Abstract
Well-differentiated neuroendocrine tumours (nets—previously called “carcinoid tumours”) are relatively rare tumours originating from the diffuse neuroendocrine system; they are found most often in the bronchial or gastrointestinal systems. In Canada, gastroenterohepatic nets represent less than 0.25% of oncology cases. Because of the relative rarity of these tumours, diagnostic and therapeutic approaches vary and are often based on individual physician experience. A number of European and North American groups have developed consensus guidelines for the diagnosis and management of well-differentiated gastroenterohepatic nets, and in 2006, Canadian consensus guidelines were published by a Canadian expert group. The updated and expanded current Canadian guidelines are based on a consensus meeting held in Paris, France, in 2008 and are based on the most current literature.
Keywords: Carcinoid tumour, carcinoid syndrome, carcinoid heart disease, neuroendocrine tumours, guidelines, clinical management, diagnosis, surgery
1. INTRODUCTION
Well-differentiated neuroendocrine tumours (nets—previously called “carcinoid tumours”) are relatively rare tumours 1 originating from the diffuse neuroendocrine system. They are found most often in the bronchial or gastrointestinal systems 2, with the gastrointestinal tract accounting for about two thirds, most of which originate in the small intestine (41.8%), rectum (27.4%), and stomach (8.7%) 3. The present article focuses on the treatment and management of well-differentiated gastroenterohepatic nets; treatment and management of other nets will be discussed in a subsequent paper.
In Canada, gastroenterohepatic nets represent less than 0.25% of oncology cases 4. Because of the relative rarity of these tumours, diagnostic and therapeutic approaches vary and are often based on individual physician experience. In recent years, a number of European and North American groups have developed consensus guidelines for the diagnosis and management of well-differentiated gastroenterohepatic nets 5–9, and in 2006, Canadian consensus guidelines were published by a Canadian net expert group 4. The updated and expanded current Canadian guidelines are based on the consensus meeting held at the European Neuroendocrine Tumour Society (enets) meeting in Paris, France, in April 2008 and are based on the most current literature.
2. CONSENSUS PROCESS
Panel members included oncologists, surgeons, radiologists, and pathologists. The recommendations arrived at represent evidence from the published literature and the collective experience of the authors. Table i describes the criteria used to rate level of consensus. All recommendations in this document fall into Category 2A, with the exception of the recommendation for octreotide as an antiproliferative agent, which falls into Category 1.
TABLE I.
Categories of consensus
| Category 1 | Uniform consensus based on high-level evidence that the recommendation is appropriate. |
| Category 2A | Uniform consensus based on lower-level evidence, including clinical experience, that the recommendation is appropriate. |
| Category 2B | Non-uniform consensus, but no major disagreement, based on lower-level evidence, including clinical experience, that the recommendation is appropriate. |
| Category 3 | Major disagreement that the recommendation is appropriate. |
3. EPIDEMIOLOGY
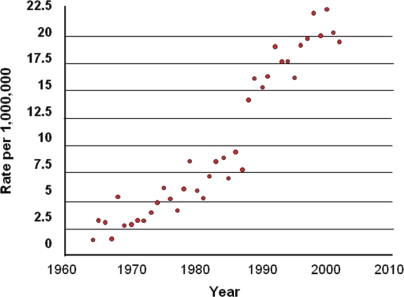
The reported incidence of gastroenterohepatic nets varies between 2.5 and 4.5 cases per 100,000 population in the United States 3,10. A trend toward rising incidence has been seen in recent years. For example, in Ontario, the incidence has risen from 2 per million population in 1964 to 22 per million in 2002 (Figure 1). Similar trends have been observed in the United States 3,12. Factors that have been hypothesized to possibly account for this rising incidence are increased clinical awareness; more thorough pathologic evaluation, including the use of immunostaining for specific molecular markers related to nets [for example, synaptophysin, chromogranin A (CgA)]; and the widespread use of proton pump inhibitors 13. Ongoing research and data collection will be important to clarify this potential change in incidence and to better understand potential causative factors.
Figure 1.
Incidence of well-differentiated neuroendocrine tumours (“carcinoid tumours”) in Ontario, 1964–2002 11. Age-adjusted rates standardized to 1991 (rate per million population), by year of diagnosis.
The appearance of these nets at the same time as other malignancies (such as adenocarcinomas) has also been demonstrated 14–16.
4. PATHOLOGY AND CLASSIFICATION
4.1. Classification
Early net classification systems focused primarily on embryologic origin (foregut, midgut, hindgut), noting differences in biochemical and histochemical properties between tumours that originated in different sites 17. The utility of this classification was limited, because it did not further characterize tumours based on their histologic appearance, prognosis, or clinical behaviour.
The 1980 World Health Organisation (who) classification scheme broadly applied the term “carcinoid” to tumours of the diffuse neuroendocrine system, but subdivided them on the basis of granulestaining techniques into enterochromaffin-cell carcinoids, gastrin-cell carcinoids, and other carcinoids. With increasing use of newer diagnostic techniques, including biochemistry, immunohistochemistry, and molecular biology, it has become apparent that this classification also does not adequately reflect the histopathologic diversity, functional behaviour, and prognosis of these tumours 18.
The most recent classification system devised by the who 19 emphasizes a prognosis-oriented definition of gastroenterohepatic nets regardless of anatomic site of origin:
- Well-differentiated nets
- Benign
- Uncertain malignant potential
Well-differentiated neuroendocrine carcinomas
Poorly-differentiated neuroendocrine carcinomas
Well-differentiated nets are often considered the “classical carcinoid” net and demonstrate a trabecular, insular, or ribbon-like architecture; minimal cellular pleomorphism; and sparse mitotic activity. Well-differentiated neuroendocrine carcinomas (sometimes called “malignant carcinoids”) have increased cellular pleomorphism and mitotic activity, and may have punctate necrosis. Tumours with well-differentiated histologic features, but also more aggressive pathologic features such as angioinvasion, also fall into this second category. Poorly differentiated neuroendocrine carcinomas show marked cellular pleomorphism, fields of necrosis, and brisk mitotic activity, and can be histopathologically similar to small-cell lung carcinoma.
4.2. Staging
The checklists of the American Joint Committee on Cancer (ajcc) and the College of American Pathologists have not previously included nets of the gastrointestinal tract 20,21. However, the ajcc included a nets classification in its latest iteration, released in late 2009 22. Before that, enets proposed tumour, node, metastasis (TNM) staging and grading systems for foregut and midgut/hindgut nets 1,23. The TNM stage is individualized by site, with separate classifications for gastric, duodenal and proximal jejunal, pancreatic, distal jejunal and ileal, appendiceal, and colorectal tumours. This proposed grading system includes proliferative markers such as mitotic count and Ki-67 index. Prospective validation of this system is underway. Problematically, the two systems do not agree in terminology and classification.
4.3. Specimen Collection, Handling, and Analysis
4.3.1. Biopsy Specimens
Biopsy specimens (needle core biopsies, endoscopic biopsies) of suspected nets should be immediately placed in formalin for proper fixation and processed according to routine laboratory protocols for small biopsies. Currently, formalin-fixed paraffin-embedded tissue for routine microscopic examination and immunohistochemical staining are adequate to provide diagnostic and prognostic information.
4.3.2. Resection Specimens
Gross assessment of large resection specimens should focus on the provision of prognostic information. Specimens should be properly fixed in formalin to ensure good histologic detail and reliable immunohistochemical staining. The following elements are recommended for inclusion in pathology reports for well-differentiated nets 23:
Tumour site
Histologic type (who classification)
Local tumour growth (T stage, size and involvement of adjacent organs)
Margins (proximal, distal, radial)
Lymphatic and vascular invasion
Necrosis (focal or zonal)
Lymph nodes (number with metastases/overall number examined)
Mitotic rate [per 10 high-power fields (hpf)]
Ki-67 index
Any other histochemical or immunohistochemical stains performed and the results
A minimum data set for pathology reporting of nets has also recently been published 24.
4.4. Ki-67 Index
The Ki-67 index, a widely used marker for cell proliferation, is essential for the management of nets. Among the commercial antibody tests available, the MIB-1 antibody is recommended by enets 1. The usefulness of Ki-67 stems from the fact that this protein is present and detectable in the nucleus during all active phases of the cell cycle, but that it is absent from resting cells 25. As a guide to medical treatment of nets 26, Ki-67 is regarded as a prognostic factor for survival and a surrogate marker of biologic behaviour 27.
It is recommended that the Ki-67 index be determined in 2000 tumour cells within areas of highest observed nuclear labelling 1,24. Computer-assisted image analysis may help to reduce inter-observer variability in Ki-67 assessment 28, but more research is required in this area.
It should be noted that, to ensure accurate Ki-67 results, adequate biopsy material is required; a pathologist should be consulted before biopsy to ensure that an adequate sample is obtained.
