Abstract
The nuclear receptor TLX (also known as NR2E1) is essential for adult neural stem cell self-renewal; however, the molecular mechanisms involved remain elusive. Here we show that TLX activates the canonical Wnt/β-catenin pathway in adult mouse neural stem cells. Furthermore, we demonstrate that Wnt/β-catenin signalling is important in the proliferation and self-renewal of adult neural stem cells in the presence of epidermal growth factor and fibroblast growth factor. Wnt7a and active β-catenin promote neural stem cell self-renewal, whereas the deletion of Wnt7a or the lentiviral transduction of axin, a β-catenin inhibitor, led to decreased cell proliferation in adult neurogenic areas. Lentiviral transduction of active β-catenin led to increased numbers of type B neural stem cells in the subventricular zone of adult brains, whereas deletion of Wnt7a or TLX resulted in decreased numbers of neural stem cells retaining bromodeoxyuridine label in the adult brain. Both Wnt7a and active β-catenin significantly rescued a TLX (also known as Nr2e1) short interfering RNA-induced deficiency in neural stem cell proliferation. Lentiviral transduction of an active β-catenin increased cell proliferation in neurogenic areas of TLX-null adult brains markedly. These results strongly support the hypothesis that TLX acts through the Wnt/β-catenin pathway to regulate neural stem cell proliferation and self-renewal. Moreover, this study suggests that neural stem cells can promote their own self-renewal by secreting signalling molecules that act in an autocrine/paracrine mode.
The finding of neurogenesis in the adult brain led to the discovery of adult neural stem cells. Neural stem cells are defined as a subset of undifferentiated precursors that retain the ability to proliferate and self-renew and have the capacity to give rise to both neuronal and glial lineages1–4. Under normal conditions, neurogenesis in the adult mammalian brain is restricted to two discrete germinal centres: the subgranular layer of the hippocampal dentate gyrus3 and the subventricular zones of the lateral ventricles5,6. A complete understanding of adult neural stem cells requires the identification of molecules that determine the self-renewal and multipotent characteristics of these cells.
TLX is an orphan nuclear receptor that is expressed in vertebrate forebrains7,8. We showed previously that TLX is an important regulator of neural stem cell maintenance and self-renewal in both embryonic and adult brains9,10. TLX maintains neural stem cells in the undifferentiated and self-renewable state by complexing with histone deacetylases to repress downstream target genes11 and forms a negative regulatory loop with the microRNA miR-9 (ref. 12). The feedback loop with miR-9 provides a strategy for controlling the balance between neural stem cell proliferation and differentiation12. The TLX-positive neural stem cells in the dentate gyrus are important in spatial learning and memory13, whereas the TLX-positive cells in the subventricular zones were identified as the slowly dividing type B neural stem cells14.
Wnt proteins represent a growing family of secreted signalling molecules that perform multiple functions15–17. In canonical Wnt signalling pathways, binding of Wnts to their receptors inhibits the phosphorylation of β-catenin by glycogen synthase kinase 3β (GSK3β). This inhibition prevents β-catenin degradation, resulting in its accumulation in the cytosol18. β-Catenin translocates to the nucleus, where it binds to the lymphoid enhancer binding factor (LEF)/T-cell transcription factor (TCF) family of transcription factors19 to induce expression of their target genes, such as cyclin D1 (refs 20–22). Wnts have been shown to regulate the self-renewal of multiple types of stem cell, including hematopoietic stem cells23,24 and epidermal/gut progenitors25,26. The role of Wnt signalling in progenitor cell expansion in the developing nervous system has also been studied extensively27–32. Recently, the role of Wnt signalling in neural stem cell proliferation in adult brains has begun to be identified33.
We show here that TLX activates the Wnt/β-catenin pathway in adult mouse neural stem cells to stimulate neural stem cell proliferation and self-renewal. Both Wnt7a and an active β-catenin rescued TLX short interfering RNA (siRNA)-induced deficiency in neural stem cell proliferation. Intracranial transduction of an active β-catenin increased cell proliferation in the neurogenic area of TLX−/− adult brains significantly. These results strongly indicate that TLX regulates neural stem cell proliferation and self-renewal through activation of the Wnt/β-catenin pathway in adult brains.
RESULTS
TLX activates Wnt/β-catenin signalling
We have shown that TLX is essential for maintenance of the undifferentiated and self-renewable state of neural stem cells in the adult brain9. To identify downstream target genes of TLX, a gene profiling analysis was performed with RNAs isolated from adult brains of wild-type and TLX-null mice. Among genes with altered expression levels, a significant downregulation of Wnt7a expression was detected in adult TLX mutant brains (Fig. 1a and Supplementary Information, Fig. S1). Neural stem cells were isolated from TLX+/− forebrains containing the hippocampal dentate gyrus and subventricular zones and cultured in N2-supplemented DMEM F12 medium containing epidermal growth factor (EGF) and fibroblast growth factor (FGF), as described9. Wnt7a was highly expressed in proliferating neural stem cells, and its expression was decreased markedly on differentiation, in a similar manner to the expression profile of TLX (Fig. 1a). Co-expression of TLX and Wnt7a in neural stem cells was confirmed by immunofluorescence analyses, showing nuclear staining of TLX and cytoplasmic/membrane staining of Wnt7a (Fig. 1b). These results suggest that TLX could be an activator of Wnt7a.
Figure 1.
