Abstract
Background
Peritoneal seeding after abdominal surgery is a well known route of metastasis in intra-abdominal solid tumours. Direct mechanical contamination, local peritoneal trauma and subsequent inflammation, postoperative immunosuppression, and laparoscopic surgery are the proposed predisposing factors for this type of metastasis. These factors probably result in enhanced adhesion or growth of tumour cells. However, this route of metastasis has not yet been reported for lymphomas. Here, we report the first case of peritoneal seeding of lymphoma cells after an abdominal surgery.
Case Description
A 47-year-old man with mantle cell lymphoma had ascites because of infiltration of the liver. He underwent debulking splenectomy. The postoperative ascites cytology and control abdominal computed tomography imaging both confirmed peritoneal involvement and lymphoma progression. Demonstration of negative peritoneal involvement before surgery and close timing of peritoneal involvement after splenectomy suggested to us that the debulking surgery was the main cause of peritoneal seeding of lymphoma cells in our case.
Conclusions
Factors similar to those in solid tumour seeding may also be valid for lymphomas. Peritoneal seeding and consequent disease progression may be a potential complication of abdominal surgery in lymphoma with extensive intra-abdominal involvement.
Keywords: Lymphoma, seeding, progression, laparotomy, laparoscopy
1. INTRODUCTION
Peritoneal seeding is a well known complication of abdominal surgery for intra-abdominal solid tumours 1,2. Although lymphomas occasionally involve the peritoneum in their course, peritoneal seeding as a route of postoperative involvement has not yet been reported. Here, we report the first case of peritoneal dissemination and further progression of lymphoma after abdominal surgery, and we discuss pathophysiologic aspects of the case.
2. CASE DESCRIPTION
In January 2008, a 47-year-old man with massive splenomegaly and multiple superficial and abdominal pathologic lymphadenopathies was diagnosed as having CD20+ mantle cell lymphoma. He also had chronic hepatitis B viral infection. Three cycles of a chop regimen (cyclophosphamide 1200 mg daily, doxorubicin 80 mg daily, vincristine 2 mg daily, and methylprednisolone 80 mg daily for 5 days) was administered with lamivudine prophylaxis. However, splenomegaly progressed and ascites developed.
Splenectomy was planned for debulking and symptomatic relief in May 2008. At this point, analysis of the ascites revealed leukocytes 2300/mm3, neutrophils 400/mm3, lymphocytes 1400/mm3, total protein 2.7 g/dL, albumin 1.5 g/dL, lactate dehydrogenase (ldh) 219 U/L (serum normal: 240–480 U/L), and glucose 96 mg/dL. Simultaneous serum albumin was 3.2 g/dL, yielding a serum–ascites albumin gradient of 1.7 g/dL. Ascites cytology revealed no atypical cells. Peritoneal lymphoma involvement was therefore excluded, and spontaneous ascites infection was suspected. Ceftriaxone 2 g daily was therefore given for 13 days.
Control ascites cytology revealed a low number of normal-appearing lymphocytes (200/mm3). Upper gastrointestinal endoscopy revealed no esophageal varices. Computed tomography (ct) imaging of the abdomen revealed splenomegaly and multiple para-aortacaval lymph node enlargements. Trucut biopsy of the liver demonstrated lymphoma infiltration without any signs of cirrhosis. Meanwhile, the fourth cycle of chemotherapy was given as cop (cyclophosphamide 1200 mg daily, vincristine 2 mg daily, and methylprednisolone 80 mg daily for 5 days).
Splenectomy was performed in June 2008. Although the operation had been planned as laparoscopic surgery, it was switched to classical abdominal splenectomy after exploration of the massively enlarged spleen. The splenic artery was injured and repaired during the operation; thereafter, the surgery was completed without any further complication.
On the fifth postoperative day, the patient developed a high fever (38°C) and abdominal tenderness. An increase in C-reactive protein (crp) to 132 mg/L was noted. Ascites cytology revealed white blood cells (wbcs) 3400/mm3 (no differential available), total protein 1.4 g/dL, albumin 0.7 g/dL, ldh 232 U/L, and glucose 101 mg/dL. Secondary bacterial peritonitis was suspected, and ampicillin–sulbactam (4×2 g daily) was initiated. The patient’s fever persisted without any change in ascites cytology on the fourth day of treatment. Antibiotherapy was switched to imipenem–cilastatin 4×500 mg daily.