4.4.1. Ki-67 as a Predictive and Prognostic Marker
Although Ki-67 is frequently used to subclassify these tumours, there are no well-established, validated cut-off points. In the most recent TNM classification of foregut nets, 3 groups are suggested: ≤ 2%, 3%–20%, and >20%. Other authors suggest that a level of ≥ 5% be considered indicative of higher-proliferating tumours more likely to benefit from chemotherapy; other treatments—somatostatin analogs (ssas), interferon, and potentially, mammalian target of rapamycin (mtor) inhibitors and anti-angiogenic therapy—are considered for tumours with Ki-67 below 5% 27.
Tumour heterogeneity and changes in proliferation between primary and metastatic sites may significantly alter the Ki-67 index and limit its utility. The Ki-67 index should be used only as a guide; the prognostic and predictive value of Ki-67 needs to be established in a prospective manner. The recent publication on pathology reporting of nets indicated considerable disparity between the European and the U.S. recommendations for Ki-67 reporting. The latter group felt that Ki-67 is not always necessary (reflecting current practice and capabilities). In the Canadian context, it is recommended that the Ki-67 index (and number of mitoses per 10 hpf) be consistently reported until further data clarify or resolve the contention. The clinical behaviour of the tumour should always be considered the guiding factor when choosing a management strategy.
4.5. Other Immunohistochemical Stains
The typical architectural and cytologic features of nets are usually recognized on routine hematoxylin and eosin preparations, but immunohistochemical stains can be useful adjuncts in confirming the neuroendocrine nature of a tumour, particularly in the setting of poor histologic differentiation. Commonly used antibodies include cytosolic (neuron-specific enolase), small vesicle (synaptophysin), secretory granule (CgA), and cell membrane (neural cell adhesion molecule, CD56) markers.
4.6. Recommendations
Use of the 2007 who classification system is recommended to ensure consistency in nomenclature.
Known or possible features of prognostic significance should be routinely reported as outlined earlier. The proposed TNM staging and grading system proposed by enets may be used, with the caveat that this system still requires prospective validation.
At high-volume centres, review of pathologic specimens is encouraged to maximize consistency in reporting and to ensure proper management. The College of American Pathologists provides a checklist online 21.
5. CLINICAL MANIFESTATIONS
The clinical manifestations of gastroenterohepatic nets can overlap with a number of benign conditions, potentially leading to significant delays in diagnosis. Primary intestinal lesions may present asymptomatically, with nonspecific abdominal complaints, or with symptoms related to mass effect or partial intestinal obstruction. Symptoms also depend on whether a net secretes biologically active hormones (serotonin, vasoactive intestinal peptide, or gastrin, for instance) or whether it is non-functional 2,6,8,29. Owing to the relatively slow-growing nature of many nets, and to the nonspecific symptomatologies caused by hormone overproduction, alternative diagnoses such as irritable bowel syndrome, colitis, menopause, and asthma are often reached in error because of a failure to consider a diagnosis of net. Primary care physicians should consider the possibility of net in the appropriate clinical settings so that early diagnosis of these tumours is not missed.
5.1. Functional Tumours
Clinical suspicion of a functional net is commonly raised because of symptomatic manifestations of excess hormone production. The most frequently observed is the carcinoid syndrome caused by high levels of circulating serotonin. Table ii presents manifestations of the carcinoid syndrome, which can include facial flushing, diarrhea, and bronchoconstriction 6,30–32. Right-sided heart failure may be present at the time of diagnosis in long-standing carcinoid syndrome 6,30–32. Flushing can be provoked by certain drugs, some foods (for example, nuts and cheese), and alcohol 32.
TABLE II.
Clinical manifestations of carcinoid syndrome 6
| Clinical presentation | Rate (%) | Examples |
|---|---|---|
| Vasomotor symptoms | 90 | Facial flushing, telangiectasia, chronic facial cyanosis, rhinitis |
| Increased intestinal motility | 80 | Diarrhea, borborygmia, abdominal pain |
| Heart failure | 40 | Endocardial fibrosis, tricuspid insufficiency, pulmonary stenosis |
| Bronchoconstriction | 15 | Asthma |
Excess serotonin [metabolized to 5-hydroxyindoleacetic acid (5-hiaa)] appears to be the major contributor to carcinoid syndrome 32,33. Carcinoid syndrome arises primarily in the setting of hepatic metastases or because of primary lesions with direct drainage into the systemic as opposed to the portal circulation (primary ovarian nets, for instance). The severity of carcinoid syndrome may correlate with urinary 5-hiaa levels 33.
5.2. Non-functional Tumours
Local growth and metastasis—and also mesenteric fibrosis—may lead to abdominal discomfort, bowel obstruction, and diarrhea 6,34. However, in many cases, non-functional tumours are asymptomatic, and because they are slow-growing, they may go undetected until they have progressed to advanced disease. Indeed, they are often found incidentally during surgery, and their neuroendocrine origin may be recognized only after histologic examination. Some patients with these tumours should remain under watchful observation, because they may progress to more aggressive disease that requires therapy.
6. BIOCHEMICAL MARKERS
The detection of substances specific for particular types of nets can facilitate a more exact diagnosis and permit earlier detection, which may contribute to improved control of syndromes related to hormone oversecretion, such as carcinoid heart disease 29. Two biochemical products that can be measured and used to aid in diagnosis and surveillance of well-differentiated gastroenterohepatic nets are 5-hiaa and CgA.
6.1. 5-HIAA
Serotonin released by functional tumours is metabolized by monoamine oxidases to 5-hiaa in the liver, lungs, and brain. When measured in a 24-hour urine collection, 5-hiaa has a sensitivity of 73% and a specificity of 100% for diagnosing well-differentiated functional gastroenterohepatic nets 35. Levels of 5-hiaa may not be elevated in non-functional tumours.
The 24-hour urinary 5-hiaa test is a part of standard testing for these tumours and is also highly sensitive for diagnosing and monitoring treatment of the carcinoid syndrome 29.
Compared with patients having normal urinary 5-hiaa levels, patients with elevated levels, whether symptomatic or not, tend to have a poorer prognosis 36–38. The severity of carcinoid syndrome symptomatology correlates with the level of 5-hiaa elevation. There is also evidence that long-standing significant elevations in 5-hiaa may be associated with carcinoid heart disease 39–42.
Levels of 5-hiaa can also be used to assess biochemical response to ssas, with a 50% reduction from pretreatment levels and control of symptoms being indicative of response. However, because flushing is mediated by different hormones in foregut and midgut tumours, some patients with a net will have symptoms of flushing with low or normal levels of 5-hiaa 43,44. Although symptoms may be controlled without normalizing urinary 5-hiaa, elevated 5-hiaa has been associated with progression of carcinoid heart disease; it is therefore important to try to normalize this value 41. Some patients with elevated 5-hiaa may be asymptomatic; the role of ssas in this group of patients is controversial.
The normal range for urinary 5-hiaa is 3–15 mg/24 h, but this figure may vary depending on the laboratory. Practical challenges associated with 5-hiaa testing include the inconvenience of collecting a 24-hour urine sample, and the fact that readings may be affected by certain foods and drugs (Table iii).
TABLE III.
|
Potentially causes |
||
|---|---|---|
| False-positive results | False-negative results | |
| Drugs | Acetaminophen Caffeine Fluorouracil Methysergide Naproxen Non-prescription serotonin Diazepam Ephedrine Glycerol guaiacolate Nicotine Phenobarbital |
Acetylsalicylic acid Adrenocorticotropic hormone Levodopa Methyldopa Phenothiazines Ethyl alcohol Imipramine Isoniazid Monoamine oxidase inhibitors Tricyclic antidepressants |
| Foods | Avocado Bananas Eggplant Pineapple Plums Walnuts Plantain Tomato |
|
6.2. CgA
Chromogranin A is found in the wall of secretory granules and is co-released with hormones. Chromogranins are precursors of various biologically active peptides, and they may play a role in the progression and metastasis of nets 29. Measurement of circulating CgA is particularly useful as a biochemical marker because, unlike 5-hiaa, CgA may be expressed by functional and non-functional tumours alike.
The sensitivity of CgA for nets has been found to be 62.9%, and the specificity, 98.4% 43,44,47. Levels of CgA are elevated in 85%–100% of patients with well-differentiated gastroenterohepatic nets, regardless of whether the tumour is functional or nonfunctional 48,49. In classical midgut nets, CgA levels are elevated to 100–1000 times normal 48,49. Serial CgA evaluations may be helpful in the monitoring of both functional and non-functional nets either being observed or on therapy.
Table iv lists factors that may cause false elevations in CgA.
TABLE IV.
Factors causing false elevations in chromogranin A
| Inflammatory conditions |
| Renal insufficiency |
| Type A gastritis |
| Treatment with proton pump inhibitors |
6.3. Recommendations
At diagnosis, 5-hiaa and CgA should be measured in all patients.
In asymptomatic patients who have undergone complete resection and show no signs of disease, CgA should be evaluated as part of annual surveillance.
