Abstract
The classical setting of evolutionary game theory, the replicator equation, assumes uniform interaction rates. The rate at which individuals meet and interact is independent of their strategies. Here we extend this framework by allowing the interaction rates to depend on the strategies. This extension leads to nonlinear fitness functions. We show that a strict Nash equilibrium remains uninvadable for non-uniform interaction rates, but the conditions for evolutionary stability need to be modified. We analyze all games between two strategies. If the two strategies coexist or exclude each other, then the evolutionary dynamics do not change qualitatively, only the location of the equilibrium point changes. If, however, one strategy dominates the other in the classical setting, then the introduction of non-uniform interaction rates can lead to a pair of interior equilibria. For the Prisoner’s Dilemma, non-uniform interaction rates allow the coexistence between cooperators and defectors. For the snowdrift game, non-uniform interaction rates change the equilibrium frequency of cooperators.
1. Introduction: replicator equation with uniform interaction rates
Consider a two strategy game with payoff matrix
Strategy A receives payoffs a and b when playing against strategy A and B respectively. Strategy B receives payoffs c and d when playing against A and B, respectively. We denote by x and y the frequency of individuals adopting strategy A and B, respectively. We have x + y = 1.
With uniform interaction rates, where players interact with each other indiscriminantly, the selection dynamics can be described by the standard replicator equation [Taylor & Jonker, 1978, Hofbauer et al., 1979, Hofbauer & Sigmund, 1998, and Hofbauer & Sigmund, 2003]:
| (1) |
The fitness of A and B players are linear functions of x, given by
The average fitness of the population is given by
The replicator equation assumes that the rate (or probability) of interaction between two players is independent of their strategies.
There are three generic evolutionary outcomes:
A dominates B: If a > c and b > d, then the entire population will eventually consist of A players. The only stable equilibrium is x = 1. A is a strict Nash equilibrium, and therefore an evolutionarily stable strategy (ESS), while B is not. We use the notation A ← B.
A and B co-exist in a stable equilibrium: If a < c and b > d, then the interior equilibrium x = (b−d)/(b + c−a−d) is stable. Neither A nor B are Nash equilibria. We use the notation A →← B.
A and B are bi-stable: If a > c and b < d, the interior equilibrium x = (d−b)/(a+d−b−c) is unstable. The two boundary points, x = 0 and x = 1 are attracting. A and B are both strict Nash equilibria. We use the notation A ←→ B. If a + b > c + d, then strategy A is risk dominant. It has the larger basin of attraction. If a > d, then strategy A is Pareto optimal, i.e. there is no other strategies the two players can employ to have payoff at least as high as a, and at least one player having payoff higher than a.
2. Replicator equation with non-uniform interaction rates
Now suppose that the probability of interaction between two players is not independent of their strategies. Analogous to a chemical reaction, an A player interacts with another A player with reaction rate r1, an A player and a B player interact with reaction rate r2, and a B player interacts with another B player with reaction rate r3.
We assume that the fitness of individuals is determined by the average payoff over a large number of interactions. Therefore, the fitness of A and B players are non-linear functions of x and y, given by
The rates r1, r2 and r3 are non-negative. The normal replicator equation with uniform interaction rates corresponds to the special case r1 = r2 = r3 > 0. If r1 > r2 and r3 > r2, then players prefer to interact with their own kind. If, however, r1 < r2 and r3 < r2, then mixed interactions (between A and B) are more likely.
2.1. A comparison with kin selection
In the context of kin selection [Hamilton, 1964], games between relatives have been studied in the late 1970’s by [Grafen, 1979], [Hines & Maynard Smith, 1979], [Mirmirani & Oster 1978], [Orlove, 1978 Orlove, 1979a, 1979], and [Treisman, 1977]. The “inclusive fitness” approach simply modifies the original payoff matrix M to
where r is the coefficient of relatedness. In this framework, all individuals of the population are assumed to be related equally. The payoff to a player in a pairwise interaction is the sum of his own payoff plus r times the opponent’s payoff. Grafen proposed a “personal fitness” approach to account for the fact that an individual is more likely to play an opponent with the same strategy. In Grafen’s model,
where r is the probability than a player will meet an opponent with the same strategy because of some genetic or social relationship.
More recently, [Tao & Lessard, 2002] developed the ESS theory for frequency-depedent selection in family-structure populations. In particular, they use the payoff matrix
where r is the probability to interact with a sib, to show the effect of kin selection involving full sibs on ESS conditions.