The fever responded partially to the new antibiotherapy, and crp declined to 55 mg/L. However, ascites cytology revealed an even higher wbc. Biochemical analysis of ascites revealed ldh 630 U/L and glucose 42 mg/dL. A peripheral smear and ascites cytology (Figure 1) showed lymphoma infiltration. During the follow-up period, the patient also developed bilateral pleural effusion attributable to lymphoma infiltration. Control abdominal ct imaging performed in July 2008 showed progression of the lymphoma, with multiple progressed lymph-node enlargements and peritonitis carcinomatosis as compared with the preoperative ct images (Figure 2).
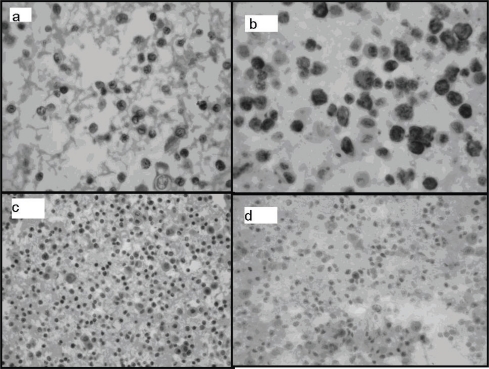
Figure 1.
Cell block sections (hematoxylin and eosin stain). (a) Atypical lymphoid cells, scarce small lymphocytes, and mesothelial cells. (b) Common immunoreactivity with CD20 in atypical cells. (c) Negative reaction with CD3 in atypical cells. (d) Bcl-1 (cyclin D1): focal nuclear immunoreactivity in atypical lymphoid cells.
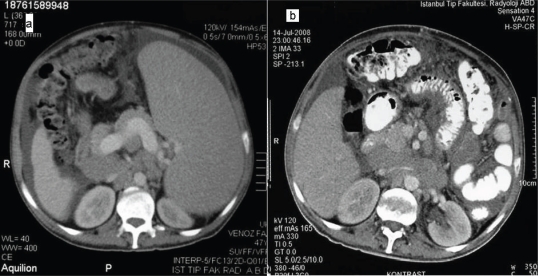
Figure 2.
(a) Preoperative and (b) postoperative computed tomography images revealing multiple progressed lymphadenomegalies and development of heterogeneous mesenteric adipose tissue suggestive of peritonitis carcinomatosa after debulking splenectomy.
The fever persisted, and the patient was given one more cycle of chop chemotherapy. On the sixth day of chemotherapy, the fever subsided, and imipenem–cilastatin therapy was therefore stopped at day 17. After a diagnosis of treatment-refractory lymphoma, the patient’s chemotherapy was switched to rituximab 375 mg/m2 daily (day 1), with bortezomib 1.3 mg/m2 and dexamethasone 40 mg on days 1, 4, 8, and 11. He received seven cycles of this chemotherapy—the last cycle in December 2008, at which time, his serosal effusions had completely disappeared.
3. DISCUSSION
Peritoneal seeding and tumour recurrence are well known complications of abdominal surgery after potentially curative surgical resection for intra-abdominal solid tumours 1,2 and were initially believed to be related to mechanical contamination of wounds. However, it is now recognized that there are other possible contributory factors, such as local peritoneal trauma and subsequent inflammation 3–5, postoperative immunosuppression 6,7, and laparoscopic mode of surgery 8. These factors probably result in enhanced adhesion or growth of tumour cells.
Initially, our patient had ascites as a result of hypoalbuminemia and liver involvement. Peritoneal involvement was also suspected, but was excluded by negative cytology, normal glucose and ldh levels in the ascites sample, portal hypertensive serum–ascites albumin gradient, and normalization of leukocyte counts after antibiotic therapy 9.
The patient was dyspneic because of massive splenomegaly and ascites. Splenectomy was therefore performed for both debulking and symptomatic relief. Development of fever and abdominal tenderness in the early postoperative period was suggestive of secondary bacterial peritonitis as a surgical complication. Ascites cytology and increased serum crp accorded with this diagnosis, and the patient partially responded with milder fever and limited crp response to the antibiotic therapy. But ascites samples remained rich in wbcs, the ascitic glucose level decreased, and the ldh level increased. We therefore suspected peritoneal involvement and confirmed it by ascites cytology.