Expression of TLX and Wnt7a in adult TLX-null brains and adult neural stem cells. (a) Northern blot analysis of Wnt7a expression in wild-type (+/+), TLX-heterozygote (+/−), and TLX-null (−/−) adult brains, or in adult mouse neural stem cells (NSCs) cultured under proliferation (P) or differentiation (D) conditions. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was included as a loading control. (b) Immunofluorescence analysis of TLX (green) and Wnt7a (red) in adult mouse neural stem cells. Scale bar, 20 μm. (c) Binding of TLX to Wnt7a promoter in gel shift assays. 32P-labelled DNA oligonucleotides containing the consensus TLX binding sites in the Wnt7a promoter were used as probe. TLX–DNA complexes and free probes are indicated. n.s., non-specific binding; C, control lysates. (d) Binding of TLX to Wnt7a promoter in chromatin immunoprecipitation analysis. DNA input was included in lane 1. Control IgG was in lane 2 and TLX antibody was used in lane 3. (e) TLX activates Wnt7a in reporter assays. Luciferase reporter gene (wnt–luc) was placed downstream of the wild-type (WT) or mutant (Mut) Wnt7a promoter. Luciferase activity in cells transfected with control vector (−TLX) or TLX-expressing vector (+TLX) was measured and normalized with β-galactosidase activity for transfection efficiency. Error bars show s.d.; assays were performed in triplicate. Uncropped images of blots in (a) and (d) are shown in Supplementary Information, Fig. S5.
Direct regulation of Wnt7a by TLX was suggested by a sequence analysis of the Wnt7a promoter, which revealed the presence of two consensus TLX binding sites (5′-AAGTCA-3′) with a six-nucleotide spacer (5′-AACTCA-3′) (−587 to −570). Gel shift and chromatin immunoprecipitation analyses revealed binding of TLX to the consensus sites in the Wnt7a promoter (Fig. 1c, d). To investigate whether TLX activates Wnt7a transcription, reporter assays were performed with a luciferase reporter gene linked to a 1.85-kilobase Wnt7a promoter (−1700 to +150) containing the consensus TLX binding sites. Transfection of TLX led to a 2.5-fold activation of the Wnt7a promoter, and mutation of the consensus TLX binding sites abolished the activation (Fig. 1e). These results demonstrate that TLX functions as a transcriptional activator of Wnt7a through direct binding to its promoter.
TLX represses transcription when bound to the p21 promoter, which contains a monomeric TLX binding site, but activates transcription when bound to the Wnt7a promoter, which has two adjacent TLX binding sites (Supplementary Information, Fig. S2). Exchanging the monomeric TLX response element from the p21 promoter with the sites contained in the Wnt7a promoter reversed the effect of TLX from a repressor to an activator of p21, whereas replacing the TLX response elements from the Wnt7a promoter with that from the p21 promoter turned TLX from an activator to a repressor (Supplementary Information, Fig. S2). These results suggest that the TLX response elements may dictate TLX transcriptional activity.
In the canonical Wnt signalling pathway, Wnt stabilizes β-catenin and activates its downstream target genes. To determine whether TLX regulates Wnt/β-catenin signalling, a β-catenin-responsive luciferase reporter Topflash was co-transfected with β-catenin or TLX, or both, into CV-1 cells. Co-transfection of TLX and β-catenin led to synergistic activation of the Topflash reporter (Fig. 2a), indicating that TLX potentiates β-catenin-responsive gene expression. To determine whether Wnt7a induction is necessary for TLX-mediated potentiation of the β-catenin reporter, we designed Wnt7a-specific siRNAs. Among the three siRNAs tested, Wnt7a siRNAs 1 and 3 had marked knockdown effect (Fig. 2b) and were used for subsequent studies. Treatment with Wnt7a siRNAs 1 and 3 led to a significant decrease in Topflash reporter activity (2.9-fold) in cells co-transfected with TLX and β-catenin (Fig. 2a). A decrease in reporter activity by Wnt7a siRNAs was also observed in β-catenin-transfected cells (1.7-fold), but this was not as marked as the decrease in activity in cells co-transfected with TLX and β-catenin. These results indicate that Wnt7a induction is important for the synergistic activation of Topflash by TLX and β-catenin.
Figure 2.
TLX activates Wnt/β-catenin/cyclin D1. (a) TLX potentiates a β-catenin-responsive reporter gene. The β-catenin-responsive reporter gene Topflash was transfected into CV-1 cells along with β-catenin or TLX, or both, in the presence of a control siRNA (C) or Wnt7a siRNAs (siWnt7a-1 and siWnt7a-3). Reporter luciferase activity was measured and normalized with β-galactosidase activity. Error bars show s.d.; assays were performed in triplicate. (b) Knockdown of Wnt7a expression with Wnt7a-specific siRNAs as revealed by luciferase assays. Wnt7a-specific siRNAs 1, 2 and 3 (si1, si2 and si3) were co-transfected with a luciferase reporter gene upstream of Wnt7a cDNA. The knockdown effect was determined by relative luciferase activity. GFP siRNA was included as a control (C). Error bars show s.d.; assays were performed in triplicate. Asterisk, P < 0.005 by Student’s t-test. (c) Expression of Wnt7a and cyclin D1 in TLX siRNA (siTLX)-treated adult mouse neural stem cells as revealed by RT–PCR analysis. Actin was included as a loading control. (d) Expression of Wnt7a and cyclin D1 in TLX-expressing 3T3 (3T3-TLX) cells and parental 3T3 cells as revealed by northern blot analysis. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was included as a loading control. (e) Expression of TLX and cytosolic β-catenin in 3T3-TLX and parental 3T3 cells demonstrated by western blot analysis. Uncropped images of blots in (c)–(e) are shown in Supplementary Information, Fig. S5.
Cyclin D1 is a well-characterized β-catenin downstream target gene that is activated by β-catenin–TCF–LEF complexes. To determine further whether TLX activates Wnt/β-catenin signalling, we examined whether TLX regulates cyclin D1 expression. siRNA knockdown of TLX in neural stem cells led to significantly decreased expression of both Wnt7a and cyclin D1 (Fig. 2c), whereas ectopic expression of TLX in 3T3 cells induced both Wnt7a and cyclin D1 expression (Fig. 2d), in parallel with an increased accumulation of β-catenin protein levels in the cytosol (Fig. 2e). These results indicate that TLX activates cyclin D1 expression, presumably through activation of Wnt7a/β-catenin.