In patients with active functional disease, 5-hiaa and CgA should be used to monitor treatment, to follow changes in symptomatology, to evaluate for tumour growth, and so on.
7. DIAGNOSTIC IMAGING
Radiographic and nuclear imaging play an important role in the diagnosis and management of gastroenterohepatic nets.
7.1. Conventional Imaging
Ultrasonography, computed tomography (ct) imaging, and magnetic resonance imaging (mri) can be used for determining the anatomic location and extent of tumours, and for monitoring response to treatment.
7.1.1. CT
Triphasic ct of the liver at diagnosis should be considered a standard component of the diagnostic imaging of nets, because the liver is the most common site of metastatic involvement and because of the wide availability of this diagnostic modality. Triphasic ct can also be used to evaluate tumours before radiofrequency ablation and hepatic arterial embolization, and it should be routinely used in the preoperative evaluation of potentially resectable primary or metastatic lesions. Sequential evaluation can assist in monitoring disease status in response to therapy or in the surveillance setting.
7.1.2. Ultrasonography
Most guidelines for the diagnosis and management of nets recommend the inclusion of ultrasonography in the range of imaging modalities used to detect and monitor nets 6,9. This technique may serve as an adjunct to ct imaging in certain clinical situations—such as assessing tumour volume before radiofrequency ablation and hepatic arterial embolization, and differentiating lesions with uncertain ct imaging characteristics.
7.1.3. MRI
In detecting nets of the pancreas, mri has a sensitivity of 74%–100% 50,51. The technique is not routinely recommended in gastroenterohepatic nets, although it can be used to characterize hepatic lesions before surgery if the diagnosis is not clear, and in general, it may be useful if ct or ultrasonography results are conflicting or unhelpful. Magnetic resonance imaging may also be used in particular cases when ct may be contraindicated or less sensitive for disease detection; in patients having contrast allergies or hepatic steatosis; and in consideration of possible liver resection or tumour-debulking surgeries.
7.2. Other Imaging Modalities
Other modalities—specifically, 111In-pentetreotide scintigraphy and meta-iodobenzylguanidine (mibg) scintigraphy—are important for identifying and staging tumours. Positron-emission tomography (pet) imaging currently has a limited role in net management.
7.2.1. 111In-Pentetreotide Scintigraphy
Scintigraphy using 111In-pentetreotide is the most important imaging investigation for identifying and staging gastroenterohepatic nets. An 111In-labelled ssa, pentetreotide, which shares the somatostatin receptor–binding profile of octreotide, concentrates in tumours with somatostatin receptor subtypes 2 and 5, the subtypes most frequently expressed in this tumour type 52.
Scintigraphy using 111In-pentetreotide can detect octreotide-avid lesions throughout the body, and therefore can assist in disease staging, preoperative evaluation, surveillance, and monitoring response to therapeutic intervention 52. Available data also suggest that somatostatin-avid disease on scintigraphy may imply a preferential benefit in disease control with therapeutic doses of octreotide (discussed later in this article), although long-term data are lacking 53–56.
It is generally suggested that octreotide therapy should be interrupted before scintigraphy. For patients treated with subcutaneous immediate-release (ir) octreotide, treatment should be stopped for 24 hours before the scan. Injections can be restarted 4–6 hours after the scan without interfering with the quality of the images. For patients treated with the long-acting release (lar) formulation, the scan should be performed just before the next lar administration. However, in patients with severe functional symptoms, data from several centres and the present group’s experience suggest that, in such situations, somatostatin therapy can be maintained 48.
7.2.2. MIBG Scintigraphy
Meta-iodobenzylguanidine concentrates in gastroenterohepatic nets, and 123I- or 131I-labelled mibg can be useful for staging, monitoring, and conducting preoperative evaluations, particularly in cases in which an 111In-pentetreotide scan is negative or unhelpful 57. Radiolabelled mibg testing may also be useful for identifying patients that may be candidates for radiolabelled mibg therapy (discussed later in this article) 56,58–60.
To minimize the risk of false-negative scans, it is important to ensure an adequate dose of 123I- or 131I-labelled mibg. Patients should also be questioned carefully about prescription and recreational drug use; false-negative scans from interference with mibg uptake can be caused by the beta-blocker labetalol and by cocaine.
About 10% of patients with a negative 111In-pentetreotide scintigraphy result have a positive mibg scan. The latter technique is therefore recommended if 111In-pentetreotide scintigraphy is negative or if the patient is a candidate for mibg 131I therapy.
7.2.3. PET
Imaging by pet is not routinely recommended for nets because of the inherently low metabolic activity of well-differentiated tumours, but it may be used in cases in which other imaging techniques are contradictory or unhelpful 56,60,61. As neuroendocrine-specific markers become available, pet imaging has the potential to become a much more sensitive technique. Indeed, a recent European study found that 18F-l-dihydroxyphenylalanine pet compared favourably with conventional imaging 62; however, the utility of the technique is currently limited because of lack of availability in Canada.
7.3. Recommendations
Baseline scanning at diagnosis should include one or more of triphasic ct imaging of the liver, 111In-pentetreotide scintigraphy, and mibg scintigraphy.
Imaging modalities for postoperative evaluation or for disease status monitoring in the metastatic setting should include the most sensitive imaging modality determined at diagnosis. Because of the highly variable clinical presentation of nets, and because of the individualization of therapeutic interventions, imaging intervals and modalities may both vary depending on the goals of care and the intensity of therapy.
8. ALGORITHM
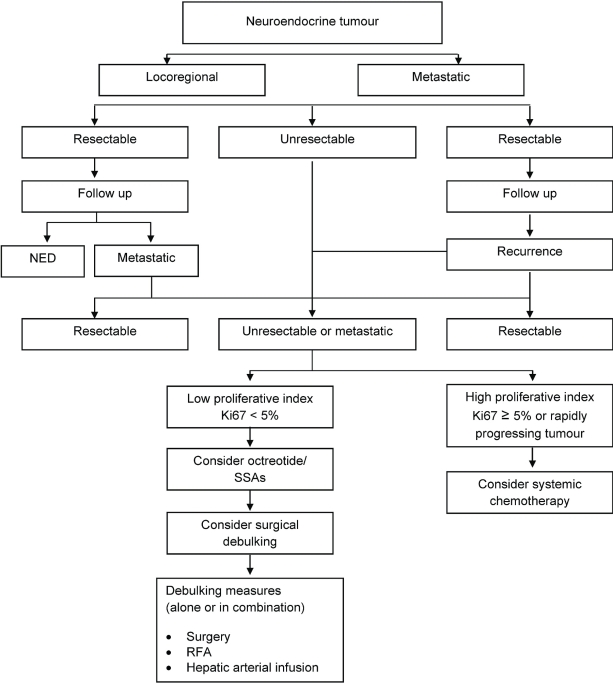
The main goals of net management are increased survival, symptom control, biochemical control (that is, lowering or even normalizing of 5-hiaa levels), objective tumour control, and improvement in quality of life. Figure 2 shows a suggested algorithm for management in the Canadian context.
Figure 2.
Suggested management algorithm for well-differentiated gastroenterohepatic neuroendocrine tumours. ned = no evidence of disease; ssas = somatostatin analogs; rfa = radiofrequency ablation.
9. SURGICAL MANAGEMENT
Surgical management of gastroenterohepatic nets can be performed with curative or palliative intent. Even in the setting of metastatic disease, most patients benefit from primary tumour resection or tumour debulking. In limited or locoregional disease, surgery has curative potential and has also been found to improve survival for patients with metastatic disease 26,48,63,64. As well, because of the intense mesenteric fibrotic changes elicited by primary lesions within the gastrointestinal tract, primary disease resection will reduce or eliminate the risk of progressive symptomatology from incomplete or complete bowel obstruction. Early intervention may also maximize the chance of successful surgery and reduce technical complications from progressive fibrosis. If technically feasible, the primary tumour should be removed by segmental resection, including lymphadenectomy.
A prophylactic cholecystectomy should be considered in every patient undergoing surgery for nets of the digestive tract. This procedure mitigates the biliary toxicity of ssa therapy and avoids chemical cholecystitis if transcatheter arterial chemoembolization (tace) is performed in the future 48,65,66.
Good communication between surgical and anesthesia teams is important to ensure that octreotide is administered perioperatively and intraoperatively. Intraoperative carcinoid crisis is a rare but serious aggravation of symptoms that can be provoked by surgery or anesthesia in patients with functional tumours 32. Preoperative ssa administration is required in cases of functional nets. In patients whose symptoms are well controlled with a long-acting ssa, a supplemental dose of short-acting octreotide should be given 1–2 hours before surgery, and in some cases, an intraoperative intravenous (IV) infusion should be considered (bolus IV doses of 500–1000 μg, repeated every 5 minutes until symptom control is achieved) 48. Intraoperative signs and symptoms can include refractory hypotension and can be treated with intraoperative intravenous octreotide infusions titrated to blood pressure control. For emergency surgery, octreotide 500–1000 μg IV bolus or 500 μg subcutaneously can be given 1–2 hours before the procedure, followed by an IV infusion of 50–200 μg/h, if needed.