All these approaches contain linear fitness functions. In contrast, our model is based on non-linear fitness functions and can therefore not be studied by a simple transformation of the payoff matrix. In our model, there will be new dynamical features which are not present in the standard replicator equation.
[Queller, 1985] used a definition of relatedness which depends on the covariance of the strategies of interacting individuals. Non-uniform interaction rates lead to high covariance of players’ strategies, and hence a degree of relatedness in Queller’s formulation.
2.2. Invariant transformations
For the standard replicator equation, there exist some useful transformations of the payoff matrix that do not change the evolutionary dynamics. Here we show that some of these transformations can also be used for non-uniform interaction rates.
Consider the equations
with φ = xfA + yfB and
-
Adding the same constant to all payoff values, a, b, c and d, does not change the evolutionary dynamics. We haveTherefore, we obtain the substitutions
The corresponding fitness of A and B players, and hence the average fitness of the population, are all increased by the base fitness f0. Therefore, the evolutionary outcome and speed remain invariant when background fitness is introduced.
-
Multiplying all payoff values, a, b, c and d, by the same factor does not change the evolutionary dynamics. We haveTherefore, we have the substitutions
The corresponding fitness of A and B players, and hence the average fitness of the population, are all multiplied by k. The evolutionary outcome is still the same, while the evolutionary speed is increased k-fold.
-
For the replicator equation with uniform interaction rates, it is possible to add arbitrary constants to each column of the payoff matrix:We have the substitutionsHence
This transformation does not change the evolutionary dynamics for uniform interaction rates. For non-uniform interaction rates, however, such transformations will change the evolutionary dynamics, in general. Only for the specific case, , the evolutionary dynamics remain invariant.
2.3. Evolutionary stability
When players do not interact with players of the opposite strategy, r2 = 0, then A dominates B if and only if a > d, while B dominates A if and only if a < d.
With uniform interaction rates, if ε many B players enter a population of 1 − ε many A players, the fitness of A and B players are given by
A is stable against invasion by B if fA > fB for small z. Hence A is an evolutionary stable strategy (ESS), if
either a > c,
or a = c and b > d.
The concept of an ESS was introduced by [Maynard Smith & Price, 1973]. With non-uniform interaction rates, if z many B players enter a population of 1 − ε many A players, the fitness of A and B players are given by
In this case, strategy A is ESS if either
a > c, or
a = c and .
Therefore, the conditions for evolutionary stability does depend on the interaction rates r1, r2, and r3.
If a > c, then A is a strict Nash equilibrium and cannot be invaded by B for any choice of r1, r2 and r3 with r1, r2, r3 > 0. Therefore, a strict Nash equilibrium remains uninvadable for non-uniform interaction rates, while the condition for ESS changes.
Consider the following example
For uniform interaction rates, strategy A is ESS and therefore cannot be invaded by B. We have A ← B. For non-uniform interaction rates, however, if
then B can invade A.
2.4. Evolutionary dynamics
From now on, we consider the case r2 > 0. Without loss of generality, let r2 = 1. We will show that for non-uniform interaction rates, there are four generic outcomes, one of which is entirely new.
Let us introduce the parameters
| (2) |
We list below the evolutionary outcomes of a deterministic two strategy game with non-uniform interaction rates. We will prove these results in the Appendix.
2.5. B dominates A
If a < c and b < d, then B dominates A in the normal replicator equation. We have to distinguish two cases:
-
If c > a > d > b andthen we haveThe stable interior equilibrium is given byThe unstable interior equilibrium is given byThe bifurcation occurs when
As r1r3 increases above this threshold, α2 − 4βγ increases.
-
If d > b > c > a andthen we again haveThe stable interior equilibrium is given byThe unstable interior equilibrium is given byThe bifurcation occurs when
As r1r3 decreases below this threshold, α2 − 4βγ increases.
Hence, for the first two classes of games where strategy B is strictly dominant, the evolutionary outcome can be altered by varying the reaction rates r1 and r3. Note, however, that the invasion dynamics do not change. B can invade A, but A cannot invade B. If initially most of the population play B, then everyone will play B. If initially most of the population play A, then A and B players will co-exist.
The bifurcation point is where the evolutionary outcome changes its course. In particular, bifurcation occurs when
When α2 − 4βγ < 0, we simply have A → B.
When α2 = 4βγ, and , we have a tangent (or fold) bifurcation point at
As α2 − 4βγ increases above zero, the two equilibria move symmetrically away from
toward the neighboring end points.
When r1 = r3, we find that the bifurcation point is at
Obviously when a > c and b > d, then the situation is similar with A and B exchanged.