Overexpression of TLX enhanced Wnt7a expression in neural stem cells and promoted neural stem cell proliferation (Supplementary Information, Fig. S3). Treatment with Wnt7a siRNAs knocked down Wnt7a expression and decreased cell proliferation in both control and TLX-overexpressing neural stem cells (Supplementary Information, Fig. S3), suggesting that Wnt7a is important for the effect of TLX on neural stem cell proliferation.
Wnt7a/β-catenin stimulates neural stem cell proliferation and self-renewal
To further determine the role of Wnt7a in neural stem cell proliferation and self-renewal, we generated Wnt7a-overexpressing cells. Wnt7a was expressed as a fusion protein to human IgG in HEK 293 cells (Fig. 3a). Neural stem cells were co-cultured with the Wnt7a-transfected cells in a transwell system34. Both cell proliferation, as revealed by cell numbers (Fig. 3b), and self-renewal, as determined by clonal rate (Fig. 3c), increased significantly in neural stem cells co-cultured with Wnt7a-expressing cells, compared with those co-cultured with control IgG-expressing cells. The clonal derivative of neural stem cells that were co-cultured with Wnt7a-expressing cells remained multipotent, with the ability to differentiate into neurons (Tuj1-positive), astrocytes (glial fibrillary acidic protein (GFAP)-positive), and oligodendrocytes (marker O4-positive) (Fig. 3d). These results suggest that Wnt7a stimulates neural stem cell proliferation and self-renewal in the presence of EGF and FGF.
Figure 3.
Wnt7a and active β-catenin stimulate neural stem cell (NSC) proliferation and self-renewal. (a) Western blot analysis of control IgG and Wnt7a–IgG expression in stable HEK 293 cells with the use of human IgG antibody. (b) Proliferation of low-density adult mouse NSCs during co-culture with control IgG (C) and Wnt7a–IgG (Wnt)-expressing cells was monitored by cell numbers in each input well. Data are means ± s.d.; asterisk, P < 0.01 by Student’s t-test, n = 6. (c) The self-renewal capacity of adult mouse NSCs co-cultured with cells expressing control IgG and Wnt7a–IgG was determined by secondary clonal rate. Data are means ± s.d.; asterisk, P < 0.05 by Student’s t-test, n = 5. (d) Multipotency of the Wnt7a-treated NSCs. Cells were immunostained for a neuronal marker (Tuj1), an astrocyte marker (GFAP) and an oligodendrocyte marker (O4). Scale bar, 100 μm. (e) Expression of β-catenin ΔN90 in transduced adult rat NSCs analysed by western blot analysis with anti-β-catenin antibody. WT, wild-type β-catenin; ΔN90, β-catenin ΔN90. (f) Cell-cycle analysis of control and β-catenin ΔN90-transduced adult rat NSCs. The percentages of cells at G1 phase and at G2/S/M phases are indicated. (g) The self-renewal capacity of adult rat NSCs transduced with control vector (C) or β-catenin ΔN90-expressing virus (ΔN90) was determined by secondary clonal rate. Data are represented as means ± s.d.; asterisk, P < 0.05 by Student’s t-test, n = 5. (h) Representative clonal derivatives of adult rat NSCs transduced with control vector or β-catenin ΔN90-expressing virus. d1, d10: days 1 and 10 of clonal analysis, respectively. Scale bar, 100 μm. (i) Multipotency of β-catenin ΔN90-transduced NSCs. Cells were differentiated and immunostained for Tuj1, GFAP, and O4. 4,6-Diamidino-2-phenylindole (DAPI) staining is shown in blue. The right-hand panel is the merged image. Representative O4-positive cells are shown in the inset. Scale bar, 50 μm. Uncropped images of blots in (a) and (e) are shown in Supplementary Information, Fig. S5.
Neural stem cells were also transduced with a constitutively active β-catenin harbouring a deletion of the first 90 amino acids (ΔN90) containing the GSK3β phosphorylation site to mimic active Wnt signalling35. Expression of the transduced ΔN90 β-catenin was shown by immunoblotting (Fig. 3e). Cell cycle analysis revealed that the active β-catenin promoted neural stem cell proliferation, resulting in an increase in cell population in the S/G2/M phases from 33.8% (control cells) to 49.4% (Fig. 3f). The β-catenin-transduced cells also self-renewed to a greater extent than control vector-transduced cells did, as revealed by an increased clonal rate and an enlarged clonal size (Fig. 3g, h). The clonal derivatives of the β-catenin-transduced cells were multipotent, with the ability to differentiate into both neurons and glia (Fig. 3i). These results suggest that the active β-catenin promotes neural stem cell proliferation and self-renewal.
To test whether Wnt signalling is required for neural stem cell growth, neural stem cells were treated with Frzb, a soluble Wnt antagonist that contains the cysteine-rich domain for Wnt interaction but lacks the transmembrane domain of the Frizzled receptors36,37. Neural stem cells were cultured in Frzb–IgG or control IgG-containing conditioned medium with EGF and FGF. The protein levels of control IgG and Frzb–IgG in the conditioned medium were determined by western blot analysis (Fig. 4a). Treatment of Frzb led to decreased levels of cytosolic β-catenin protein (Fig. 4b) and significant inhibition of neural stem cell growth but had no effect on cell survival (Fig. 4c, d, and Supplementary Information, Fig. S4a, b).
Figure 4.