9.1. Curative Surgery
Resection of the primary tumour should be accompanied by careful intraoperative evaluation for synchronous tumours or metastatic disease in the liver. Resectable liver metastasis should be resected 67.
In cases having borderline liver reserve, selective portal-vein embolization is considered so as to induce hypertrophy of the future remaining liver. Liver transplantation can also be considered in young patients (below 50 years of age) when the primary tumour originates in the gastrointestinal tract, is drained by the venous portal system, and has been previously removed with curative intent, and when disease progression is controlled for at least 6 months before transplantation 68.
No currently available data support the use of adjuvant systemic or radioisotope treatment after complete resection.
9.2. Palliative Surgery
For patients with nets of the gastrointestinal tract in whom complete resection is not possible, cytoreductive resection should be considered. The goals of non-curative surgery include stabilization or improvement of symptoms and lowering or normalization of 5-hiaa levels. Palliative surgery in the appropriate clinical context can reduce tumour bulk, prevent or delay complications from local or distant disease, and reduce 5-hiaa levels 2,26.
The rationale for palliative surgical intervention derives from the fact that nets of the gastrointestinal tract are usually slow-growing, and patients may experience prolonged survival even with metastatic disease. A meta-analysis of cytoreductive partial hepatectomy in patients with malignant nets observed a 5-year survival rate of 71% and complete resolution of carcinoid syndrome symptoms lasting 4–120 months in 86% of cases 69.
Cytoreductive surgery can consist of a combination of multiple techniques, such as hepatectomy, local ablative therapies (radiofrequency ablation, cryotherapy, or microwave therapy), intra-abdominal organ resection, and bypass procedures 48. Patients considered for cytoreductive surgery should be assessed by a multidisciplinary team that includes both medical and surgical oncologic expertise to ensure coordination of care and exploration of case-specific palliative options.
Hepatic arterial embolization, with or without chemotherapy, represents a further cytoreductive option for patients who are not candidates for surgery. A recent report of 122 patients undergoing this procedure revealed radiographic tumour regression in 82% of patients, with stabilization of disease in 12% 70. Median duration of ct response was 19 months; improvement in symptoms occurred in 92% of patients for a median duration of 13 months 70. In addition, recent reports suggest that radioembolization for unresectable metastatic liver nets may also be effective. In one study, 148 patients were treated with 185 separate procedures. Imaging response was stable in 22.7% of patients, partial in 60.5%, and complete in 2.7%, with progressive disease occurring in 4.9%. No radiation-induced liver failure occurred, and median survival was 70 months. The advantage of radioembolization appears to be a morbidity rate lower than that seen with tace 71. Short-acting ssas must also be used immediately before and during hepatic arterial embolization in patients with functional nets.
9.3. Recommendations
Definitive resection of the primary tumour should always be performed whenever technically feasible.
Prophylactic cholecystectomy should be considered during surgery for nets.
In patients with functional tumours, short-acting ssas should be given peri- and intraoperatively during invasive surgery to prevent carcinoid crisis.
In patients with localized, locoregional, or resectable metastatic disease, curative surgery should be considered if technically and clinically feasible.
If technically feasible and clinically appropriate, cytoreductive surgery to achieve maximal debulking and palliation of symptoms should be considered in all patients with unresectable metastatic disease.
Surgical approaches should be individualized, with the ultimate therapeutic decisions and approaches decided after a full multidisciplinary evaluation.
Surgical treatment of complex cases should be undertaken by surgeons having expertise and experience with these tumours.
Unresectable liver disease may be considered for tace or radioembolization.
10. MEDICAL MANAGEMENT
10.1. SSAs
Somatostatin analogs bind selectively to somatostatin receptors and are indicated in the management of symptomatic functional gastroenterohepatic nets to lower hormone production, to provide symptom control, and to reduce the risk of carcinoid crises and other severe events 32.
10.1.1. Functional Tumours
Somatostatin analogs have been used primarily to relieve symptoms of carcinoid syndrome; they can significantly improve symptoms in most patients by reducing or normalizing circulating 5-hiaa levels 32. As noted earlier, short-acting octreotide may also be used to prevent carcinoid crisis during procedures such as surgery or hepatic arterial infusion 5,26,48,63.
Treatment of Symptomatic Patients: Patients with symptomatic tumours should be treated with ssa therapy to manage symptoms, reduce 5-hiaa levels, and stabilize tumour growth 4,48,72. Treatment initiation usually involves subcutaneous administration of short-acting octreotide for 3–7 days to ensure tolerability, followed by administration of the more convenient lar formulation 4. Lifelong treatment is likely. Octreotide is currently the only ssa approved for the treatment of nets in Canada (other ssas can be considered in cases of intolerance to octreotide):
Octreotide ir: 100–500 μg subcutaneously three times daily
Octreotide lar: intramuscularly starting at 30 mg every 4 weeks; titrate up as required
The usual starting dose for octreotide lar of 30 mg every 4 weeks may be titrated up to 60 mg for breakthrough symptoms, if needed. It may be necessary to go beyond 60 mg based on perceived patient benefit. Patients with “breakthrough” symptoms during the 4th week of therapy may also be considered for injections every 3 weeks.
Side effects may include nausea, abdominal pain, flatulence, vomiting, and diarrhea, which usually resolve within days of starting therapy. In patients with steatorrhea, pancreatic enzyme therapy should be considered. Cholelithiasis and biliary sludge can develop as a long-term complication in up to 50% of patients 73. Bile acid colitis from previous terminal ileal resection should be treated with cholestyramine. Tachyphylaxis and resistance to ssa therapy can occur, but other causes should be considered, including progressive disease.
Treatment of Asymptomatic Patients with Elevated 5-HIAA: Patients with elevated 5-hiaa levels remain at risk for carcinoid heart disease 33,39–42,74,75. Case series of patients with carcinoid heart disease have described an association between elevated 5-hiaa and the development and pathogenesis of carcinoid heart disease 33,39–42. Therapy with ssa reduces circulating serotonin levels and may stabilize the progression of carcinoid heart disease 74,75.
Because elevated 5-hiaa is almost universally viewed as a predictor of cardiac complications and a marker of tumour growth or progression, the consensus of the present expert group was that all patients with elevated 5-hiaa levels (>70 mg/24 h)—even those who are asymptomatic—should be considered for ssa therapy.
10.1.2. Non-functional Tumours
The role of ssas in non-functional disease has been under debate; however, recent evidence from the Placebo-Controlled, Double-Blind, Prospective Randomized Study of the Effect of Octreotide lar in the Control of Tumour Growth in Patients with Metastatic Neuroendocrine Midgut Tumours (promid) trial has demonstrated the utility of octreotide lar in these patients for tumour stabilization. Based on the promid data, asymptomatic patients with progressive disease should be monitored closely with serial 5-hiaa, imaging (ct and mri), and CgA evaluations, and they should be treated with octreotide lar (evidence: Level 1).
Antitumour Effects: The results of the promid trial were presented in January 2009 72 and subsequently published 76. The intent of the study was to evaluate the potential antitumour effect of octreotide lar in newly diagnosed, treatment-naïve patients with well-differentiated midgut nets, both functional and non-functional. Patients were randomized to receive octreotide lar 30 mg or placebo intramuscularly every 4 weeks, and the primary endpoint was time to tumour progression (ttp). Overall, the results demonstrated that octreotide lar significantly increased ttp. Median ttp was 14.3 months [95% confidence interval (ci): 11.0 to 28.8 months] for octreotide lar compared with 6.0 months (95% ci: 3.7 to 9.4 months) for placebo. This effect represents a 66% reduction in disease progression (hazard ratio: 0.34; 95% ci: 0.20 to 0.59; p = 0.000072).
Tumour stabilization was shown in patients with functional and non-functional nets, regardless of CgA levels. The effect was most evident in patients with hepatic loads below 10%; however, patients with a hepatic tumour load above 10% experienced a clinical benefit as well.
The evidence is now sufficient to recommend the use of octreotide lar for tumour stabilization in newly diagnosed treatment-naïve patients with well-differentiated midgut nets. Octreotide lar should be strongly considered for patients with fore- and hindgut nets as well.
10.2. Other Treatment Alternatives
10.2.1. Interferon alfa
Interferon alfa inhibits protein and hormone synthesis in tumour cells, inhibits angiogenesis, and stimulates the immune system. It can be used for low-proliferating nets, either alone or in combination with ssas. Interferon therapy requires careful monitoring; toxicities include severe fatigue, neutropenia, hepatotoxicity, autoimmune disorders, and depression or other mental disturbances 6,32,77. The recommended doses for gastroenterohepatic nets are lower than for most other indications, and therefore tolerance may be improved.
The recommended dose of interferon alfa is 3–5 million units subcutaneously, 3–5 times per week. The dose should be individually titrated, aiming for a reduction in leukocyte count to approximately 3×109/L.