2.6. A and B coexist
If a < c and b > d, then A and B co-exist in a stable equilibrium,
The interior stable equilibrium is given by
The evolutionary dynamics do not change by introducing non-uniform interaction rates. However, the location of the interior stable equilibrium, x*, can be shifted by varying r1 and r3.
We find that x* increases monotonically with respect to r1 and r3. As r3 → ∞, x* → 1; as r3 → 0, x* → 0. In particular, we can increase the equilibrium frequency of A by increasing r1 and r3, and we can increase the equilibrium frequency of B by decreasing r1 and r3.
2.7. A and B are bi-stable
If a > c and b < d, then A and B are bi-stable,
Both strategies are strict Nash equilibria. The unstable interior equilibrium is given by
For a bi-stable game, the evolutionary outcome stays the same with nonuniform interaction rates. However, the location of the interior unstable equilibrium, x*, can be shifted by varying r1 and r3.
We find that x* decreases monotonically with respect to r1 and r3. As r1 → ∞, x* → 0; as r1 → 0, x* → 1. In particular, we can minimize the invasion barrier for A by increasing r1 and r3, and we can minimize the invasion barrier for B by decreasing r1 and r3.
For uniform interaction rates, A is risk dominant if
This implies x* < 1/2 and, hence, A has a larger basin of attraction.
For non-uniform interaction rates, A has the larger basin of attraction if
This inequality can be written as
This is the condition that the fitness an A player is greater than that of a B player when the proportion of A and B players are equal. We argue that this condition is the relevant criterion for risk-dominance in the context of non-uniform interaction rates as it generates the larger basin of attraction.
Given a > c and d > b, it is not possible that a strategy is risk-dominant for any choice of r1 and r3.
Let us focus on the special case where r1 = r3 = r > 0 and r2 = 1. Strategy A is risk dominant if
Consider the example
For uniform interaction rates, B is risk dominant. For non-uniform interaction rates, if r < 2, B is risk dominant, and if r > 2, then A is risk dominant. If a > d > b > c, then A is risk dominant for any choice of r.
3. Application to Prisoner’s Dilemma
In the Prisoner’s Dilemma, the two strategies C and D denote cooperation and defection. The payoff matrix is given by
The Prisoner’s Dilemma is defined by T > R > P > S and R > (T + S)/2, and it corresponds to the case outlined in case (1) in section 2.5.
For uniform interaction rates r1 = r2 = r3, defection is the dominant strategy. Hence, the entire population will consist of defectors eventually. We have C → D.
However, if players only interact with opponents of the same strategy, then cooperators cannot be exploited by defectors. In this case, where r2 = 0 and r1, r3 > 0, cooperation is the dominant strategy, because R > P. Hence C ← D.
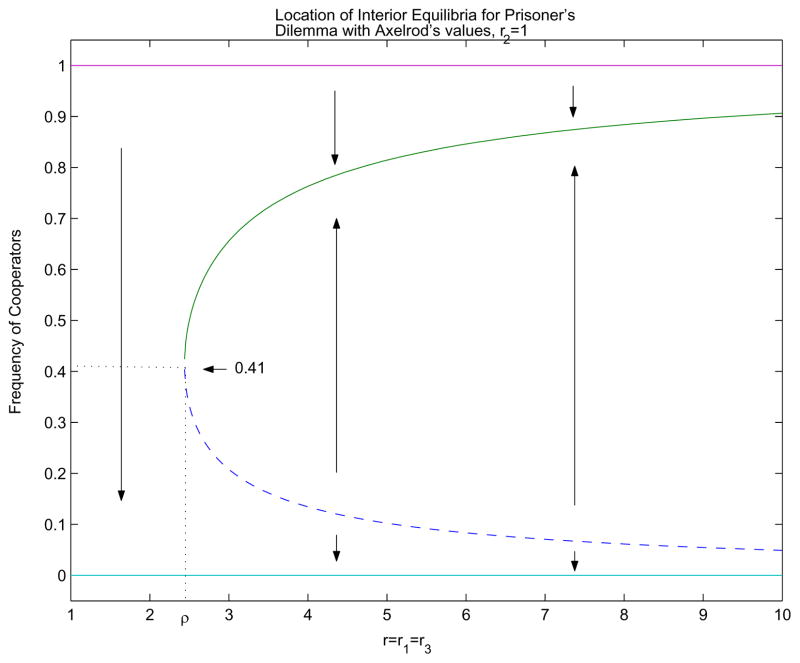
Assume r2 ≠ 0, which means that cooperators and defectors do interact. Without loss of generality, assume that r2 = 1. The selection dynamics depend on the size of r1r3 relative to ρ2, where
Note that ρ is always greater than 1. We use the values set in [Axelrod & Hamilton, 1981], T = 5, R = 3, P = 1, S = 0, we obtain ρ ≃ 2.44.