Inhibition of adult neural stem cell growth by Frzb and Axin. (a) Western blot analysis of control IgG and Frzb–IgG expression in stable HEK 293-cell-conditioned medium. (b) Expression of cytosolic β-catenin in adult mouse neural stem cells (NSCs) treated with control IgG (C) or Frzb–IgG (Frzb), demonstrated by western blot analysis. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was included as a loading control. (c) Proliferation of low-density adult mouse NSCs that were treated with control IgG (C) or Frzb–IgG (Frzb). (d) Self-renewal of adult mouse NSCs that were treated with control IgG (C) or Frzb–IgG (Frzb) is shown by secondary clonal rate. (e) Northern blot analysis of viral axin expression in axin-transduced adult rat NSCs. C, control cells; axin, axin-transduced cells. (f) Expression of cytosolic β-catenin in control vector (C) or axintransduced adult rat NSCs demonstrated by western blot analysis. GAPDH was included as a loading control. (g) Proliferation of low-density adult rat NSCs that were transduced with control vector (C) or axin-expressing virus. (h) Self-renewal of control vector (C) or axin-transduced adult rat NSCs as revealed by secondary clonal rate. Data are represented as means ± s.d.; asterisk, P < 0.01 by Student’s t-test for (c) and (g) (n = 6); two asterisks, P < 0.05 by Student’s t-test for (d) and (h) (n = 5). Uncropped images of blots in (a) and (e) are shown in Supplementary Information, Fig. S5.
In addition to Frzb, Wnt signalling was also inhibited by the expression of axin, an intracellular β-catenin inhibitor38. Neural stem cells were transduced with control vector or axin-expressing retrovirus. The expression of viral axin was shown by northern blot analysis (Fig. 4e). Decreased cytosolic β-catenin protein levels were observed on treatment with axin (Fig. 4f). The axin-transduced cells showed a marked decrease in cell growth and self-renewal with no significant difference in cell survival (Fig. 4g, h, and Supplementary Information, Fig. S4c, d). These results further support the notion that Wnt/β-catenin signalling is required for neural stem cell proliferation.
Wnt7a/β-catenin rescues TLX siRNA-induced proliferation deficiency in neural stem cells
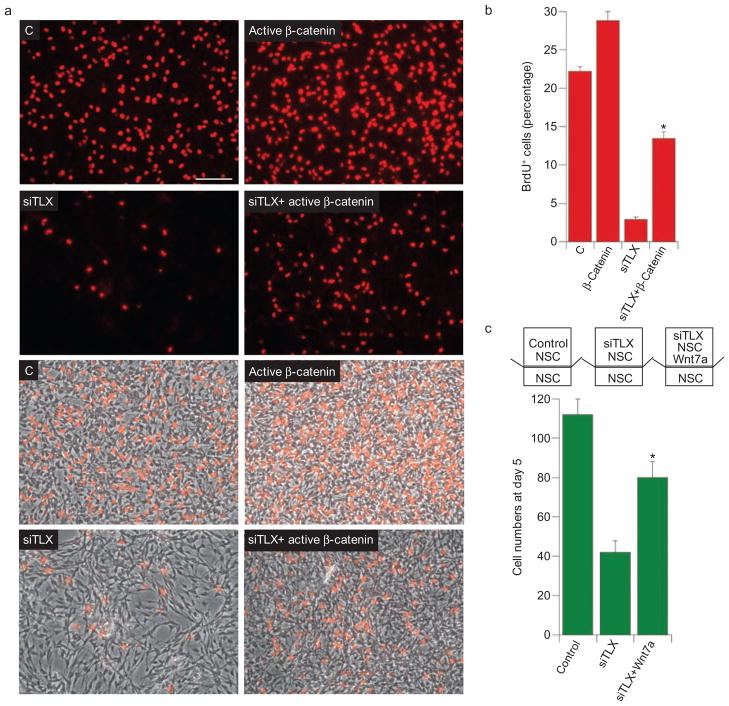
To determine whether Wnt/β-catenin acts downstream of TLX to regulate neural stem cell proliferation and self-renewal, neural stem cells were treated with TLX siRNA-expressing lentivirus, which led to significant inhibition of neural stem cell proliferation. Introduction of the constitutively active β-catenin ΔN90 into TLX siRNA-transduced neural stem cells led to an up to 60% rescue of neural stem cell proliferation in the presence of EGF and FGF (Fig. 5a, b). These results strongly support the hypothesis that TLX acts in part through Wnt/β-catenin to regulate neural stem cell proliferation.
Figure 5.
Constitutively active β-catenin and Wnt7a rescued TLX siRNA-mediated proliferation deficiency in neural stem cells (NSCs). (a) Active β-catenin rescued TLX siRNA-induced NSC proliferation deficiency. Control vector (C), TLX siRNA (siTLX), active β-catenin, or siTLX and β-catenin double-transduced adult mouse NSCs were cultured in N2-supplemented medium containing EGF and FGF for 46 h and pulse-labelled with BrdU for 2 h. Cell proliferation was determined by BrdU labelling (red). Merged images of BrdU labelling and phase contrast images (grey) are shown in the lower panels. Scale bar, 100 μm. (b) Quantification of the percentage of BrdU-positive (BrdU+) cells in total living cells of control vector (C), active β-catenin (β-cat.), TLX siRNA (siTLX), or both TLX siRNA and active β-catenin-transduced NSCs. Data are represented as means ± s.d.; asterisk, P < 0.01 by Student’s t test, (n = 3). (c) Recovery of TLX siRNA-mediated NSC proliferation deficiency by Wnt7a. Untreated adult mouse NSCs were plated at low cell density at the base of transwells and co-cultured with control vector or TLX siRNA-transduced NSCs, or co-cultured with TLX siRNA-transduced NSCs plus Wnt7a–IgG (Wnt7a)-expressing cells that were seeded in the upper chambers of transwells. Cells were cultured in N2-supplemented medium containing EGF and FGF. NSC proliferation was monitored by cell numbers in each input well at the base of transwells on day 5 of co-culture. Data are represented as means ± s.d.; asterisk, P < 0.05 by Student’s t test, (n = 3).