A suggested dose (not yet established) for pegylated interferon alfa is 75–150 μg subcutaneously per week.
10.2.2. Cytotoxic Treatments
Cytotoxic treatment is usually used for tumours with a high proliferative capacity (Ki-67 ≥ 5%); it is of less use in low-proliferating gastroenterohepatic nets. Indeed, these tumours are often resistant to chemotherapy, with tumour response rates of only 10%–16% 78.
Streptozocin in combination with 5-fluorouracil or doxorubicin is the most commonly reported regimen 27. Poorly differentiated nets are associated with an up to 67% response rate to etoposide plus cisplatin, but prognosis is poor, with a 2-year survival rate of less than 20% 7,60,79. Epirubicin, cisplatin, and fluorouracil combination therapy has been used, but results with that regimen are inconclusive. There is increasing evidence that regimens of temozolomide plus capecitabine 80,81 may be beneficial. A regimen of temozolomide and thalidomide has also been shown to be beneficial 82, but thalidomide is not currently available in Canada.
Given their side-effects profiles, most cytotoxic therapies should be used only when they are most likely to have an effect. Cytotoxic therapy may be considered to lower the proliferative capacity of highly proliferative disease, potentially improving the effectiveness of other treatment options, including resection or debulking, hepatic arterial infusion, and ssa, interferon alfa, or radioisotope therapy.
10.2.3. New Agents
New agents such as imatinib and mtor inhibitors (for example, RAD001) are being evaluated. Anti-angiogenic substances such as Endostatin (Entremed, Rockville, MD, U.S.A.), angiostatin, the new compound 2004-01-13IZD6126, and a new ssa that binds to somatostatin receptors 1, 2, 3, and 5 (SOM230) may also have future roles.
10.2.4. Other Agents
Other agents may be administered as required, depending on symptomatology. Examples include loperamide or diphenoxylate to treat diarrhea, and H1 or H2 blockers (or both) for histamine-secreting tumours.
10.3. Recommendations
For patients with symptomatic secretory tumours, ssas are the primary treatment.
Octreotide lar is recommended to stabilize tumour growth in patients with asymptomatic progressive disease.
Somatostatin analogs should be used to prevent or treat carcinoid crises before, during, and after procedures such as surgery and hepatic arterial embolization.
Interferon alfa (alone or in combination with ssas) may be used for low-proliferating gastroenterohepatic nets.
Cytotoxic treatment may be a first-line treatment for nets with a high proliferation index (Ki-67 ≥ 5%).
11. RADIATION THERAPY/RADIOISOTOPES
11.1. Radiation Therapy
External radiation therapy has limited value in nets; it often results in fibrosis and may therefore interfere with tumour evaluation. It may also cause loss of somatostatin receptors on tumour cell surfaces, thereby reducing the effectiveness of ssa therapy. For these reasons, radiotherapy is recommended only for bone and brain metastases.
11.2. Radioisotope Therapy
Tumour-targeted treatment with radioactive octreotide derivatives [111In-d-Phe(1)-Tyr(3)-octreotide (111In-dota-octreotide) or 90Y-dota-octreotide and 177Lu-dota-octreotate] and with 131I-mibg have been associated with varied response rates and clinical benefit. One large single-institution study of 310 patients with gastroenteropancreatic nets treated with 177Lu-dota-octreotate documented a 28% response rate and a median overall survival from start of treatment of almost 4 years 83. Because of regulatory constraints associated with the synthesis of 177Lu-dota-octreotate, the treatment is not currently available in Canada.
12. MONITORING AND FOLLOW-UP
Generally, nets are slow-growing, but they may progress faster if they have a high Ki-67 index (≥5%) or are poorly differentiated. Patients should be followed more closely during the first year after diagnosis to establish the “tempo” of the disease. If disease is stable or gradually progressive, subsequent investigations may occur less frequently. Occasionally, nets may become more aggressive, requiring a change in frequency of follow-up. If a patient’s status shows a significant clinical change, an increase in biomarkers, or new sites of disease, a complete reassessment is required, and more frequent follow-up tests may be needed.
Routine evaluations to detect carcinoid heart disease in its early stages can improve prognosis 45. Follow-up should include early echocardiograms for patients with elevated 5-hiaa.
Quality of life should also be regularly assessed during treatment. A specific quality-of-life score is currently being developed for patients with nets; at present, a useful tool is the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–C30 9.
Table v stratifies patients according to potential risk of recurrence and outlines suggested follow-up schedules.
TABLE V.
Monitoring and follow-up
| Disease type | Recommended follow-up | |
|---|---|---|
| Resected disease | ||
| Low-risk | All of:
|
Risk of recurrence is low (for example, carcinoid appendix); follow-up is at the discretion of the physician |
| High-risk | Any one or more of:
|
Close follow-up tailored to the patient’s clinical presentation. |
Year 1:
| ||
Years 2–5:
| ||
| Metastatic disease or post-debulking (post-resection where macroscopic residual disease is not present) | Close follow-up tailored to the patient’s clinical presentation. | |
Year 1:
| ||
Years 2 and 3:
| ||
Years 4 and 5:
| ||
| Unresected disease | ||
| Metastatic disease or post-debulking (where macroscopic residual disease is present) | Close follow-up tailored to the patient’s clinical presentation. | |
Year 1:
| ||
Years 2–5:
| ||
5-hiaa = 5-hydroxyindoleacetic acid; CgA = chromogranin A; ct = computed tomography; mibg = meta-iodobenzylguanidine.
12.1. Recommendations
Patients should be stratified according to risk and followed accordingly—more frequently in the first year after diagnosis to determine the “tempo” of disease, and then at regular intervals thereafter depending on the risk of recurrence.
Patients with elevated 5-hiaa (>70 mg/24 h) should be routinely evaluated for carcinoid heart disease.
13. CARCINOID HEART DISEASE
Carcinoid syndrome is associated with the release of serotonin and other vasoactive substances by the tumour; exposure of the heart to high levels of these substances can result in endocardial damage, most commonly involving the right side of the heart, including the tricuspid valves, the pulmonary valves, and the endocardium. Heart failure is one of the most serious manifestations of carcinoid syndrome, and it occurs in 20%–70% of patients with metastatic disease 40,42. It is associated with a mortality rate as high as 50% of the mortality associated with these tumours, right ventricular failure being a major cause of death 33,84.
The role of serotonin in the development of carcinoid-related heart disease is controversial, but the indirect evidence appears compelling. Only patients with elevated 5-hiaa levels develop this complication 39–42; serotonin receptors have been identified in the heart 85–88; experimentally, stimulation of these receptors causes cardiac cell proliferation 89–91; and two drugs, fen-phen and methysergide, can cause identical cardiac pathology (both have chemical structures analogous to serotonin, and both can be shown to bind to heart serotonin receptors) 92.
13.1. Diagnosis
Early diagnosis of carcinoid heart disease is essential. The symptoms of carcinoid heart disease may be so subtle as to be attributed to noncardiac causes; as a result, it is advisable that patients with carcinoid syndrome have an echocardiogram at diagnosis. Patients who are predisposed to carcinoid heart disease should have an annual echocardiogram and follow-up with a cardiologist.
Carcinoid heart disease can appear in asymptomatic patients and in those with small increases in 5-hiaa, but it is more commonly associated with carcinoid syndrome and chronic elevation of 5-hiaa. Carcinoid heart disease is likely to develop in patients with longstanding elevations of 5-hiaa; it is uncommon in patients without elevated 5-hiaa 39–42. Asymptomatic patients with elevated 5-hiaa should also be closely monitored for the development of carcinoid heart disease.
13.2. Prevention
In patients at risk, routine examinations (for example, echocardiography, mri) should be conducted every 6–12 months to detect cardiac involvement in its early stages and to possibly initiate treatment 45.
Some indirect evidence supports the use of ssas to prevent or minimize carcinoid heart disease, but the efficacy of such therapy has not been demonstrated conclusively. Indeed, carcinoid heart disease may continue to progress even if 5-hiaa is carefully controlled 33. Nevertheless, patients with elevated 5-hiaa levels should be strongly considered for octreotide therapy.
13.3. Treatment
Once carcinoid heart disease is diagnosed, treatment should be initiated, and referral to a cardiologist should be arranged. Treatment is generally initiated according to Canadian Cardiovascular Society recommendations 93. Initial measures for heart failure should include education, risk-factor reduction, lifestyle modifications, restriction of salt and water intake, and monitoring of fluid balance and weight 93. Patients with heart failure and a left-ventricular ejection fraction below 40% should be treated with an angiotensin converting-enzyme (ace) inhibitor in combination with a beta-blocker unless a specific contraindication exists 93. Angiotensin receptor ii antagonists may be used in those who cannot tolerate ace inhibitors 93. Digoxin (with or without nitrates), diuretics, and spironolactone may be added in those with New York Heart Association class iii–iv heart failure and persistent symptoms 93. Some patients with carcinoid heart disease may benefit from cardiac valve replacement 94, but the correct timing of surgical valve replacement is not clear. As well, cardiac surgeons may be reluctant to treat patients with cancer. Nevertheless, adequate treatment of right-sided heart failure has been associated with improvements in symptomatology and quality of life 73.