If r1r3 < ρ2, then defection is the dominant strategy. If r1r3 > ρ2, then there are two interior equilibria, one stable and the other unstable, in addition to the two equilibria on the boundary. We have C → · ← · → D.
At the bifurcation point, we have r1r3 = ρ2. As r1r3 increases above ρ2, the two interior equilibria move further apart from the bifurcation point, given by
Now let r = r1 = r3. Thus the cooperator-cooperator interaction rate is the same as that of defector-defector. When r = ρ, we have the bifurcation point at
The frequency of cooperators, x*, at the bifurcation point is independent of any parameter. As r increases, the interior stable equilibrium point moves closer toward 1, while the unstable equilibrium moves closer toward 0. So the proportion of cooperators tend to increase monotonically as r increases. As r → ∞, we recover the case where r2 = 0, and cooperation is the dominant strategy, C ← D, as defectors can no longer exploit cooperators. See Figure 1.
Figure 1.
4. Application to the Snowdrift Game
In a snowdrift game [Hauert & Doebeli, 2004], two drivers who are caught in a blizzard and trapped on either side of a snowdrift. They can shovel (cooperate) or remain in the car (defect). If both cooperate, they have the benefit e of getting home while sharing the labor cost f, so the net payoff to each player is e−f/2. If both defect, they do not get home, and the net payoff is 0. If one cooperates while the other defects, the cooperator receives e − f, and the defector receives e.
If e > f > 0, the payoffs generate the snowdrift game, in which it is best to play the strategy different from one’s opponent: stay in the car if the other is shoveling, and shovel if the other is idle in the car. This corresponds the situation outlined in section 2.6. The equilibrium frequence of cooperators is 1 − f/(2e − f), where f/(2e − f) is the cost-to-benefit ratio of mutual cooperation. The average payoff of the population at equilibrium is 2e(e − f)/(2e − f), which is smaller than the average population payoff, e − f/2, if the entire population consists of cooperators only.
The equilibrium frequency of cooperators, x*, is always an increasing function of r1 and r3. Hence the equilibrium frequency of cooperators can be maximized by increasing the interaction rates between players with the same strategies.
5. Conclusion
Evolutionary game theory was pioneered by John Maynard Smith [Maynard Smith & Price, 1973, Maynard Smith, 1982]. His ideas brought game theory to biology and population thinking to game theory. Maynard Smith invented the important concept of an evolutionarily stable strategy (ESS), which can resist invasion of other strategies in infinitely large populations. Evolutionary game theory has been used to study the interaction among genes, cells, viruses, animals and humans. For a recent review see [Nowak & Sigmund, 2004]. Evolutionary game theory offers an framework to explore the evolution of altruistic behavior [Trivers, 1971, Axelrod & Hamilton, 1981, Nowak & Sigmund, 1992, Killingback & Doebeli, 2002] and human language [Nowak et al., 2002]. Mathematical approaches to evolutionary game dynamics are based on ordinary differential equations [Taylor & Jonker, 1978, Hofbauer et al., 1979, Zeeman, 1980, Fudenberg & Tirole, 1991, Weibull, 1995, Tao & Lessard, 2000], partial differential equations [Hutson & Vickers, 1993], stochastic differential equation [Imhof, 2004, Fudenberg & Imhof, 2004], cellular automata [Nowak & May, 1992, Herz, 1994, Lindgren & Nordahl, 1994, Killingback & Doebeli, 1996, Mitteldor & Wilson 2000, Irwin & Taylor 2001, Hauert et al., 2002, Le Galliard et al., 2003], and stochastic processes [Nowak et al., 2004, Taylor et al., 2004]. There is much current interest to study evolutionary game dynamics on graphs, which also leads to non-uniform interaction rates. [Ellison, 1993, Nakamaru et al., 1997 & 1998, Epstein, 1998, Abramson & Kuperman, 2001, Ebel & Bornholdt, 2002, Szabo & Vukov, 2004, Ifti & et al., 2004, Nakamaru & Iwasa, 2005, Lieberman et al., 2005] The fundamental Lotka-Volterra equation of ecology is equivalent to the replicator equation of evolutionary game theory [Hofbauer & Sigmund, 2003].