The fact that TLX activates a secreted Wnt molecule suggests that TLX may stimulate neural stem cell proliferation through an autocrine/paracrine mode, in addition to the cell-autonomous regulation. To test this hypothesis, neural stem cells were infected with control vector or TLX siRNA-expressing lentivirus and seeded in the upper chambers of transwells. Non-transduced neural stem cells were plated at low cell densities at the base of transwells. Co-culture with TLX siRNA-transduced cells led to a significantly decreased proliferation of non-transduced neural stem cells, in comparison with co-culture with control vector-transduced cells (Fig. 5c). Addition of Wnt7a-expressing cells to the co-culture resulted in a significant recovery of cell proliferation (Fig. 5c), suggesting that TLX stimulates neural stem cell proliferation in part through activating a secreted Wnt7a molecule.
Regulation of neural stem cell proliferation by Wnt7a and β-catenin in vivo
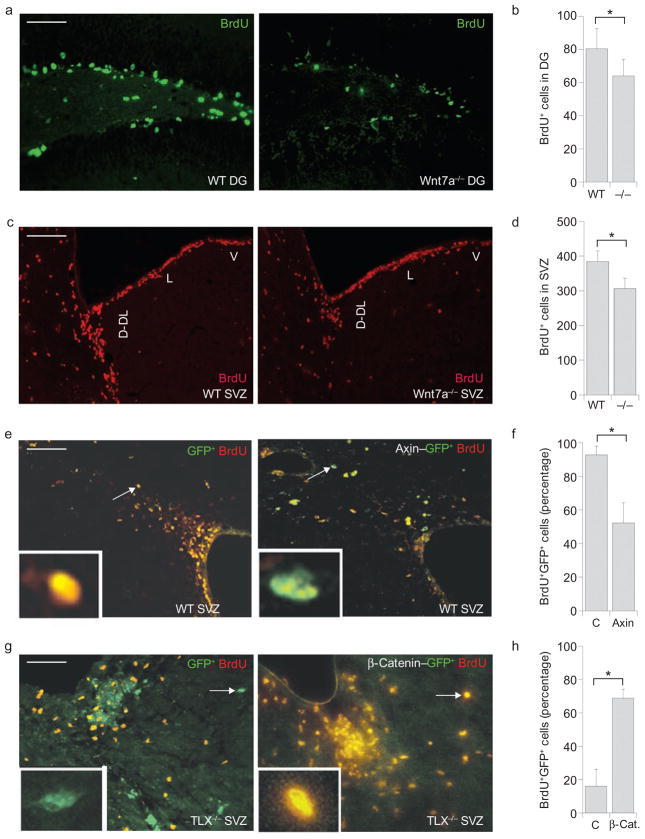
The dentate gyrus of the hippocampus and the subventricular zone are two active adult neurogenic areas. Bromodeoxyuridine (BrdU) labelling analyses in Wnt7a knockout mice39 revealed that loss of Wnt7a expression led to significant decrease in BrdU incorporation in the hippocampal dentate gyrus of adult brains (Fig. 6a, b), suggesting decreased cell proliferation. Decreased BrdU labelling was also observed in the subventricular zone of adult Wnt7a−/− mice (Fig. 6c, d). These results suggest that Wnt7a is important for adult neural stem cell proliferation in vivo.
Figure 6.
Regulation of cell proliferation by Wnt/β-catenin in adult brains. (a) Decreased BrdU staining in the dentate gyrus (DG) of Wnt7a−/− adult brains. Hippocampal DG sections of adult wild-type (WT) and Wnt7a−/− mice were immunostained for BrdU (green). Scale bar, 100 μm for all panels. (b) Quantification of BrdU-positive (BrdU+) cells in hippocampal DG of adult WT and Wnt7a−/− mice. Data are represented as means ± s.d.; asterisk, P < 0.05 by Student’s t-test, n = 12. (c) Decreased BrdU staining in the subventricular zone (SVZ) of Wnt7a−/− adult brains. SVZ sections of adult WT and Wnt7a−/− mice were immunostained for BrdU (red). V, ventral wall; L, lateral wall; D-DL, dorsal and dorsal–lateral region of the lateral ventricle. (d) Quantification of BrdU-positive (BrdU+) cells in SVZ of adult WT and Wnt7a−/− mice. Data are represented as means ± s.d.; asterisk, P < 0.05 by Student’s t-test, n = 3. (e) Decreased BrdU staining of axin-transduced cells in the SVZ of adult brains. SVZ sections from control vector (GFP) or axin (axin–GFP)-transduced and BrdU-treated mice were immunostained for BrdU (red). (f) Quantification of GFP-positive and BrdU-positive (GFP+BrdU+) cells from GFP+ cells in the SVZ of viral-infected mice. Data are represented as means ± s.d.; asterisk, P < 0.05 by Student’s t-test, n = 6. (g) Rescue of cell proliferation in the SVZ of TLX−/− adult brains with the use of active β-catenin ΔN90. SVZ sections from control vector (GFP) or β-catenin ΔN90 (β-catenin–GFP)-transduced and BrdU-treated TLX−/− mice were immunostained for BrdU (red). Rescue of cell proliferation was revealed by the increased percentage of GFP+BrdU+ cells out of total GFP+ cells. The insets are enlarged images of the cells indicated by arrows. (h) Quantification of GFP+BrdU+ cells in the SVZ of virus-transduced TLX−/− brains. Data are represented as means ± s.d.; asterisk, P < 0.05 by Student’s t-test, n = 8 for control GFP-transduced brains, n = 5 for β-catenin–GFP-transduced brains.