13.4. Recommendations
Patients with carcinoid syndrome should have an echocardiogram at diagnosis. All patients should have an annual echocardiogram. Referral to a cardiologist or cardiac surgeon should be arranged if cardiac abnormalities are diagnosed.
Early intervention with octreotide should be considered for patients with persistently elevated 5-hiaa. Because long-term low-level exposure to serotonin may cause cardiac complications, reduction of 24-hour 5-hiaa urine excretion—even normalization, if possible—is an appropriate goal.
14. CONCLUSIONS
Gastroenterohepatic nets are uncommon tumours and early diagnosis remains a challenge, particularly given that some of the diagnostic and imaging tests recommended in these guidelines may not be available at all institutions. A high index of suspicion by primary care physicians in the appropriate clinical setting can facilitate early diagnosis, and early referral to centres of excellence should be considered. This neoplasm should not be regarded as an indolent tumour. Recent reviews of 5-year survival have shown improvements in recent years, with more striking improvements in single-institution 5-year survival rates at centres of expertise.
Optimal therapy delivered by a multidisciplinary team should include surgical and medical modalities as needed. Therapy with ssas is the primary treatment choice for patients with functional tumours, and octreotide lar is recommended for patients with non-functional tumours, given the growing evidence of its antiproliferative effect. However, regardless of the options ultimately chosen, a treatment plan—facilitated by the algorithm and the recommendations outlined in the present guideline—should be developed early so as to minimize symptoms, slow tumour progression, and ultimately improve quality of life for the patient.
16. CONFLICT OF INTEREST DISCLOSURES
All authors received support from Novartis for travel to and participation in the consensus meeting. In the past, several authors have received honoraria or travel reimbursements for educational work, speaking engagements, or consultancy: WK (Novartis, Pfizer, Canadian Medical Protective Association, AstraZeneca), JM (Novartis, Roche, Sanofi–Aventis), HK (Novartis, Pfizer, Roche, Sanofi–Aventis), JFO (Novartis), CR (Novartis), SVU (Novartis), RW (Novartis).
15. ACKNOWLEDGMENTS
Editing and writing assistance was provided by CME Solutions Canada. Funding was received from Novartis Pharmaceuticals Canada (Montreal, QC). The funding source was not involved in the design of the article, nor in the analysis and interpretation of data.
17. REFERENCES
- 1.Rindi G, Klöppel G, Alhman H, et al. all other Frascati Consensus Conference participants; and the European Neuroendocrine Tumour Society (enets) TNM staging of foregut (neuro) endocrine tumours: a consensus proposal including a grading system. Virchows Arch. 2006;449:395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen RT, Doherty GM. Carcinoid tumors and the carcinoid syndrome. In: DeVita VT Jr, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 6th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2001. pp. 1813–33. [Google Scholar]
- 3.Modlin IM, Lye KDA, Kidd M. A 5-decade analysis of 13,715 carcinoid tumours. Cancer. 2003;97:934–59. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 4.Maroun J, Kocha W, Kvols L, et al. Guidelines for the diagnosis and management of carcinoid tumours. Part 1: the gastrointestinal tract. A statement from a Canadian National Carcinoid Expert Group. Curr Oncol. 2006;13:67–76. doi: 10.3390/curroncol13020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Öberg K, Astrup L, Eriksson B, et al. on behalf of the Nordic NE Tumour Group Guidelines for the management of gastroenteropancreatic neuroendocrine tumours (including bronchopulmonary and thymic neoplasms). Part i—general overview. Acta Oncol. 2004;43:617–25. doi: 10.1080/02841860410018575. [DOI] [PubMed] [Google Scholar]
- 6.Öberg K, Astrup L, Eriksson B, et al. on behalf of the Nordic NE Tumour Group Guidelines for the management of gastroenteropancreatic neuroendocrine tumours (including bronchopulmonary and thymic neoplasms). Part ii—specific ne tumour types. Acta Oncol. 2004;43:626–36. doi: 10.1080/02841860410018584. [DOI] [PubMed] [Google Scholar]
- 7.Plöckinger U, Rindi G, Arnold R, et al. Guidelines for the diagnosis and treatment of neuroendocrine gastrointestinal tumours. A consensus statement on behalf of the European Neuroendocrine Tumour Society (enets) Neuroendocrinology. 2004;80:394–424. doi: 10.1159/000085237. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Neuroendocrine Tumours. V.1.2010 Jenkintown, PA: nccn2010[Available online at: www.nccn.org/professionals/physician_gls/PDF/neuroendocrine.pdf; cited April 15, 2010] [Google Scholar]
- 9.Ramage JK, Davies AH, Ardill J, et al. on behalf of the UK-NETwork for Neuroendocrine Tumours Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours. Gut. 2005;54(suppl 4):iv1–16. doi: 10.1136/gut.2004.053314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- 11.Ontario Cancer Registry (ocr) Cancer Incidence, Mortality, Survival, and Prevalence in Ontario [ocr and seer*Stat cd-rom] Toronto, ON: ocr2004 [Google Scholar]
- 12.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 13.Modlin IM, Lye KD, Kidd M. A 50-year analysis of 562 gastric carcinoids: small tumour or larger problem? Am J Gastroenterol. 2004;99:23–32. doi: 10.1046/j.1572-0241.2003.04027.x. [DOI] [PubMed] [Google Scholar]
- 14.Maggard M, O’Connell JB, Ko CY. Updated population-based review of carcinoid tumours. Ann Surg. 2004;240:117–22. doi: 10.1097/01.sla.0000129342.67174.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumours. Cancer. 1997;79:813–29. doi: 10.1002/(sici)1097-0142(19970215)79:4<813::aid-cncr19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Levi F, Te VC, Randimbison L, Rindi G, La Vecchia C. Epidemiology of carcinoid neoplasms in Vaud, Switzerland, 1974–97. Br J Cancer. 2000;83:952–5. doi: 10.1054/bjoc.2000.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams ED, Sandler M. The classification of carcinoid tumours. Lancet. 1963;1:238–9. doi: 10.1016/s0140-6736(63)90951-6. [DOI] [PubMed] [Google Scholar]
- 18.Capella C, Heitz PU, Höfler H, Solcia E, Klöppel G. Revised classification of neuroendocrine tumours of the lung, pancreas and gut. Digestion. 1994;55(suppl 3):11–23. doi: 10.1159/000201197. [DOI] [PubMed] [Google Scholar]
- 19.Kloppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the who classification. Ann N Y Acad Sci. 2004;1014:13–27. doi: 10.1196/annals.1294.002. [DOI] [PubMed] [Google Scholar]
- 20.Greene FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. 6th ed. New York: Springer–Verlag; 2002. [Google Scholar]
- 21.College of American Pathologists (cap) Home > CAP Reference Resources and Publications > Cancer > Cancer Protocols and Checklists [Web resource] Northfield, IL: cap2010[Available at: www.cap.org/apps/cap.portal?_nfpb=true&cntvwrPtlt_actionOverride=%2Fportlets%2FcontentViewer%2Fshow&_windowLabel=cntvwrPtlt&cntvwrPtlt{actionForm.contentReference}=committees%2Fcancer%2Fcancer_protocols%2Fprotocols_index.html&_state=maximized&_pageLabel=cntvwr; cited April 15, 2010] [Google Scholar]
- 22.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th ed. New York: Springer–Verlag;; 2009. [Google Scholar]
- 23.Rindi G, Klöppel G, Couvelard A, et al. TNM staging of midgut and hindgut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2007;451:757–62. doi: 10.1007/s00428-007-0452-1. [DOI] [PubMed] [Google Scholar]
- 24.Klimstra DS, Modlin IR, Adsay NV, et al. Pathology reporting of neuroendocrine tumors: application of the Delphic Consensus Process to the development of a minimum pathology data set. Am J Surg Pathol. 2010;34:300–13. doi: 10.1097/PAS.0b013e3181ce1447. [DOI] [PubMed] [Google Scholar]