In this paper, we have studied the effect of non-uniform interaction rates on evolutionary game dynamics. In the classical approach of the replicator equation, the rate of interaction between any two individuals is the same and does not depend on the strategies (phenotypes) of these individuals. Here we assume that the interaction rates are not uniform. For example, players who use the same strategy might interact more frequently than players who use different strategies. Non-uniform interaction rates lead to nonlinear fitness functions and therefore allow richer dynamics than the classical replicator equation, which is based on linear fitness functions. We have analyzed the evolutionary dynamics of all symmetric two-strategy games.
If strategy A is a strict Nash equilibrium, then it remains uninvadable for positive non-uniform interaction rates. If A dominates B then non-uniform interaction rates can introduce a pair of interior equilibria; one of them is stable the other one unstable. If A and B coexist, then non-uniform interaction rates cannot change the qualitative dynamics, but alter the location of the stable equilibrium. If A and B are bi-stable, then again non-uniform interaction rates cannot change the qualitative dynamics, but alter the location of the unstable equilibrium. There is a new condition for risk dominance that depends on the interaction rates.
For the non-repeated Prisoner’s Dilemma, coexistence between cooperators and defectors is possible if the ratio of homogeneous (C − C, D − D) over heterogeneous (C − D) interaction rates exceeds a critical value. If C − C interactions are as likely as D − D interactions, then the pair of equilibria v arises at a cooperator frequency of which is entirely independent of the payoff matrix, as long as T > R > P > S. Both equilibria are stable, one consists of defectors alone, and the other consists of a mixture of defectors and cooperators.
For the snowdrift game, the equilibrium of frequency of cooperators is increased if homogeneous interactions are more likely than heterogeneous ones.
Spatial dynamics of the Prisoner’s Dilemma [Nowak & May, 1992, Killingback & Doebeli, 1999, 2002] leads to clustering of cooperators and therefore always favors cooperators. Spatial dynamics of the snowdrift game, however, can lead to intricate patterns of cooperators intermixed with defectors and can therefore enhance heterogeneous interactions. This effect can reduce the equilibrium frequency of cooperators [Hauert & Doebeli, 2004]. Both phenomena are in accordance with the findings of the present paper.
The analysis of non-uniform interaction rates should be extended to stochastic game dynamics of finite populations. Furthermore, we can distinguish the rate, rAB, a strategy A player interacts with a strategy B player, and the rate, rBA, a strategy B player interacts with strategy A player. Here we have analyzed rAA = r1, rBB = r3, and rAB = rBA = r2. It would also be interesting to study evolutionary dynamics for rAB ≠ rBA.
Acknowledgments
The Program for Evolutionary Dynamics is supported by Jeffrey Epstein. The authors would like to thank the referees for careful reading and suggestions.
7. Appendix
We prove our main results concerning non-uniform reaction rates. We first consider the generic case where none of the reaction rates ri is zero.
Since fA and fB are homogeneous in ri’s, after a change of variable (dividing the denominator and nominator by r2, assuming r2 ≠ 0), we can write
The replicator equations can be reduced to
where x is the proportion of A players. and
The equilibrium points are either on the boundary or in the interior.
At x = 0, the Jacobian is
So x = 0 is a stable equilibrium if b < d, and an unstable equilibrium if b > d.
At x = 1, the Jacobian is
So x = 1 is a stable equilibrium if a > c, and an unstable equilibrium if a < c.
At the interior equilibrium x*, where x* is the polynomial root of the nominator of fA − fB, call it h(x), where
where
At x = x*, the Jacobian is directly proportional to
depending on the root x*.
For x* to be an interior equilibrium, we require that
This condition and the sign of the Jacobian at x = x* will help us to determine the evolutionary outcomes of this game.
In order for both roots to be in (0, 1), α2 > 4βγ must hold. In addition, we find that either
or
We focus on the case where β and γ are both positive, hence with uniform reaction rates, A is a strict Nash equilibrium. However, with non-uniform reaction rates, the selection dynamics depends on the magnitude of r1r3 versus .
Under the conditions that a > c and b > d, β, γ > 0. Hence, we have A ← · → · ← B when a < 2β, 2γ. Since α2 > 4αβ, α < 0 must hold. Since α < 0, we find if a > d, then b < c, so a > c > b > d; if a < d, since b > d and a > c, we have b > d > a > c. The conditions α < 0 and α2 > 4βγ together imply that A ← · → · ← B if and only if one of the following holds:
The two interior equilibria are located at
In summary, the two-strategy games whose evolutionary outcome could be altered by non-uniform reaction rates fall in one of the following four categories, where either A or B is a dominant strategy:
b > d > a > c, and .
a > c > b > d, and .
a < c < b < d, and .
b < d < a < c, and .