During follow-up, the patient’s disease progressed by pleural involvement and progressive enlargement of lymph nodes. His fever subsided only after chemotherapy. Demonstration of negative peritoneal involvement before surgery and close timing of peritoneal involvement after surgery suggest peritoneal seeding of lymphoma attributable to the surgery.
During removal of a tumour, tumour cells may spill into the abdominal cavity. The proliferative and metastatic potentials of these spilled cells are very well preserved 10. Consequently, exfoliated tumour cells may undergo further division and give rise to metastases. However, implantation of spilled tumour cells on intact surfaces is an inefficient process compared with very efficient implantation on damaged surfaces 1,11.
Areas of trauma are preferred sites for metastatic tumour growth 4. A correlation between peritoneal surgical trauma and regional tumour recurrence has been observed 10,12. The dynamic process of peritoneal healing after ischemic damage of the peritoneal surfaces may be important in adhesion and growth of spilled tumour cells 13. An acute inflammatory reaction occurs even in the absence of any apparent bacterial contamination 14. The inflammatory sequelae after abdominal surgery promotes tumour recurrence based on the cellular component of the inflammatory process (especially neutrophils) 5,10. Also, surgical trauma may promote tumour recurrence by producing locally active tumour-promoting agents 13,15. Furthermore, the enhancing effect of trauma is not restricted solely to the inflicted site, but has a generalized character 10. Avoidance of unnecessary surgical trauma by using gentle techniques and materials is therefore indicated 10.
In our patient, the surgery was difficult and more technically traumatic because of massive splenomegaly. Also, the splenic artery was iatrogenically injured, permitting more tumoural cells to be spilled into the abdomen. These traumatic factors might have induced subsequent dissemination of lymphoma in our patient. Additionally, our patient experienced secondary bacterial peritonitis causing an additional inflammatory reaction. We suggest that the increased inflammatory reaction from this infectious complication might also have promoted adhesion and growth of spilled tumour cells.
Postoperative immunosuppression is another factor for tumour recurrence or dissemination 6,7. Our patient was already immunosuppressed because of his underlying malignancy. The additional immunosuppression from the preceding surgery might also have enhanced peritoneal dissemination of the lymphoma.
It has been suggested that laparoscopy increases peritoneal seeding and recurrence of gastrointestinal cancers 8. Recent studies reported higher ratios of tumour seeding—both peritoneal and at port sites—in laparoscopic surgeries 3,8,16,17. Exposure to a pneumoperitoneum–laparoscopic environment is shown to enhance the invasive capacity of colonic adenocarcinomas by a protease-determined pathway 8. Also, blood flow within the peritoneum may be influenced by insufflation with carbon dioxide. Such hyperemia could increase the propensity of implanted tumour cells to metastasize in these sites 16. Tumour cells released into the operative field can therefore be made increasingly aggressive by a laparoscopic operative environment and can thus contribute to disease dissemination 8. Also, in laparoscopic surgeries, the risk of dissemination appears higher when a large number of malignant cells are present 17. Our patient had bulky intra-abdominal involvement. The surgery was intended to be laparoscopic and pneumoperitoneum was initially performed. The laparoscopic techniques and the bulky disease may therefore also be contributing factors for peritoneal dissemination.
Two other factors in our patient predisposed to peritoneal involvement. Preoperatively, the patient underwent liver biopsy and experienced spontaneous ascites infection. Liver biopsy, by causing mechanical contamination of the peritoneum with lymphoma cells, and spontaneous ascites infection, by causing inflammation at the peritoneum, might have facilitated the predisposing effects of the surgery for peritoneal lymphoma involvement in this particular patient.
4. CONCLUSIONS
We conclude that, as in our patient, peritoneal seeding and disease progression may be a potential complication of debulking splenectomy in lymphoma patients. Not only mechanical contamination, but also local peritoneal trauma with subsequent inflammation, postoperative immunosuppression, and factors related to laparoscopic techniques are potential causes of peritoneal seeding.
5. CONFLICT OF INTEREST DISCLOSURE
The authors declare no competing financial interests.