- 25.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Öberg K. Diagnosis and treatment of carcinoid tumours. Expert Rev Anticancer Ther. 2003;3:863–77. doi: 10.1586/14737140.3.6.863. [DOI] [PubMed] [Google Scholar]
- 27.Vilar E, Salazar R, Pérez–García J, Cortes J, Oberg K, Tabernero J. Chemotherapy and role of the proliferation marker Ki-67 in digestive neuroendocrine tumours. Endocr Relat Cancer. 2007;14:221–32. doi: 10.1677/ERC-06-0074. [DOI] [PubMed] [Google Scholar]
- 28.Elie N, Plancoulaine B, Signolle JP, Herlin P. A simple way of quantifying immunostained cell nuclei on the whole histologic section. Cytometry A. 2003;56:37–45. doi: 10.1002/cyto.a.10075. [DOI] [PubMed] [Google Scholar]
- 29.Bajetta E, Ferrari L, Martinetti A, et al. Chromogranin A, neuron specific enolase, carcinoembryonic antigen, and hydroxyindole acetic acid evaluation in patients with neuroendocrine tumours. Cancer. 1999;86:858–65. doi: 10.1002/(sici)1097-0142(19990901)86:5<858::aid-cncr23>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 30.van der Horst–Schrivers AN, Wymenga AN, Links TP, Willemse PH, Kema IP, de Vries EG. Complications of midgut carcinoid tumours and carcinoid syndrome. Neuroendocrinology. 2004;80(suppl 1):28–32. doi: 10.1159/000080737. [DOI] [PubMed] [Google Scholar]
- 31.Lips CJ, Lentjes EG, Hoppener JW.The spectrum of carcinoid tumours and carcinoid syndromes Ann Clin Biochem 200340(pt 6)612–27. [DOI] [PubMed] [Google Scholar]
- 32.de Herder WW. Tumours of the midgut (jejunum, ileum and ascending colon, including carcinoid syndrome) Best Pract Res Clin Gastroenterol. 2005;19:705–15. doi: 10.1016/j.bpg.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Møller JE, Connolly HM, Rubin J, Seward JB, Modesto K, Pellikka PA. Factors associated with progression of carcinoid heart disease. N Engl J Med. 2003;348:1005–15. doi: 10.1056/NEJMoa021451. [DOI] [PubMed] [Google Scholar]
- 34.Plöckinger U, Wiedenmann B. Diagnosis of non-functioning neuro-endocrine gastro-enteropancreatic tumours. Neuroendocrinology. 2004;80(suppl 1):35–8. doi: 10.1159/000080739. [DOI] [PubMed] [Google Scholar]
- 35.Feldman JM, O’Dorisio TM. Role of neuropeptides and serotonin in the diagnosis of carcinoid tumours. Am J Med. 1986;81:41–8. doi: 10.1016/0002-9343(86)90583-8. [DOI] [PubMed] [Google Scholar]
- 36.Rorstad O. Prognostic indicators for carcinoid neuroendocrine tumors of the gastrointestinal tract. J Surg Oncol. 2005;89:151–60. doi: 10.1002/jso.20179. [DOI] [PubMed] [Google Scholar]
- 37.Agranovich AL, Anderson GH, Manji M, Acker BD, Macdonald WC, Threlfall WJ. Carcinoid tumour of the gastrointestinal tract: prognostic factors and disease outcome. J Surg Oncol. 1991;47:45–52. doi: 10.1002/jso.2930470111. [DOI] [PubMed] [Google Scholar]
- 38.Janson ET, Holmberg L, Stridsberg M, et al. Carcinoid tumors: analysis of prognostic factors and survival in 301 patients from a referral center. Ann Oncol. 1997;8:685–90. doi: 10.1023/a:1008215730767. [DOI] [PubMed] [Google Scholar]
- 39.Pellikka PA, Tajik AJ, Khandheria BK, et al. Carcinoid heart disease. Clinical and echocardiographic spectrum in 74 patients. Circulation. 1993;87:1188–96. doi: 10.1161/01.cir.87.4.1188. [DOI] [PubMed] [Google Scholar]
- 40.Lundin L, Norheim I, Landelius J, Oberg K, Theodorsson– Norheim E. Carcinoid heart disease: relationship of circulating vasoactive substances to ultrasound-detectable cardiac abnormalities. Circulation. 1988;77:264–9. doi: 10.1161/01.cir.77.2.264. [DOI] [PubMed] [Google Scholar]
- 41.Denney WD, Kemp WE, Jr, Anthony LB, Oates JA, Byrd BF., 3rd Echocardiographic and biochemical evaluation of the development and progression of carcinoid heart disease. J Am Coll Cardiol. 1998;32:1017–22. doi: 10.1016/s0735-1097(98)00354-4. [DOI] [PubMed] [Google Scholar]
- 42.Robiolio PA, Rigolin VH, Wilson JS, et al. Carcinoid heart disease. Correlation of high serotonin levels with valvular abnormalities detected by cardiac catheterization and echocardiography. Circulation. 1995;92:790–5. doi: 10.1161/01.cir.92.4.790. [DOI] [PubMed] [Google Scholar]
- 43.Eriksson B, Öberg K, Stridsberg M. Tumour markers in neuroendocrine tumours. Digestion. 2000;62(suppl 1):33–8. doi: 10.1159/000051853. [DOI] [PubMed] [Google Scholar]
- 44.Öberg K, Janson ET, Eriksson B. Tumour markers in neuroendocrine tumours. Ital J Gastroenterol Hepatol. 1999;31(suppl 2):S160–2. [PubMed] [Google Scholar]
- 45.Zuetenhorst JM, Taal BG. Metastatic carcinoid tumors: a clinical review. Oncologist. 2005;10:123–31. doi: 10.1634/theoncologist.10-2-123. [DOI] [PubMed] [Google Scholar]
- 46.Joy T, Walsh G, Tokmakejian S, Van Uum SH. Increase of urinary 5-hydroxyindoleacetic acid excretion but not serum chromogranin A following over-the-counter 5-hydroxytryptophan intake. Can J Gastroenterol. 2008;22:49–53. doi: 10.1155/2008/472159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nehar D, Lombard–Bohas C, Olivieri S, et al. Interest of chromogranin A for diagnosis and follow-up of endocrine tumours. Clin Endocrinol. 2004;60:644–52. doi: 10.1111/j.1365-2265.2004.02030.x. [DOI] [PubMed] [Google Scholar]
- 48.Öberg K, Kvols L, Caplin M, et al. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumours of the gastroenteropancreatic system. Ann Oncol. 2004;15:966–73. doi: 10.1093/annonc/mdh216. [DOI] [PubMed] [Google Scholar]
- 49.Jensen TB, Hilsted L, Rehfeld JF. Library of sequence-specific radioimmunoassays for human chromogranin A. Clin Chem. 1999;45:549–660. [PubMed] [Google Scholar]
- 50.Ichikawa T, Peterson MS, Federle MP, et al. Islet cell tumor of the pancreas: biphasic ct versus mr imaging in tumor detection. Radiology. 2000;216:163–71. doi: 10.1148/radiology.216.1.r00jl26163. [DOI] [PubMed] [Google Scholar]
- 51.Vick C, Zech CJ, Höpfner S, Waggershauser T, Reiser M. Imaging of neuroendocrine tumours of the pancreas [German] Radiologe. 2003;43:293–300. doi: 10.1007/s00117-003-0885-8. [DOI] [PubMed] [Google Scholar]
- 52.Reubi JC. Somatostatin and other peptide receptors as tools for tumor diagnosis and treatment. Neuroendocrinology. 2004;80(suppl 1):51–6. doi: 10.1159/000080742. [DOI] [PubMed] [Google Scholar]
- 53.Frilling A, Malago M, Martin H, Broelsch CE. Use of somatostatin receptor scintigraphy to image extrahepatic metastases of neuroendocrine tumours. Surgery. 1998;124:1000–4. doi: 10.1067/msy.1998.93919. [DOI] [PubMed] [Google Scholar]
- 54.Kisker O, Bartsch D, Weinel RJ, et al. The value of somatostatin-receptor scintigraphy in newly diagnosed endocrine gastroenteropancreatic tumors. J Am Coll Surg. 1997;184:487–92. [PubMed] [Google Scholar]
- 55.Kwekkeboom DJ, Krenning EP. Somatostatin receptor scintigraphy in patients with carcinoid tumours. World J Surg. 1996;20:157–61. doi: 10.1007/s002689900024. [DOI] [PubMed] [Google Scholar]
- 56.Kaltsas G, Rockall A, Papadogias D, Reznek R, Grossman AB. Recent advances in radiological and radionuclide imaging and therapy of neuroendocrine tumours. Eur J Endocrinol. 2004;151:15–27. doi: 10.1530/eje.0.1510015. [DOI] [PubMed] [Google Scholar]
- 57.Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumours. Endocr Rev. 2004;25:458–511. doi: 10.1210/er.2003-0014. [DOI] [PubMed] [Google Scholar]
- 58.Le Rest C, Bomanji JB, Costa DC, Townsend CE, Visvikis D, Ell PJ. Functional imaging of malignant paragangliomas and carcinoid tumours. Eur J Nucl Med. 2001;28:478–82. doi: 10.1007/s002590100475. [DOI] [PubMed] [Google Scholar]
- 59.Kaltsas G, Korbonits M, Heintz E, et al. Comparison of somatostatin analog and meta-iodobenzylguanidine radionuclides in the diagnosis and localization of advanced neuroendocrine tumors. J Clin Endocrinol Metab. 2001;86:895–902. doi: 10.1210/jcem.86.2.7194. [DOI] [PubMed] [Google Scholar]