The location of interior equilibria at
depend largely on the reaction rates r1, r2, and r3, as well as the signs of a −d and b − c.
When , we need to compare the payoffs a and d:
-
d > a:
- If b > d, we have an interior stable equilibrium at
we can make by increasing r1.
- If a > c, we have an interior unstable equilibrium at
we can make by increasing r3.
-
a > d:
- If c > a, we have an interior stable equilibrium at
we can make by increasing r3.
- If d > b, we have an interior unstable equilibrium at
we can make by increasing r1.
When , we need to compare the payoffs b and c.
-
b > c:
- If c > a, we have an interior stable equilibrium at
we can make by decreasing r1.
- If d > b, we have an interior unstable equilibrium at
we can make by decreasing r3.
-
b < c:
- If b > d, we have an interior stable equilibrium at
we can make by decreasing r3.
- If a > c, we have an interior unstable equilibrium at
we can make by decreasing r1.
Finally we consider the special cases when ri = 0 when i = 1, 2, or 3.
If r1 = 0, r2 = 1, then A dominates B if and only if b > c, d. If b < c, d, then B dominates A. If however, c < b < d, then we have a bi-stable game where x = 0, 1 are both Nash equilibria, and (d−b)r3/((d−b)r3+(b−c)) is an unstable interior equilibrium. Otherwise, when d < b < c, we have a mixed strategy game where x = 0, 1 are unstable equilibria, but (d−b)r3/((d−b)r3 + (b−c)) is a stable interior equilibrium.
Similarly, if r3 = 0, r2 = 1, we have A ← B when c < a, b; A → B when c > a, b; A ← · → B when b < c < a; and A → · ← B when a < c < b. The interior equilibrium is at (c−b)/(c−b + (a−c)r1). Here, the greater r1 is, the closer the interior equilibrium is to x = 0.
References
- Abramson G, Kuperman M. Social games in social network. Phys Rev E. 2001;63:030901. doi: 10.1103/PhysRevE.63.030901. [DOI] [PubMed] [Google Scholar]
- Axelrod R, Hamilton WD. The evolution of cooperation. Science. 1981;211:1390–1396. doi: 10.1126/science.7466396. [DOI] [PubMed] [Google Scholar]
- Cohen D, Levin SA. The interaction between dispersal and dormancy strategies in varying and heterogeneous environments. In: Teramoto E, Yamaguti M, editors. Mathematical Topics in Population Biology, Morphogenesis and Neurosciences, Proc Kyoto 1985. Springer-Verlag; Heidelberg: 1987. [Google Scholar]
- Doebeli M, Hauert C, Killingback T. The evolutionary origin of cooperators and defectors. Science. 2004;306:859–862. doi: 10.1126/science.1101456. [DOI] [PubMed] [Google Scholar]
- Ebel H, Bornholdt S. Coevolutionary games on networks. Phys Rev E. 2002;66:056118. doi: 10.1103/PhysRevE.66.056118. [DOI] [PubMed] [Google Scholar]
- Ellison G. Learning, local interaction, and coordination. Econometrica. 1993;61(5):1047–1071. [Google Scholar]
- Epstein JM. Zones of cooperation in demographic prisoner’s dilemma. Complexity. 1998;4(2):36–48. [Google Scholar]
- Ewens WJ. Mathematical population genetics. Springer-Verlag; 2004. [Google Scholar]
- Fudenberg D, Tirole J. Game Theory. MIT Press; 1991. [Google Scholar]
- Fudenberg D, Imhof L. Imitation processes with small mutations. 2004 Preprint. [Google Scholar]
- Grafen A. The hawk-dove game played between relatives. Anim Behv. 1979;27:905–907. [Google Scholar]
- Grafen A. Natural selection, kin selection and group selection. In: Krebs J, Davies N, editors. Behavioural Ecology: An evolutionary approach. Oxford: Blackwell Scientific Publications; 1984. pp. 62–84. [Google Scholar]
- Hamilton WD. The evolution of social behavior. J Theor Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Hauert C, De Monte S, Hofbauer J, Sigmund K. Volunteering as Red Queen mechanism for cooperation in public goods game. Science. 2002;296:1129–1132. doi: 10.1126/science.1070582. [DOI] [PubMed] [Google Scholar]