Furthermore, the β-catenin inhibitor axin was transduced into the sub-ventricular zone of adult wild-type brains by intracranial lentiviral delivery. Viral expression of axin was monitored by a co-expressed green fluorescent protein (GFP). Transduction of axin led to significantly decreased cell proliferation, as revealed by decreased numbers of GFP+BrdU+ cells compared with that in the control vector-transduced brains (Fig. 6e, f). These results further support the concept that Wnt/β-catenin signalling is important for neural stem cell proliferation in adult brains.
To determine whether Wnt/β-catenin acts downstream of TLX to regulate neural stem cell proliferation in vivo, the active β-catenin ΔN90 was introduced into the subventricular zone of TLX−/− adult brains by stereotaxic lentiviral transduction. Viral expression of β-catenin was monitored by co-expressed GFP. Intracranial injection of β-catenin ΔN90 lentivirus led to considerable rescue of cell proliferation in the subventricular zone of TLX−/− adult brains, as revealed by a marked increase in the percentage of GFP+BrdU+ cells out of total GFP+ cells, compared with that in control GFP-transduced TLX−/− brains (Fig. 6g, h). These results provide strong in vivo evidence that TLX regulates Wnt/β-catenin signalling to control neural stem cell proliferation in the neurogenic areas of adult brains.
GFAP+ type B cells have been proposed to be neural stem cells in the subventricular zone of adult brains40. Next we examined the effect of Wnt signalling on GFAP+ type B cells by lentiviral transduction of the active β-catenin ΔN90 into the subventricular zone of wild-type adult brains. Viral expression of the active β-catenin was monitored by co-expressed GFP. Transduction of β-catenin ΔN90 lentivirus led to a significant increase in the percentage of GFAP+GFP+ cells out of total GFP+ cells in the subventricular zone, compared with that in control GFP-transduced brains (Fig. 7a, b). These results suggest that Wnt/β-catenin signalling acts on type B neural stem cells in the subventricular zone of adult brains, in addition to its effect on type C cells33.
Figure 7.
TLX–Wnt/β-catenin signalling regulates neural stem cell population in the adult brain. (a) Lentiviral tranduction of active β-catenin increases the numbers of GFAP+ B cells in the SVZ of wild-type (WT) brains. Brains were injected with control GFP lentivirus (GFP) or β-catenin ΔN90–GFP virus (β-catenin–GFP). Scale bar, 10 μm. (b) Quantification of the percentage of GFAP+GFP+ cells out of total GFP+ cells in control GFP (C) and β-catenin–GFP (β-cat.)-transduced SVZ. Data are represented as means ± s.d.; asterisk, P < 0.05 by Student’s t-test, n = 10. (c) Decrease in number of BrdU-label-retaining cells in the SVZ of Wnt7a−/− brains. BrdU-label-retaining cells were labelled in green. S100β staining (red) is included to show the structure of the SVZ. Scale bar, 20 μm. (d) Quantification of the BrdU-label-retaining (BrdU+) cells in the SVZ of WT and Wnt7a−/− (Wnt−/−) brains. The fold change in BrdU+ cells in the SVZ of WT mice is calculated by dividing the number of BrdU+ cells in the SVZ of WT mice by the number of BrdU+ cells in the SVZ of Wnt7a−/− SVZ. The fold change in Wnt7a−/− SVZ is expressed as 1. Data are represented as means ± s.d.; asterisk, P < 0.001 by Student’s t-test, n = 8 for WT brains and n = 12 for Wnt7a−/− brains. (e) Decrease in number of BrdU-label-retaining cells in the SVZ of TLX−/− brains. BrdU labelling is shown in green and S100β in red. Scale bar, 20 μm. (f) Quantification of BrdU-label-retaining (BrdU+) cells in the SVZ of WT and TLX−/− brains. The fold change in BrdU+ cells in the SVZ is calculated in the same way as in (d). Data are represented as means ± s.d.; asterisk, P < 0.001 by Student’s t-test, n = 5 for both wild-type and TLX−/− brains.
It has been shown that the slowly dividing, BrdU-label-retaining cells correspond to neural stem cells in the adult brain41. To determine whether the TLX–Wnt signalling regulates neural stem cells in vivo, we treated 6-week-old Wnt7a-null mice and their wild-type littermates with BrdU for 7 days. The treated mice were allowed to survive for 3 weeks. The number of BrdU-label-retaining cells was markedly lower in the subventricular zone of Wnt7a-null mice than in their wild-type littermates (Fig. 7c, d), suggesting that Wnt7a is an important regulator of neural stem cells in the adult brain. A substantial decrease in BrdU-label-retaining cells was also detected in the subventricular zone of adult TLX−/− mice (Fig. 7e, f), which is consistent with a recent observation that TLX is expressed in type B neural stem cells in the subventricular zone of adult brains and is essential for the self-renewal of these cells14. The results from Wnt7a-null mice and TLX-null mice together provide strong evidence that the TLX–Wnt signalling cascade regulates neural stem cell population in the adult brain.
DISCUSSION
TLX has been shown to be essential in neural stem cell maintenance and self-renewal in adult brains9. To uncover the molecular mechanisms involved, we have shown here that TLX activates the canonical Wnt/β-catenin signalling in adult neural stem cells. Wnt/β-catenin in turn regulates neural stem cell proliferation and self-renewal in the presence of EGF and FGF. Our observations that both Wnt7a and a constitutively active β-catenin rescued TLX siRNA-induced proliferation deficiency in neural stem cells strongly support the notion that TLX acts in part by activating the Wnt/β-catenin pathway to regulate neural stem cell proliferation and self-renewal.