- 60.Öberg K, Eriksson B. Nuclear medicine in the detection, staging and treatment of gastrointestinal carcinoid tumours. Best Pract Res Clin Endocrinol Metab. 2005;19:265–76. doi: 10.1016/j.beem.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 61.Gibril F, Jensen RT. Comparative analysis of diagnostic techniques for localization of gastrointestinal neuroendocrine tumours. Yale J Biol Med. 1997;70:509–22. [PMC free article] [PubMed] [Google Scholar]
- 62.Koopmans KP, de Vries EG, Kema IP, et al. Staging of carcinoid tumours with 18F-dopa pet: a prospective diagnostic accuracy study. Lancet Oncol. 2006;7:728–34. doi: 10.1016/S1470-2045(06)70801-4. [DOI] [PubMed] [Google Scholar]
- 63.Schnirer II, Yao JC, Ajani JA. Carcinoid—a comprehensive review. Acta Oncol. 2003;42:672–92. doi: 10.1080/02841860310010547. [DOI] [PubMed] [Google Scholar]
- 64.Woodside KJ, Townsend CM, Jr, Mark Evers B. Current management of gastrointestinal carcinoid tumours. J Gastrointest Surg. 2004;8:742–56. doi: 10.1016/j.gassur.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 65.Trendle MC, Moertel CG, Kvols LK. Incidence and morbidity of cholelithiasis in patients receiving chronic octreotide for metastatic carcinoid and malignant islet cell tumours. Cancer. 1997;79:830–4. doi: 10.1002/(sici)1097-0142(19970215)79:4<830::aid-cncr20>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 66.Sakamoto I, Aso N, Nagaoki K, et al. Complications associated with transcatheter arterial embolization for hepatic tumors. Radiographics. 1998;18:605–19. doi: 10.1148/radiographics.18.3.9599386. [DOI] [PubMed] [Google Scholar]
- 67.Elias D, Lasser P, Ducreux M, et al. Liver resection (and associated extrahepatic resections) for metastatic well-differentiated endocrine tumors: a 15-year single center prospective study. Surgery. 2003;133:375–82. doi: 10.1067/msy.2003.114. [DOI] [PubMed] [Google Scholar]
- 68.Coppa J, Pulvirenti A, Schiavo M, et al. Resection versus transplantation for liver metastases from neuroendocrine tumors. Transplant Proc. 2001;33:1537–9. doi: 10.1016/s0041-1345(00)02586-0. [DOI] [PubMed] [Google Scholar]
- 69.Que FG, Sarmiento JM, Nagorney DM, et al. Hepatic surgery for metastatic gastrointestinal neuroendocrine tumours. Cancer Control. 2002;9:67–79. doi: 10.1177/107327480200900111. [DOI] [PubMed] [Google Scholar]
- 70.Bloomston M, Al-Saif O, Klemanski D, et al. Hepatic artery chemoembolization in 122 patients with metastatic carcinoid tumor: lessons learned. J Gastrointest Surg. 2007;11:264–71. doi: 10.1007/s11605-007-0089-z. [DOI] [PubMed] [Google Scholar]
- 71.Kennedy AS, Dezarn WA, McNeillie P, et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol. 2008;31:271–9. doi: 10.1097/COC.0b013e31815e4557. [DOI] [PubMed] [Google Scholar]
- 72.Arnold R, Müller H, Schade–Brittinger C, et al. Placebocontrolled, double-blind, prospective, randomized study of the effect of octreotide lar in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the promid study group [abstract 121] Proc Am Soc Clin Oncol Gastrointest Cancer Symp 2009:. [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=63&abstractID=10363; cited March 23, 2010]
- 73.Modlin IM, Latich I, Kidd M, Zikusoka M, Eick G. Therapeutic options for gastrointestinal carcinoids. Clin Gastroenterol Hepatol. 2006;4:526–47. doi: 10.1016/j.cgh.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 74.Bhattacharyya S, Davar J, Dreyfus G, Caplin ME. Carcinoid heart disease. Circulation. 2007;116:2860–5. doi: 10.1161/CIRCULATIONAHA.107.701367. [DOI] [PubMed] [Google Scholar]
- 75.Gustafsson BI, Hauso O, Drozdov I, Kidd M, Modlin IM. Carcinoid heart disease. Int J Cardiol. 2008;129:318–24. doi: 10.1016/j.ijcard.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 76.Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the promid Study Group. J Clin Oncol. 2009;27:4656–63. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 77.Shah T, Caplin M. Endocrine tumours of the gastrointestinal tract. Biotherapy for metastatic endocrine tumours. Best Pract Res Clin Gastroenterol. 2005;19:617–36. doi: 10.1016/j.bpg.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 78.Sun W, Lipsitz S, Catalano P, Mailliard JA, Haller DG, on behalf of the Eastern Cooperative Oncology Group Phase ii/iii study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J Clin Oncol. 2005;23:4897–904. doi: 10.1200/JCO.2005.03.616. [DOI] [PubMed] [Google Scholar]
- 79.Florman S, Toure B, Kim L, et al. Liver transplantation for neuroendocrine tumours. J Gastrointest Surg. 2004;8:208–12. doi: 10.1016/j.gassur.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 80.Fine RL, Fogelman DR, Schreibman DM.Effective treatment of neuroendocrine tumors with temozolomide and capecitabine [abstract 4216] Proc Am Soc Clin Oncol 200523:. [Available online at: meeting.ascopubs.org/cgi/content/abstract/23/16_suppl/4216; cited March 30, 2010] [Google Scholar]
- 81.Isacoff WH, Moss RA, Pecora AL, Fine RL.Temozolomide/capecitabine therapy for metastatic neuroendocrine tumors of the pancreas. A retrospective review [abstract 14023] Proc Am Soc Clin Oncol 200624suppl [Available online at: meeting.ascopubs.org/cgi/content/abstract/24/18_suppl/14023; cited March 30, 2010] [Google Scholar]
- 82.Kulke MH, Stuart K, Enzinger PC, et al. Phase ii study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. J Clin Oncol. 2006;24:401–6. doi: 10.1200/JCO.2005.03.6046. [DOI] [PubMed] [Google Scholar]
- 83.Kwekkeboom DJ, de Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177Lu-dota 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–30. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 84.Quaedvlieg PF, Lamers CB, Taal BG. Carcinoid heart disease: an update. Scand J Gastroenterol Suppl. 2002;(236):66–71. doi: 10.1080/003655202320621481. [DOI] [PubMed] [Google Scholar]
- 85.Kaumann AJ, Levy FO. 5-Hydroxytryptamine receptors in the human cardiovascular system. Pharmacol Ther. 2006;111:674–706. doi: 10.1016/j.pharmthera.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 86.Roy A, Brand NJ, Yacoub MH. Expression of 5-hydroxytryptamine receptor subtype messenger rna in interstitial cells from human heart valves. J Heart Valve Dis. 2000;9:256–60. [PubMed] [Google Scholar]
- 87.Rajamannan NM, Caplice N, Anthikad F, et al. Cell proliferation in carcinoid valve disease: a mechanism for serotonin effects. J Heart Valve Dis. 2001;10:827–31. [PubMed] [Google Scholar]
- 88.Xu J, Jian B, Chu R, et al. Serotonin mechanisms in heart valve disease ii: the 5-HT2 receptor and its signaling pathway in aortic valve interstitial cells. Am J Pathol. 2002;161:2209–18. doi: 10.1016/S0002-9440(10)64497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gustafsson BI, Tommeras K, Nordrum I, et al. Long-term serotonin administration induces heart valve disease in rats. Circulation. 2005;111:1517–22. doi: 10.1161/01.CIR.0000159356.42064.48. [DOI] [PubMed] [Google Scholar]
- 90.Hauso Ø, Gustafsson BI, Loennechen JP, Stunes AK, Nordrum I, Waldum HL. Long-term serotonin effects in the rat are prevented by terguride. Regul Pept. 2007;143:39–46. doi: 10.1016/j.regpep.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 91.Musunuru S, Carpenter JE, Sippel RS, Kunnimalaiyaan M, Chen H. A mouse model of carcinoid syndrome and heart disease. J Surg Res. 2005;126:102–5. doi: 10.1016/j.jss.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 92.Rothman RB, Baumann MH, Savage JE, et al. Evidence for possible involvement of 5-HT2B receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102:2836–41. doi: 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]
- 93.Arnold JMO, Liu P, Demers C, et al. Canadian Cardiovascular Society consensus conference recommendations on heart failure 2006: diagnosis and management. Can J Cardiol. 2006;22:23–45. doi: 10.1016/s0828-282x(06)70237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wilhelmi M, Fritz MK, Fischer S, Haverich A, Harringer W. Triple valve replacement in a patient with severe carcinoid heart disease. Cardiovasc Surg. 2002;10:287–90. doi: 10.1016/s0967-2109(01)00137-5. [DOI] [PubMed] [Google Scholar]