- Hauert C, Doebeli M. Spatial structure often inhibits the evolution of cooperation in the Snowdrift game. Nature. 2004;428:643–646. doi: 10.1038/nature02360. [DOI] [PubMed] [Google Scholar]
- Herz AVM. Collective phenomena in spatially extended evolutionary games. J Theor Biol. 1994;169:65–87. doi: 10.1006/jtbi.1994.1130. [DOI] [PubMed] [Google Scholar]
- Hines WGS, Maynard Smith J. Games between relatives. J Theor Biol. 1979;79:19–30. doi: 10.1016/0022-5193(79)90254-6. [DOI] [PubMed] [Google Scholar]
- Hofbauer J, Schuster P, Sigmund K. A note on evolutionary stable strategies and game dynamics. J Theor Biology. 1979;81:609–612. doi: 10.1016/0022-5193(79)90058-4. [DOI] [PubMed] [Google Scholar]
- Hofbauer J, Sigmund K. Evolutionary Games and Population Dynamics. Cambridge University Press; 1998. [Google Scholar]
- Hofbauer J, Sigmund K. Evolutionary Game Dynamics. Bull Am Math Society. 2003;40:479–519. [Google Scholar]
- Hutson V, Vickers GT. Traveling waves and dominance of ESS’s. J Math Biol. 1993;30:457–471. [Google Scholar]
- Hutson V, Lou Y, Mischaikow K. Spatial heterogeneity of resources versus Lotka-Volterra dynamics. J Diff Equations. 2002;185:97–136. [Google Scholar]
- Iftin M, Killingback T, Doebeli M. Effects of neighbourhood size and connectivity on the spatial continuous Prisoner’s Dilemma. J Theor Biol. 2004;231:97–106. doi: 10.1016/j.jtbi.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Imhof L. Evolutionary game dynamics under stochastic influences. 2004 Preprint. [Google Scholar]
- Irwin J, Taylor PD. Evolution of altruism in stepping-stone populations with overlapping generations. Theor Pop Biol. 2001;60:315–325. doi: 10.1006/tpbi.2001.1533. [DOI] [PubMed] [Google Scholar]
- Killingback T, Doebeli M. Spatial evolutionary game theory: Hawks and Doves revisited. Proc Roy Soc Lond Bio. 1996;263(1374):1135–1144. [Google Scholar]
- Killingback T, Doebeli M. ’Raise the stakes’ evolves into a defector. Nature. 1999;400(6744):518–518. doi: 10.1038/22913. [DOI] [PubMed] [Google Scholar]
- Killingback T, Doebeli M. The continuous Prisoner’s Dilemma and the evolutionary of cooperation through reciprocal altruism with variable investment. AM NAT. 2002;160(4):421–438. doi: 10.1086/342070. [DOI] [PubMed] [Google Scholar]
- Le Galliard JF, Ferrière R, Dieckmann U. The adaptive dynamics of altruism in spatially heterogeneous populations. Evolution. 2003;57:1–17. doi: 10.1111/j.0014-3820.2003.tb00211.x. [DOI] [PubMed] [Google Scholar]
- Lessard S. Evolutionary stability: one concept, several meannings. Theor Pop Biol. 1990;37:159–170. [Google Scholar]
- Lessard S. Relatedness and inclusive fitness with inbreeding. Theor Pop Biol. 1992;42:284–307. doi: 10.1016/0040-5809(92)90016-m. [DOI] [PubMed] [Google Scholar]
- Levin SA. Some approaches to the modeling of coevolutionary interactions. In: Nitecki M, editor. Coevolution. Univ. of Chicago Press; 1983. [Google Scholar]
- Lieberman E, Hauert C, Nowak MA. Evolutionary dynamics on graphs. Nature. 2005;433:312–316. doi: 10.1038/nature03204. [DOI] [PubMed] [Google Scholar]
- Lindgren K, Nordahl MG. Evolutionary dynamics of spatial games. Physica D. 1994;75:292–309. [Google Scholar]
- Maynard Smith J. Evolution and the Theory of Games. Cambridge University Press; 1982. [Google Scholar]
- Maynard Smith J, Price GR. The logic of animal conflict. Nature. 1973;246:15–18. [Google Scholar]
- Mirmirani M, Oster G. Competition, kin selection, and evolutionary stable strategies. Theor Popul Biol. 1978;13:304–339. doi: 10.1016/0040-5809(78)90049-7. [DOI] [PubMed] [Google Scholar]
- Mitteldorf J, Wilson DS. Population viscosity and the evolution of altruism. J Theor Biol. 2000;204:481–496. doi: 10.1006/jtbi.2000.2007. [DOI] [PubMed] [Google Scholar]
- Nakamaru M, Matsuda H, Iwasa Y. The evolution of cooperation in a lattice-structured population. J Theor Biol. 1997;184:65–81. doi: 10.1006/jtbi.1996.0243. [DOI] [PubMed] [Google Scholar]