6. REFERENCES
- 1.Sugarbaker PH. A perspective on clinical research strategies in carcinoma of the large bowel. World J Surg. 1991;15:609–16. doi: 10.1007/BF01789207. [DOI] [PubMed] [Google Scholar]
- 2.Yeh CN, Chen MF, Jeng LB. Resection of peritoneal implantation from hepatocellular carcinoma. Ann Surg Oncol. 2002;9:863–8. doi: 10.1007/BF02557522. [DOI] [PubMed] [Google Scholar]
- 3.Neuhaus SJ, Watson DI, Ellis T, Rofe AM, Jamieson GG. The effect of immune enhancement and suppression on the development of laparoscopic port site metastases. Surg Endosc. 2000;14:439–43. doi: 10.1007/s004640000157. [DOI] [PubMed] [Google Scholar]
- 4.Lawrance RJ, Loizidou M, Cooper AJ, Alexander P, Taylor I. Importance of the omentum in the development of intra-abdominal metastases. Br J Surg. 1991;78:117–19. doi: 10.1002/bjs.1800780135. [DOI] [PubMed] [Google Scholar]
- 5.van den Tol MP, ten Raa S, van Grevenstein WM, van Rossen ME, Jeekel J, van Eijck CH. The post-surgical inflammatory response provokes enhanced tumour recurrence: a crucial role for neutrophils. Dig Surg. 2007;24:388–94. doi: 10.1159/000107781. [DOI] [PubMed] [Google Scholar]
- 6.Mels AK, Statius Muller MG, van Leeuwen PA, et al. Immunestimulating effects of low-dose perioperative recombinant granulocyte–macrophage colony–stimulating factor in patients operated on for primary colorectal carcinoma. Br J Surg. 2001;88:539–44. doi: 10.1046/j.1365-2168.2001.01722.x. [DOI] [PubMed] [Google Scholar]
- 7.Sietses C, Beelen RH, Meijer S, Cuesta MA. Immunological consequences of laparoscopic surgery, speculations on the cause and clinical implications. Langenbecks Arch Surg. 1999;384:250–8. doi: 10.1007/s004230050200. [DOI] [PubMed] [Google Scholar]
- 8.Paraskeva PA, Ridgway PF, Jones T, Smith A, Peck DH, Darzi AW. Laparoscopic environmental changes during surgery enhance the invasive potential of tumours. Tumour Biol. 2005;26:94–102. doi: 10.1159/000085816. [DOI] [PubMed] [Google Scholar]
- 9.Runyon BA, Hoefs JC. Peritoneal lymphomatosis with ascites. A characterization. Arch Intern Med. 1986;146:887–8. [PubMed] [Google Scholar]
- 10.van den Tol PM, van Rossen EE, van Eijck CH, Bonthuis F, Marquet RL, Jeekel H. Reduction of peritoneal trauma by using nonsurgical gauze leads to less implantation metastasis of spilled tumor cells. Ann Surg. 1998;227:242–8. doi: 10.1097/00000658-199802000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher B, Fisher ER, Fedustea N. Trauma and localization of the tumor cells. Cancer. 1967;20:23–30. doi: 10.1002/1097-0142(1967)20:1<23::aid-cncr2820200103>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 12.Bouvy ND, Marquet RL, Jeekel J. Laparoscopic surgery is associated with less tumour growth stimulation than conventional surgery: an experimental study. Br J Surg. 1997;84:358–61. [PubMed] [Google Scholar]
- 13.Eggermont AMM, Steller EP, Sugarbaker PH. Laparotomy enhances intraperitoneal tumor growth and abrogates the antitumour effects interleukin-2 and lymphokine-activated killer cells. Surgery. 1987;102:71–8. [PubMed] [Google Scholar]
- 14.Kuraoka S, Campeau JD, Nakamura RM. Modulation of postsurgical macrophage function by early postsurgical polymorphonuclear leukocytes. J Surg Res. 1992;53:245–50. doi: 10.1016/0022-4804(92)90042-x. [DOI] [PubMed] [Google Scholar]
- 15.Weese JL, Ottery FD, Emoto SE. Do operations facilitate tumor growth? An experimental model in rats. Surgery. 1986;100:273–7. [PubMed] [Google Scholar]
- 16.Brundell SM, Tsopelas C, Chatterton B, Touloumtzoglou J, Hewett PJ. Experimental study of peritoneal blood flow and insufflation pressure during laparoscopy. Br J Surg. 2002;89:617–22. doi: 10.1046/j.1365-2168.2002.02071.x. [DOI] [PubMed] [Google Scholar]
- 17.Canis M, Botchorishvili R, Wattiez A, et al. Cancer and laparoscopy, experimental studies: a review. Eur J Obstet Gynecol Reprod Biol. 2000;91:1–9. doi: 10.1016/s0301-2115(99)00251-1. [DOI] [PubMed] [Google Scholar]