.4049/jimmunol.180.10.6439. [DOI] [PubMed] [Google Scholar]
- 30.Chon H, Verhaar MC, Koomans HA, Joles JA, Braam B. Role of circulating karyocytes in the initiation and progression of atherosclerosis. Hypertension. 2006;47:803–810. doi: 10.1161/01.HYP.0000210554.61293.90. [DOI] [PubMed] [Google Scholar]