Wnt signalling has been shown to regulate the self-renewal of multiple types of stem cell. The role of Wnt signalling in the expansion of stem cells in developing brains is supported by studies in several mouse models27,29,30. Our study demonstrated that the Wnt/β-catenin signalling is also important for adult neural stem cell proliferation and self-renewal. Wnt signalling has multiple functions in stem cells: in addition to its essential role in neural stem cell proliferation and self-renewal, Wnt signalling has also been shown to regulate neuronal differentiation of neural precursor cells42–44. How stem cells respond to Wnts is likely to depend not only on cell-intrinsic properties but also on their specialized microenvironment45. It is possible that Wnt/β-catenin signalling stimulates neural stem cell proliferation and self-renewal in the presence of mitogens and promotes neurogenesis on receiving differentiation cues. The presence of multiple Wnts also allows various outcomes of Wnt signalling. The Wnt pathways are therefore poised at a critical crossroads in balancing neural stem cell self-renewal versus differentiation.
The ability of TLX to repress transcription of the genes encoding intracellular molecules, such as GFAP9 and p21 (ref. 11), indicates that TLX is a cell-autonomous regulator of neural stem cell self-renewal. Regulation of a secreted Wnt-encoding gene by TLX suggests that TLX may also function through an autocrine/paracrine mode in neural stem cells, in addition to its cell-autonomous actions. In this sense, our results support a novel concept that neural stem cells can stimulate their own proliferation by secreting signalling molecules, in addition to garnering support from other cell types, such as endothelial cells34 and astrocytes46, in the progenitor niche. The difficulty in culturing neural stem cells at a low cell density supports the concept that neural stem cell growth requires stimulation by autocrine effect or by cell–cell contact. The fact that CCg, a molecule secreted by neural stem cells, stimulates neural stem cell proliferation and self-renewal47 lends additional support to this concept.
TLX has been shown to function as a transcription factor to repress the expression of its downstream target genes, such as p21. However, we show here that TLX activates Wnt7a expression. This activation could occur by direct binding to the Wnt7a promoter or by repression of a Wnt7a repressor. Binding of TLX to the consensus sites in the Wnt7a promoter has been observed in our study, although this does not exclude the possibility that TLX also activates Wnt7a by repressing a Wnt7a repressor. An explanation for the activation of Wnt7a by TLX binding could be the fact that DNA can act as an allosteric regulator to provide gene-specific regulation, the best-studied example of which is the glucocorticoid receptor48. Our TLX-binding-element swap experiment suggests that a specific configuration of the TLX response elements may dictate TLX to be a Wnt7a activator.
A substantial decrease in the number of neural stem cells retaining BrdU label was detected in the subventricular zone of both Wnt7a−/− and TLX−/− adult brains, providing strong evidence that TLX–Wnt signalling regulates the neural stem cell population in the adult brain. The decrease in the number of cells retaining BrdU label in the subventricular zone of adult TLX−/− mice is more severe than that in Wnt7a−/− mice. This discrepancy could be explained by the fact that TLX, as a transcription factor, regulates a spectrum of downstream target genes. Other functional targets, in addition to Wnt7a, may contribute to the substantial depletion of neural stem cell pool in TLX−/− brains. Alternatively, the possible existence of a Wnt7a-independent stem cell population may also provide an explanation for the less severe decrease in neural stem cell numbers in Wnt7a−/− brains.
Stem cell technology holds great promise for the treatment of many diseases that currently lack effective therapies. Identifying factors that control stem cell maintenance and self-renewal is an important step in moving stem cell technology from bench to bedside. Our finding that TLX activates Wnt to stimulate neural stem cell self-renewal suggests that purified Wnt proteins or molecules activating Wnt signalling can be developed as tools to facilitate the expansion of neural stem cells as a source for cell replacement therapies in the treatment of neurodegenerative diseases and brain injury.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/naturecellbiology/.
Supplementary Material
Acknowledgments
We thank Jeremy Stark and John Zaia for a critical reading of the manuscript; Shengxiu Li and Richard Stewart for technical help; Mariko Lee and Brian Armstrong for microscopy support; Angela Barth (Stanford University) for the constitutively active β-catenin construct; Frank Costantini (Columbia University) for providing the axin cDNA; Andrew Lassar (Harvard Medical School) and Eldad Tzahor (Weizmann Institute) for providing the IgG and Frzb–IgG expression vectors and stably transfected cells; and Bert Vogelstein (Johns Hopkins University) for Topflash plasmid. Y.S. is a Kimmel Scholar. G.S. is a Herbert Horvitz Postdoctoral Fellow. F.H.G. is the Adler Professor of Age-Related Neurodegenerative Diseases and is supported by the National Institutes of Health (NIH) and the Picower Foundation. R.M.E. is an Investigator of the Howard Hughes Medical Institute at the Salk Institute and March of Dimes Chair in Molecular and Developmental Biology. This work was supported by the Whitehall Foundation, the Margaret E. Early Medical Trust and the NIH National Institute of Neurological Disorders and Stroke RO1 NS059546 (to Y.S.).
Footnotes
Note: Supplementary Information is available on the Nature Cell Biology website.