- Nakamaru M, Nogami H, Iwasa Y. Score-dependent fertility model for the evolution of cooperation in a lattice. J Theor Biol. 1998;194:101–124. doi: 10.1006/jtbi.1998.0750. [DOI] [PubMed] [Google Scholar]
- Nakamaru M, Iwasa Y. The evolution of altruism by costly punishment in lattice-structured populations: score-dependent viability versus score-dependent fertility. Evolutionary ecology research. 2005;7:853–870. [Google Scholar]
- Nowak MA, May RM. Evolutionary games and spatial chaos. Nature. 1992;359:826–829. [Google Scholar]
- Nowak MA, Sigmund K. Tit for Tat in heterogeneous Populations. Nature. 1992;355:250–253. [Google Scholar]
- Nowak MA, Komarova NL, Niyogi P. Computational and evolutionary aspects of language. Nature. 2002;417:611–617. doi: 10.1038/nature00771. [DOI] [PubMed] [Google Scholar]
- Nowak MA, Sigmund K. Population Dynamics in Evolutionary Ecology. In: Keinan E, Schechter I, Sela M, editors. Life Sciences for the 21st Century. Wiley-VCH Verlag GmbH & Co; 2004. [Google Scholar]
- Nowak MA, Sasaki A, Taylor C, Fudenberg D. Emergency of cooperation and evolutionary stability in finite populations. Nature. 2004;428:646–650. doi: 10.1038/nature02414. [DOI] [PubMed] [Google Scholar]
- Orlove MJ. Coefficients of relationship and coefficients of relatedness in kin selection: a covariance form for the RHO formula. J Theor Biol. 1978;73:679–686. doi: 10.1016/0022-5193(78)90129-7. [DOI] [PubMed] [Google Scholar]
- Orlove MJ. Putting the diluting effect into inclusive fitness. J Theor Biol. 1979;78:449–450. doi: 10.1016/0022-5193(79)90344-8. [DOI] [PubMed] [Google Scholar]
- Orlove MJ. A reconciliation of inclusive fitness and personal fitness approaches: a proposed correcting term for the inclusive fitness formula. J Theor Biol. 1979;81:577–586. doi: 10.1016/0022-5193(79)90055-9. [DOI] [PubMed] [Google Scholar]
- Queller D. Kinship, reciprocity, and synergism in the evolution of social behavior. Nature. 1985;318:366–367. [Google Scholar]
- Szabo G, Vukov J. Cooperation for volunteering and partially random partnerships. Phys Rev E. 2004;69:036107. doi: 10.1103/PhysRevE.69.036107. [DOI] [PubMed] [Google Scholar]
- Tao Y, Lessard S, Lemire M. Evolutionarily stable strategy in a sex- and frequency- dependent selection model. Journal of Theoretical Biology. 2000;204:191–200. doi: 10.1006/jtbi.2000.1098. [DOI] [PubMed] [Google Scholar]
- Tao Y, Lessard S. Fundamental theorem of natural selection and frequency-dependent selection: Analysis of the diploid matrix game model. Journal of Theoretical Biology. 2000;206:17–25. doi: 10.1006/jtbi.2000.2097. [DOI] [PubMed] [Google Scholar]
- Tao Y, Lessard S. Frequency-dependent selection in sexual family-structure populations. J Theor Biol. 2002;217:525–534. doi: 10.1006/jtbi.2002.3041. [DOI] [PubMed] [Google Scholar]
- Taylor PD, Jonker L. Evolutionary stable strategies and game dynamics. Math Biosciences. 1978;40:145–156. [Google Scholar]
- Taylor C, Fudenberg D, Sasaki A, Nowak MA. Evolutionary game dynamics in finite populations. Bull Math Biol. 2004;66(6):1621–1644. doi: 10.1016/j.bulm.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Treisman M. The evolutionary restriction of aggression within a species: a game theory analysis. J Math Psychol. 1977;16:167–203. [Google Scholar]
- Trivers RL. The evolution of reciprocal altruism. Quarterly Review of Biology. 1971;46:35–37. [Google Scholar]
- Weibull J. Evolutionary game theory. MIT Press; 1995. [Google Scholar]
- Zeeman EC. Global theory of dynamical systems. Springer; 1980. Population dynamics from game theory; p. 819. Lecture Notes in Mathematics. [Google Scholar]
- Zeeman EC, Zeeman ML. From local to global behavior in competitive Lotka-Volterra systems. Trans Am Math Soc. 2003;355:713–734. [Google Scholar]