AUTHOR CONTRIBUTIONS
Y.S. conceived and designed the study. Y.S., Q.Q., G.S., W.L., S.Y., P.Y. and C.Z. performed the experiments and analysed the data. Y.S., Q.Q., G.S., F.H.G. and R.M.E. interpreted the data. Y.S. wrote the paper with comments from Q.Q., G.S., R.T.Y., F.H.G. and R.M.E.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Published online at http://www.nature.com/naturecellbiology
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Weiss S, van der Kooy D. CNS stem cells: where’s the biology (a.k.a. beef)? J Neurobiol. 1998;36:307–314. doi: 10.1002/(sici)1097-4695(199808)36:2<307::aid-neu14>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Buylla A, Temple S. Stem cells in the developing and adult nervous system. J Neurobiol. 1998;36:105–110. [PubMed] [Google Scholar]
- 3.Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.McKay R. Stem cells in the central nervous system. Science. 1997;276:66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richards LJ, Kilpatrick TJ, Bartlett PF. De novo generation of neuronal cells from the adult mouse brain. Proc Natl Acad Sci USA. 1992;89:8591–8595. doi: 10.1073/pnas.89.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu RT, McKeown M, Evans RM, Umesono K. Relationship between Drosophila gap gene tailless and a vertebrate nuclear receptor Tlx. Nature. 1994;370:375–379. doi: 10.1038/370375a0. [DOI] [PubMed] [Google Scholar]
- 8.Monaghan AP, Grau E, Bock D, Schutz G. The mouse homolog of the orphan nuclear receptor tailless is expressed in the developing forebrain. Development. 1995;121:839–853. doi: 10.1242/dev.121.3.839. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, et al. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427:78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- 10.Li W, et al. Nuclear Receptor TLX Regulates cell cycle progression in neural stem cells of the developing brain. Mol Endocrinol. 2008;22:56–64. doi: 10.1210/me.2007-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun G, Yu RT, Evans RM, Shi Y. Orphan nuclear receptor TLX recruits histone deacetylases to repress transcription and regulate neural stem cell proliferation. Proc Natl Acad Sci USA. 2007;104:15282–15287. doi: 10.1073/pnas.0704089104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao C, Sun G, Li S, Shi Y. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nature Struct Mol Biol. 2009;16:365–371. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1008. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 14.Liu HK, et al. The nuclear receptor tailless is required for neurogenesis in the adult subventricular zone. Genes Dev. 2008;22:2473–2478. doi: 10.1101/gad.479308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nusse R, Varmus HE. Wnt genes. Cell. 1992;69:1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- 16.McMahon AP, Gavin BJ, Parr B, Bradley A, McMahon JA. The Wnt family of cell signalling molecules in postimplantation development of the mouse. Ciba Found Symp. 1992;165:199–212. doi: 10.1002/9780470514221.ch12. [DOI] [PubMed] [Google Scholar]
- 17.Moon RT, Brown JD, Torres M. WNTs modulate cell fate and behavior during vertebrate development. Trends Genet. 1997;13:157–162. doi: 10.1016/s0168-9525(97)01093-7. [DOI] [PubMed] [Google Scholar]
- 18.Willert K, Nusse R. Beta-catenin: a key mediator of Wnt signaling. Curr Opin Genet Dev. 1998;8:95–102. doi: 10.1016/s0959-437x(98)80068-3. [DOI] [PubMed] [Google Scholar]
- 19.Clevers HC, Grosschedl R. Transcriptional control of lymphoid development: lessons from gene targeting. Immunol Today. 1996;17:336–343. doi: 10.1016/0167-5699(96)10019-0. [DOI] [PubMed] [Google Scholar]
- 20.Molenaar M, et al. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 21.Behrens J, et al. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–342. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 22.Tetsu O, McCormick F. β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 23.Reya T, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 24.Willert K, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 25.Zhu AJ, Watt FM. β-Catenin signalling modulates proliferative potential of human epidermal keratinocytes independently of intercellular adhesion. Development. 1999;126:2285–2298. doi: 10.1242/dev.126.10.2285. [DOI] [PubMed] [Google Scholar]
- 26.Korinek V, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nature Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 27.Lee SM, Tole S, Grove E, McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- 28.Viti J, Gulacsi A, Lillien L. Wnt regulation of progenitor maturation in the cortex depends on Shh or fibroblast growth factor 2. J Neurosci. 2003;23:5919–5927. doi: 10.1523/JNEUROSCI.23-13-05919.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 30.Zechner D, et al. β-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 31.Zhou CJ, Zhao C, Pleasure SJ. Wnt signaling mutants have decreased dentate granule cell production and radial glial scaffolding abnormalities. J Neurosci. 2004;24:121–126. doi: 10.1523/JNEUROSCI.4071-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu HM, Liu B, Costantini F, Hsu W. Impaired neural development caused by inducible expression of Axin in transgenic mice. Mech Dev. 2007;124:146–156. doi: 10.1016/j.mod.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adachi K, et al. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- 34.Shen Q, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 35.Barth AI, Pollack AL, Altschuler Y, Mostov KE, Nelson WJ. NH2-terminal deletion of β-catenin results in stable colocalization of mutant β-catenin with adenomatous polyposis coli protein and altered MDCK cell adhesion. J Cell Biol. 1997;136:693–706. doi: 10.1083/jcb.136.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsieh JC, et al. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398:431–436. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- 37.Cadigan KM, Fish MP, Rulifson EJ, Nusse R. Wingless repression of Drosophila frizzled 2 expression shapes the Wingless morphogen gradient in the wing. Cell. 1998;93:767–777. doi: 10.1016/s0092-8674(00)81438-5. [DOI] [PubMed] [Google Scholar]
- 38.Zeng L, et al. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 39.Parr BA, McMahon AP. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature. 1998;395:707–710. doi: 10.1038/27221. [DOI] [PubMed] [Google Scholar]
- 40.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 41.Jackson EL, et al. PDGFRα-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51:187–199. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 42.Hirabayashi Y, et al. The Wnt/β-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- 43.Lie DC, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 44.Kuwabara T, et al. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nature Neurosci. 2009;12:1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 46.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 47.Taupin P, et al. FGF-2-responsive neural stem cell proliferation requires CCg, a novel autocrine/paracrine cofactor. Neuron. 2000;28:385–397. doi: 10.1016/s0896-6273(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 48.Lefstin JA, Yamamoto KR. Allosteric effects of DNA on transcriptional regulators. Nature. 1998;392:885–888. doi: 10.1038/31860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.